Abstract
Tuberculosis is a major health concern. Non-living tuberculosis (TB) vaccine candidates may not only be safer than the current vaccine (BCG) but could also be used to boost BCG to enhance or elongate protection. No subunit vaccines, however, are currently available for TB. To address this gap and to improve the global TB situation, we have generated a defined subunit vaccine by genetically fusing the genes of 3 potent protein Mtb antigens, Rv2875, Rv3478 and Rv1886, into a single product: ID87. When delivered with a TLR4 agonist-based adjuvant, GLA-SE, ID87 immunization reduced Mtb burden in the lungs of experimentally-infected mice. The reduction in bacterial burden of ID87/GLA-SE immunized mice was accompanied by an early and significant leukocyte infiltration into the lungs during the infectious process. ID87/GLA-SE appears to be a promising new vaccine candidate that warrants further development.
Keywords: tuberculosis, mycobacteria, vaccine, adjuvant
Introduction
Two million people die from Mycobacterium tuberculosis (Mtb) infection each year and it is estimated that one third of the world’s population is infected [1]. The live, attenuated vaccine, M. bovis Bacillus Calmette-Guérin (BCG), has been routinely administered to infants and children since 1921, preventing tuberculosis (TB) dissemination and TB meningitis in children, but not preventing infection or active pulmonary TB (reviewed in [2, 3]). Results from BCG efficacy trials indicate disparate levels of protection, ranging from a complete lack of protection to protection for more than 50 years (reviewed in [4]). This variability may be attributed to the use of different BCG vaccine strains or immunization strategies, both of which vary in distinct geographic regions [4]. Regardless, the prevalent belief is that protection afforded by childhood BCG vaccination wanes over the course of a couple of decades such that young adults are no longer protected. The lack of a consistently protective vaccine highlights the need for additional strategies to prevent TB.
BCG currently remains the only vaccine recommended by the World Health Organization for the control of TB. Proposed TB vaccination strategies include administration of genetically-modified recombinant BCG (rBCG) incorporating Mtb proteins that are otherwise absent in the live, attenuated bacteria; Mtb DNA delivered with or without an adjuvant; and Mtb proteins delivered with an adjuvant. Each of these strategies have demonstrated the ability to reduce Mtb bacterial burden in preclinical models of TB [5–19]. Although rBCG are advancing toward large scale evaluations, their progression could be limited by safety concerns regarding their use in immune compromised individuals. Indeed, the WHO recommends that BCG not be administered to HIV+ individuals [20, 21]. A defined subunit vaccine may represent a better candidate for widespread use, To date, three subunit vaccines (MVA85B; Mtb72F formulated with AS02A or AS01 adjuvant; and AERAS-402) have advanced to phase 1 clinical trials where they have demonstrated good safety profiles [22–24].
Recently we characterized the responses of human peripheral blood mononuclear cells (PBMC) from tuberculin skin test-positive, TB disease free individuals to a large panel of Mtb proteins and then evaluated the ability of a selected antigen panel to protect mice from experimental Mtb infection [25]. Among the selected antigens, Rv3478, a protein from the PE/PPE family, and two secreted/membrane proteins, Rv2875 and Rv1886, were recognized by PPD+ but not PPD- individuals and demonstrated protective efficacy in mice [25]. Protection afforded by Rv2875 and Rv3478 were among the highest observed in the study, and Rv1886, also known as Ag85B, has previously been demonstrated to provide protection against Mtb [7, 14, 26]. As it has been demonstrated that delivery of Mtb antigens in the form of a fusion molecule is more efficacious than delivery of pooled antigens [26], we hypothesized that combining these antigens into a single fusion molecule, could enhance the protective efficacy. We therefore combined Mtb proteins Rv2875, Rv3478 and Rv1886 to create a single fusion molecule called ID87. Our data indicate that administration of ID87 formulated with the TLR4-based adjuvant, GLA-SE [27], promotes a strong Th1 immune response that reduces the lung Mtb burden following experimental infection.
Materials and Methods
Animals
Six week old, female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in the Infectious Disease Research Institute (IDRI) animal facility under specific pathogen free conditions. Animals were maintained under BSL3 conditions according to the regulations and guidelines of the IDRI Institutional Animal Care and Use Committee.
ID87 cloning and purification
ID87 was generated through a tandem fusion of the individual cloned and amplified genes of Rv2875, Rv3478, and Rv1886 using restriction site linkers. The recombinant pET28a plasmids (Novagen, Madison, WI) containing the individual Rv2875, Rv3478, and Rv1886 genes were previously described [25]. ID87 PCR primers were designed to incorporate specific restriction enzyme sites 5’ and 3’ of the gene of interest with primer sequences as follows: Rv2875-5’-NdeI, CAATTACATATGGGTAC-CCATCTCGCCAACGGTTCGATG; Rv2875-3’-SacI, CAATTAGAGCTCGTTGCAC-GCCCAGTTGACGAT; Rv3478-5’-SacI, CAATTAGAGCTCATGACCTCGCGTTTT-TGACG; Rv3478-3’-SalI, CAATTAGTCGACGCTGCTGAGGATCTGCTGGGA; Rv1886-5’-SalI, CAATTAGTCGACATGAATTTCGCCGTTTTGCCG; Rv1886-3’-HindIII, CAATTAAAGCTTTTAAGTACTGAAAAGTCGGGGTAGCGCCG. The DNA sequences were amplified from plasmid DNA templates using Pfx DNA polymerase (Invitrogen, Carlsbad, CA) with 30 cycles at 94 C for 15 s, 60 C for 30 s and 68 C for 1 h 30 min. The Rv2875 PCR product was digested with NdeI/SacI restriction enzymes then cloned into the pET28a vector. Rv3478 and the pET28a-Rv2875 plasmid were restriction digested with SacI/SalI and cloned to generate pET28a-Rv2875-3478 plasmid vector. The Rv1886 PCR product was digested with SalI/HindIII and ligated into the Sa1I/HindIII-cut pET28a-Rv2875-3478 vector. The resulting plasmid construct was named pET28a-ID87 and DNA sequence verified. The fusion gene product Rv2875-Rv3478-Rv1886 was renamed ID87, and encodes an 87 kDa protein containing an N-terminal six-histidine tag followed by a thrombin cleavage site and the M. tuberculosis genes of interest separated by restriction site linkers.
ID87 was expressed in E. coli host strainHMS-174 grown in 2xYS media at 37°C. Expression was induced with 0.5mM IPTG at an OD600= 0.6 and growth continued for 4 hours. Cultures were centrifuged at 6,000 × g and the cell pellet was resuspended in lysis buffer (20mM Tris pH 8.0, 5 mM EDTA, 100mM NaCl, Protease Inhibitor Cocktail (Sigma-Aldrich) and stored at −20°C. Cell pellets were thawed on ice and lysed using a M110S Microfluidizer (Microfluidics Corp.) with 3 passes at 60psi, and spun at 30,000 × g for 45 minutes. The ID93 fusion protein remained in the insoluble inclusion body fraction and was purified under denaturing conditions. The inclusion body was washed with 0.5% deoxycholate, 25 mM Tris pH 8.0, centrifuged at 10000 × g, solubilized in binding buffer (8M urea, 20 mM Tris pH 8, 25 mM DTT) and purified by Q Sepharose (GE Healthcare) and CHT Ceramic Hydroxyapatite (BioRad) column chromatography. Purified ID87 protein was quantified using the BCA protein assay (Pierce, Rockford, IL) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 4 – 20% Tris glycine gel (Invitrogen). The absence of E. coli contamination was confirmed by immunoblotting with horseradish peroxidase-conjugated rabbit polyclonal anti-E. coli antibody (1:1000, ViroStat, Inc.). Residual LPS contamination was determined to be less than 15 EU/mg of protein by the Limulus amoebocyte lysate assay (Cambrex Corp.).
Immunization of experimental animals
Mice were immunized subcutaneously at the base of tail with 0.5μg of recombinant ID87 in the presence or absence of 5μg GLA-SE. Immunizations were performed three times, with two weeks between each injection. Negative control mice received phosphate buffered saline (PBS). Mice were rested for one month after the final immunization prior to Mtb infection. Vaccine control mice received Pasteur strain BCG (Sanofi Pasteur, Paris, France) intradermally in a single dose of 5×104 colony forming units (CFU) two months prior to Mtb infection.
Aerosol infection of experimental animals
Mice were infected with Mtb strain H37Rv (American Type Tissue Culture Collection no. 35718) in an aerosol exposure chamber designed by the University of Wisconsin, College of Engineering Shops (Madison, WI). A low dose aerosol (50–100 CFU) of Mtb was delivered to the lungs of each animal. The average number of CFU delivered to the lungs of experimental animals was determined 24 hours post-infection enumerating CFU of 2–3 representative animals from each round of infection.
Organ homogenization, bacterial burden and cellular enumeration
The lungs and spleens were harvested from 4–7 mice per group. One lobe of the lung was fixed in 10% normal buffered formalin (NBF) for histological analysis. The remainder of the lung or spleen was homogenized through a 45μM cell strainer into cold media. An aliquot of organ homogenate was serially diluted in 0.1% Tween80 in PBS, plated on 7H10 plates (Molecular Toxicology, Boone, NC) and incubated for 14–21 days at 37ºC with 5% CO2 to determine bacterial burden. On day 4 post-infection, prior to extraction of the lungs, bronchioalveolar lavage was performed on deceased mice by insertion of flexible tubing into the trachea. The lungs were washed 5 times with 1mL sterile PBS, which was homogenized through a 45μM syringe filter (VWR) prior to freezing. Red blood cells in the remaining lung homogenate were lysed using red blood cell lysis buffer (EBioscience, San Diego, CA). The total number of cells in organ homogenates was counted using a Guava Cell Counter (Millipore) and a Guava Count reagent (Millipore) according to manufacturer instructions.
Flow cytometry
For cell surface staining directly ex vivo, aliquots of organ homogenates were fixed in 4% paraformaldehyde (PFA) (VWR, West Chester, PA) for at least 10 minutes at room temperature prior to removal from BSL3 conditions. Cells were stained in 20% normal mouse serum (VWR) as a blocking agent in PBS using fluorophore-conjugated monoclonal antibodies purchased from eBioscience (San Diego, CA) for 15 minutes at room temperature. For intracellular cytokine staining, aliquots of organ homogenates were re-stimulated for 1–2 hours at 37ºC with Mtb lysate (10μg/mL) or with ID87 fusion protein at a final concentration of 1μg/mL. GolgiPlug (BD Biosciences, San Jose, CA) was then added at a concentration of 1μg/mL and cells were incubated for a further 8 hours at 37ºC. Cells were fixed and permeablized using Cytofix/Cytoperm (BD Biosciences) for 30 minutes at room temperature and stained for 15 minutes at room temperature with fluorchrome conjugated flow cytometry antibodies purchased from EBioscience. Cells were washed 2 times with BD permeablization buffer prior to data collection using BD FacsDiva software (BD Biosciences) on a BD LSRII or Fortessa cytometer and analyzed using FlowJo software.
Histology
NBF fixed lung lobes were embedded in paraffin, sectioned and stained with hematoxylin and eosin as a purchased service by the Benaroya Research Institute Histology Core (Seattle, WA). Images were obtained at 10× magnification using a Nikon DS Camera Control Unit DS-L2 on a Nikon Eclipse E400 compound microscope.
ID87 antibody ELISA
Serum antibody levels were determined by ELISA. PolySorp plates (Nunc, Rochester, NY) were coated with 2μg/mL of ID87 in 0.1M bicarbonate buffer and incubated at 4ºC overnight. Plates were washed using 0.1% Tween20 in PBS and blocked for at least 2 hour at room temperature or overnight at 4ºC with 1% bovine serum albumin (BSA) in 0.25% Tween20/PBS. Serial dilutions of mouse serum were incubated on coated/blocked plates for 2 hours at room temperature, washed and incubated for at least 1 hour with anti-mouse IgG, IgG1 or IgG2a conjugated to streptavidin (VWR). Plates were washed using 0.1% Tween20/PBS and developed using SureBlue tetramethylbenzidine (TMB) substrate (Kirkegarrd & Perry Laboratories Inc, Gaithersburg, MD). The reaction was stopped after 1–2 minutes in 1 N H2SO4 and data were collected using an ELISA reader at 450nM wavelength, with a correction factor reading of 570nM.
Cytokine Expression
To assess cytokine levels in the airways 4 days following Mtb infection, the bronchioalveolar lavage fluid was analyzed by a Quantikine assay kit purchased from Pamomics (Fremont, CA). Fluid was incubated with polystyrene beads coated with antibodies against 12 different cytokines followed by secondary antibodies, and developed using a secondary antibody as per manufacturer recommendations. Florescence was measured on a Luminex 200 Multi-Analyte system and data were analyzed by Masterplex QT software (MiraiBio, Austin, TX).
Statistics
Statistical analyses were performed using Microsoft Excel or GraphPad Prism software. When analyzing parametric or log transformed data, Student’s T-test was performed to compare 2 groups of mice, while an ANOVA with a Dunnett post-test was used to compare more than 2 groups of mice. 4–7 mice per group per time point were included in all experiments, and data reported here are representative of at least 2 experiments.
Results
Immunogenicity: ID87/GLA-SE induces a Th1 skewed immune response
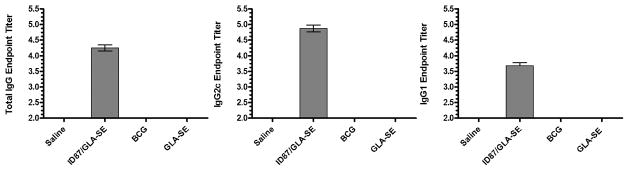
Mice were immunized with ID87 formulated in GLA-SE and the anti-ID87 IgG responses were assessed. As anticipated, one month after the three immunizations, significant levels of anti-ID87 IgG antibodies were detected in the serum of ID87/GLA-SE immunized, but not saline or GLA-SE only or BCG injected animals (Figure 1). Antibodies of both the IgG1 and IgG2c isotypes were elicited (Figure 1), although the IgG2c:IgG1 ratio indicated that the antibody response was skewed toward Th1 type immunity.
Figure 1. Antibody responses following ID87/GLA-SE immunization.
Mice were immunized with ID87/GLA-SE or injected with saline or GLA-SE alone, a total of 3 times with 2 weeks between injections. Four weeks after the final immunization ID87-specific serum antibody responses were determined by ELISA. Data are representative of at least 4 mice per group and of 2 independent experiments.
We also examined the systemic antigen-specific recall response by stimulating spleen cells ex vivo with ID87. While no difference was observed between groups in the percent of the spleen cells that were CD4+, CD8+ T cells constituted a lower proportion of the cells in the spleens of immunized mice (BCG = 7.93%, p-value = 0.0027 and ID87/GLA-SE = 10.43% p-value = 0.1257) than control, saline-injected mice (11.8%). Flow cytometry analyses indicated significant production of IFN-γ in antigen-experienced, memory (CD44+) T cells from ID87/GLA-SE immunized mice, but not from control, saline-injected or BCG-immunized mice (Figure 2A). Similarly, CD8+CD44+ T cells from the spleens of ID87/GLA-SE immunized mice produced significantly more IFN-γ in response to ID87 re-stimulation than those saline-injected or BCG-immunized mice (Figure 2B). Taken together, these data indicate that immunization with ID87/ GLA-SE promotes an antigen-specific Th1 response, the type believed to be highly relevant for protection against Mtb.
Figure 2. Antigen-specific Th1 responses are promoted by ID87/GLA-SE immunization.
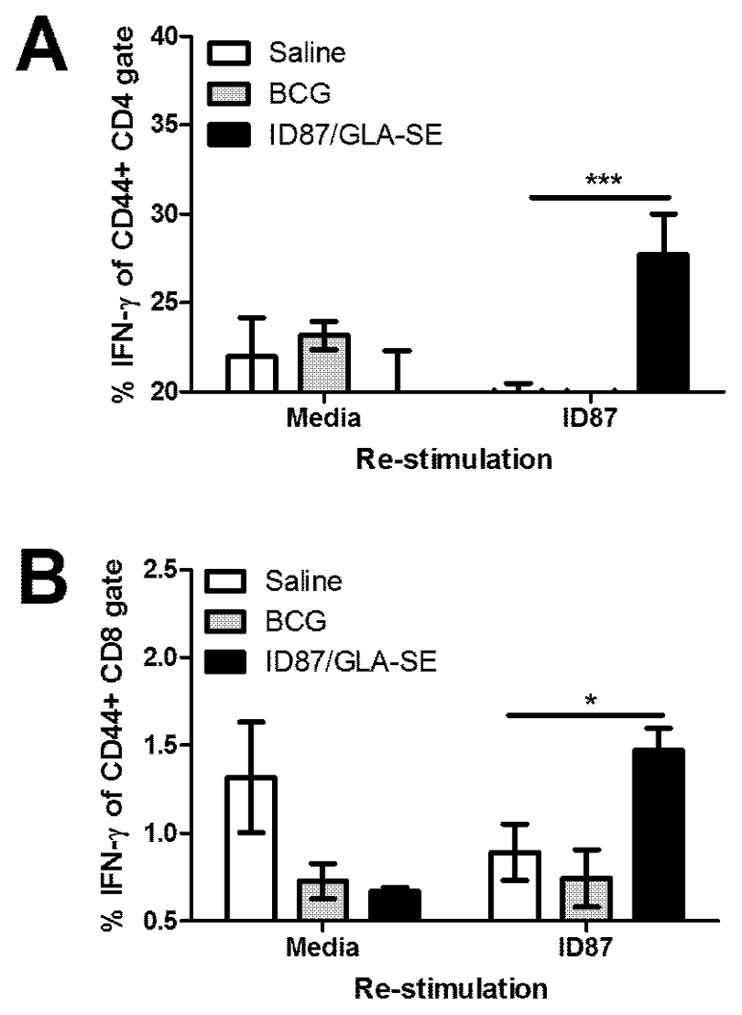
Four weeks after the second boost, spleen cells were stimulated with ID87 and IFN-γ production assessed by intracellular cytokine staining and flow cytometry. IFN-γ expression was assessed in memory (CD44+) (B) CD4+ and (C) CD8+ T cells. n = 4–7 mice per group, *p<0.05 and ***p< 0.001. Results are representative of 2 similar experiments.
ID87/GLA-SE immunization reduces bacterial burden in the lungs and spleens following M. tuberculosis infection
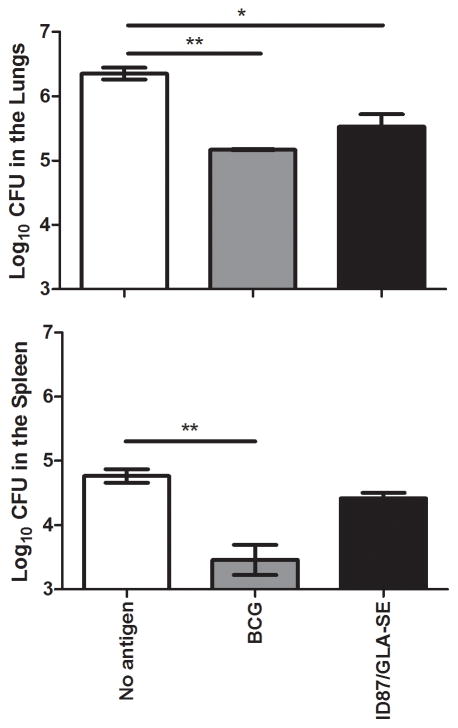
ID87/GLA-SE immunized mice, BCG immunized mice or saline injected control mice were infected with a low dose (50–100 CFU) of Mtb H37Rv, and bacterial burden in the lungs and spleens was assessed four weeks post-infection. As expected, the Mtb burden was reduced in the lungs (p<0.01) and spleens (p<0.01) of BCG immunized mice compared to saline injected mice 4 weeks post-infection. Bacterial burden was reduced in the lungs of ID87/GLA-SE immunized mice (p<0.05), but in this case, the reduction of bacterial burden in the spleens was not statistically significant (Figure 3).
Figure 3. ID87/GLA-SE immunization reduces bacterial burden in Mtb infected mice.
Bacterial burden was assessed in the lungs and spleens of mice immunized with ID87/GLA-SE or BCG, or injected with saline following low dose Mtb H37Rv aerosol infection. n = 4–7 mice per group, *p<0.05, **p<0.01 and ***p< 0.001. Results are representative of at least 2 similar experiments.
Greater early cellular infiltration of the lungs following Mtb infection in ID87/GLA-SE immunized mice
As accelerated granuloma formation can be predictive of vaccine-induced protection [28], we also assessed lung pathology during Mtb infection. Two weeks after infection, cellular infiltrates were not apparent in the control mice (saline or GLA-SE injected) but a cellular infiltration of the lungs was clearly visible in mice immunized with ID87/GLA-SE or BCG (Figure 4). Although granulomas were not detected at this time point, the cellular infiltrate in the immunized mice appeared to be loosely aggregating. Four weeks into infection, granulomas were detected in the lungs of mice from all experimental groups (Figure 4). At this time point, no differences in the size, composition or number of granulomas detected in the lungs from any group were detected.
Figure 4. Early cellular infiltration and granuloma in the lungs of ID87/GLA-SE immunized mice.
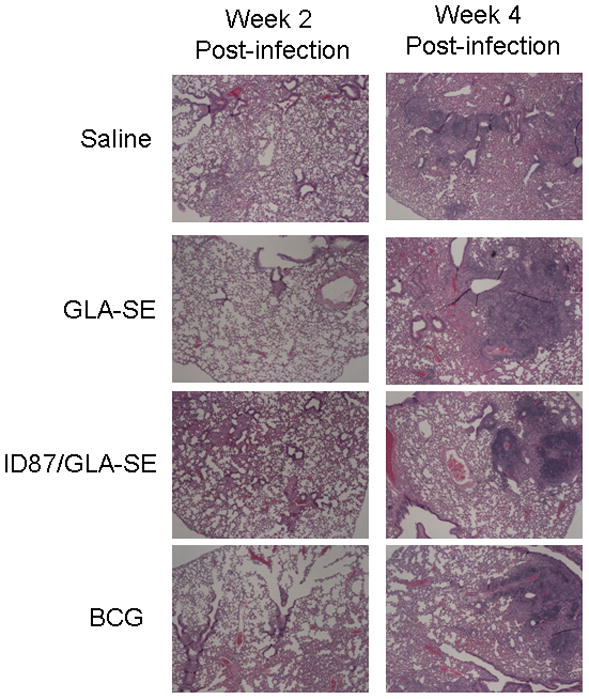
Histopathology was assessed in NBF fixed, paraffin embedded tissue sections by H&E staining. Lungs were examined at (A) 2 weeks and (B) 4 weeks following Mtb infection. Representative 4× magnified representative images from each group are shown.
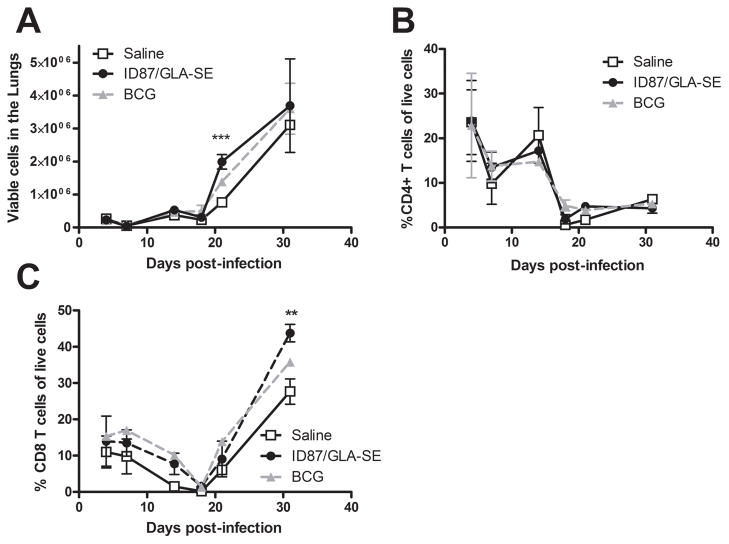
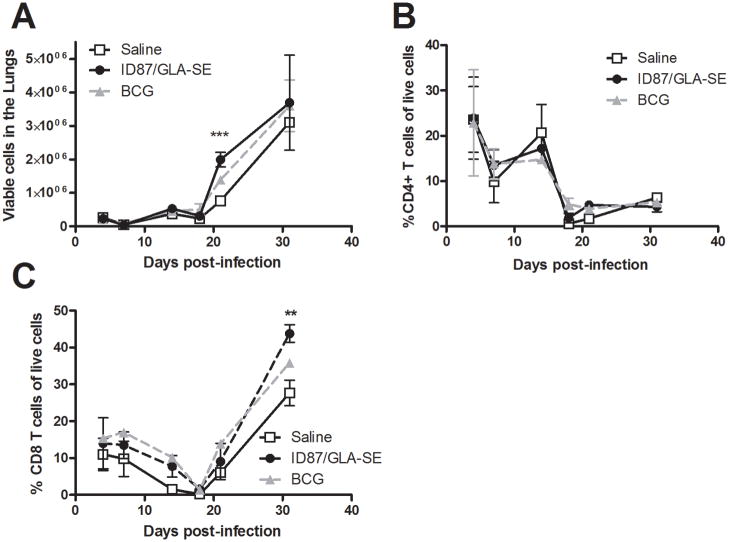
To investigate whether early inflammatory events within the lungs might be associated with vaccine-induced protection, inflammatory cytokines in bronchoalveolar lavage fluid collected 4 days post-Mtb infection were determined. We found a trend elevated IL-6 (Figure 5), but not TNF-α (data not shown), levels in the lungs of ID87/GLA-SE or BCG immunized mice compared to saline injected controls. To further characterize the lung infiltrate, cells were collected and phenotyped by flow cytometry. Significantly more viable cells were detected in the lungs of ID87/GLA-SE immunized mice than saline injected mice 3 weeks post-infection (Figure 6A, p<0.001). The increase in cellularity 3 weeks post-infection was not due to infiltration of any one population of cells, as flow cytometry did not show a significant increase in the total CD4+ T cells, CD8+ T cells, neutrophils, macrophages, dendritic cells or B cells at this time point (Figures 6B, 6C and data not shown). Interestingly, significantly more CD8+ T cells were detected in the lungs of ID87/GLA-SE immunized mice 4 weeks post-infection (Figure 5C, p<0.01).
Figure 5. Early IL-6 production in the lungs of immunized mice during Mtb infection.
Mice were infected with Mtb following immunization. IL-6 secretion into BAL fluid was assessed 4 days after infection by Luminex assay.
Figure 6. ID87/GLA-SE immunization accelerates cellular infiltration.
Mice were infected with Mtb following immunization. In (A), live cells in the lungs were enumerated. Cells were then subjected to flow cytometry to determine the total (B) CD4+ T cells and (C) CD8+ T cells.
Discussion
An effective subunit TB vaccine could provide a more defined and consistent vaccine. In this report, we have expanded upon our previous data to demonstrate that a fusion protein (ID87) consisting of Rv2875, Rv3875 and Rv1886, when formulated with the adjuvant GLA-SE, reduces bacterial burden in the lungs of Mtb-infected mice with similar efficacy to BCG. This work suggests that ID87/GLA-SE is a good candidate for further development.
Although the components of ID87 were previously validated as vaccine candidates using CpG as an adjuvant [25], in this study we used GLA-SE as it is similar in mode of action to the TLR4 agonist MPL that has been approved for use in human vaccines. We found that vaccination with ID87/ GLA-SE skewed responses toward a Th1 profile, with elevated IgG2c and IFNγ responses.
Because the C57BL/6 mouse is relatively resistant to TB, it is difficult to use a reduction in bacterial burden alone as an indicator of protection from Mtb infection. For this reason, Agger et al [28] have suggested that early granuloma formation may in fact be more predictive of protection from TB than a decline in bacterial burden when using the C57BL/6 mouse model of TB infection. In this study, both histology and flow cytometry indicated greater cellular infiltration occurred in the lungs of ID87/GLA-SE immunized mice compared to saline controls, and this may indicate the initiation of granulomas. While an increase in TNF-α, which is required for granuloma formation and maintenance, was not detected very early in BAL, a moderate increase in the pro-inflammatory cytokine IL-6 was detected in BAL from both ID87/GLA-SE and BCG immunized mice. The increased IL-6 may contribute to the early leukocyte infiltration into the lungs, and therefore may be associated with early control of TB and the subsequent reduction in bacterial burden. Ladel et al. have demonstrated that IL-6 production is required for control of TB, and this may be due to macrophage activation or due to a requirement for IL-6 in supporting a Th1 type immune response [29]. Future studies in our laboratory will be geared toward further resolving the mechanism of protection of mice from Mtb through subunit vaccine immunization.
Highlights
ID87/GLA-SE immunization yields IFN-γ producing memory T cells
ID87/GLA-SE immunization reduces Mycobacterium tuberculosis bacterial burden in C57BL/6 mice
Acknowledgments
The work presented in this manuscript was supported by National Institutes of Health grants AI-044373; AI-078054; AI-067251 and NIH contract HHSN272200800045C. All authors on this manuscript actively participated in experimental planning and/or data analysis and all authors participated in writing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Global tuberculosis control: surveillance, planning, financing. World Health Orgainization; 2009. [Google Scholar]
- 2.Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: efficacy and indications for vaccination and revaccination. J Pediatr (Rio J) 2006 Jul;82(3 Suppl):S45–54. doi: 10.2223/JPED.1499. [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. May 15;50(Suppl 3):S184–94. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 4.Singhal N, Bisht D, Joshi B. Immunoprophylaxis of tuberculosis: an update of emerging trends. Arch Immunol Ther Exp (Warsz) 2010 Apr;58(2):97–106. doi: 10.1007/s00005-010-0068-z. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, et al. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009 May 18;27(23):3063–71. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg TP, Vanonckelen A, et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998 Apr;66(4):1527–33. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derrick SC, Yang AL, Morris SL. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine. 2004 Dec 21;23(6):780–8. doi: 10.1016/j.vaccine.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, Deck RR, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996 Aug;2(8):893–8. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 9.Langermans JA, Doherty TM, Vervenne RA, van der Laan T, Lyashchenko K, Greenwald R, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005 Apr 15;23(21):2740–50. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 10.Lozes E, Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, et al. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997 Jun;15(8):830–3. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 11.McShane H, Behboudi S, Goonetilleke N, Brookes R, Hill AV. Protective immunity against Mycobacterium tuberculosis induced by dendritic cells pulsed with both CD8(+)- and CD4(+)-T-cell epitopes from antigen 85A. Infect Immun. 2002 Mar;70(3):1623–6. doi: 10.1128/IAI.70.3.1623-1626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4(+) t-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun. 2001 Feb;69(2):681–6. doi: 10.1128/IAI.69.2.681-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollenkopf HJ, Grode L, Mattow J, Stein M, Mann P, Knapp B, et al. Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect Immun. 2004 Nov;72(11):6471–9. doi: 10.1128/IAI.72.11.6471-6479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 2004 Oct;72(10):6148–50. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, et al. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One. 2009;4(4):e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, et al. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A. 2009 Feb 17;106(7):2301–6. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsenova L, Harbacheuski R, Moreira AL, Ellison E, Dalemans W, Alderson MR, et al. Evaluation of the Mtb72F polyprotein vaccine in a rabbit model of tuberculous meningitis. Infect Immun. 2006 Apr;74(4):2392–401. doi: 10.1128/IAI.74.4.2392-2401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasser Eddine A, Kaufmann SH. Improved protection by recombinant BCG. Microbes Infect. 2005 May;7(5–6):939–46. doi: 10.1016/j.micinf.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, et al. A Defined Tuberculosis Vaccine Candidate Boosts BCG and Protects Against Multidrug-Resistant Mycobacterium tuberculosis. Science Translational Medicine. 2010 October 13;2(53):53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, et al. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007 Jan 2;25(1):14–8. doi: 10.1016/j.vaccine.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Safety GACoV. Global Advisory Committee on Vaccine Safety. 2006. Nov 29–30, [Google Scholar]
- 22.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004 Nov;10(11):1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 23.Von Eschen K, Morrison R, Braun M, Ofori-Anyinam O, De Kock E, Pavithran P, et al. The candidate tuberculosis vaccine Mtb72F/AS02A: Tolerability and immunogenicity in humans. Hum Vaccin. 2009 Jul;5(7):475–82. doi: 10.4161/hv.8570. [DOI] [PubMed] [Google Scholar]
- 24.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, et al. The Novel TB Vaccine, AERAS-402, Induces Robust and Polyfunctional CD4 and CD8 T Cells in Adults. Am J Respir Crit Care Med. Feb 18; doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008 Dec 1;181(11):7948–57. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, et al. Exchanging ESAT6 with TB10.4 in an Ag85B Fusion Molecule-Based Tuberculosis Subunit Vaccine: Efficient Protection and ESAT6-Based Sensitive Monitoring of Vaccine Efficacy. The Journal of Immunology. 2005 May 15;174(10):6332–9. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 27.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLOS One. 2011;6(1):e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agger EM, Cassidy JP, Brady J, Korsholm KS, Vingsbo-Lundberg C, Andersen P. Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology. 2008 Jun;124(2):175–85. doi: 10.1111/j.1365-2567.2007.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladel C, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann S. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997 November 1;65(11):4843–9. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]