Abstract
Rodent models of absence seizures are used to investigate the network properties and regulatory mechanisms of the seizure's generalized spike and wave discharge (SWD). As rats age, SWDs occur more frequently, suggesting aging-related changes in the regulation of the corticothalamic mechanisms generating the SWD. We hypothesized that brain resetting mechanisms - how the brain “resets” itself to a more normal functional state following a transient period of abnormal function, e.g., a SWD - are impaired in aged animals and that brain infarction would further affect these resetting mechanisms. The main objective of this study was to determine the effects of aging, infarction, and their potential interaction on the resetting of EEG dynamics assessed by quantitative EEG (qEEG) measures of linear (signal energy measured by amplitude variation; signal frequency measured by mean zero-crossings) and nonlinear (signal complexity measured by the pattern match regularity statistic and the short-term maximum Lyapunov exponent) brain EEG dynamics in 4- and 20-month-old F344 rats with and without brain infarction. The main findings of the study were: 1) dynamic resetting of both linear and nonlinear EEG characteristics occurred following SWDs; 2) animal age significantly affected the degree of dynamic resetting in all four qEEG measures: SWDs in older rats exhibited a lower degree of dynamic resetting; 3) infarction significantly affected the degree of dynamic resetting only in terms of EEG signal complexity: SWDs in infarcted rats exhibited a lower degree of dynamic resetting; and 4) in all four qEEG measures, there was no significant interaction effect between age and infarction on dynamic resetting. We conclude that recovery of the brain to its interictal state following SWDs was better in young adult animals compared with aged animals, and to a lesser degree, in age-matched controls compared with infarction-injured animal groups, suggesting possible effects of brain resetting mechanisms and/or the disruption of the epileptogenic network that triggers SWDs.
Keywords: Absence seizures, spike wave discharges, photothrombosis, cortical infarction, aging, signal energy, signal frequency, signal complexity, EEG Dynamics, dynamic resetting
Introduction
Numerous studies have used rodent models of absence (petit mal) seizures to investigate the network properties and regulatory mechanisms of the seizure's ictal discharge. The defining EEG ictal event in these models is a 7-12 Hz generalized spike and wave discharge (SWD), characterized by abrupt onset, variable duration (seconds to minutes), and abrupt termination. SWDs typically occur during passive wakefulness and light sleep and at transitions of sleep states (Coenen et al., 1992; Willoughby and Mackenzie, 1992). Episodes of SWDs are characterized primarily by behavioral arrest and decreased responsiveness of the animal, with or without additional behavioral features (Buzsaki et al., 1990; Coenen et al., 1992; Vergnes et al., 1982; Willoughby and Mackenzie, 1992).
Two categories of animal models involving generalized SWDs exist: acquired SWD models and spontaneous SWD models. Acquired SWD models employ chemical agents to provoke SWDs or decrease the threshold for their expression. Alternatively, spontaneous SWD models utilize inherited factors leading to the development of spontaneous SWD activity, which more closely resembles spontaneous human epilepsy (Coenen et al., 1992). In the latter category of studies, WAG/Rij rats (Wistar Albino Glaxo strain, bred in Rijswijk, Netherlands) and genetic absence epilepsy rats from Strasbourg (GAERS) are commonly used because of the selective inbreeding that increases their predisposition to express generalized SWDs. While these particular rat strains appear most often in SWD studies, SWDs occur in many common inbred and outbred laboratory rat strains, which are not well recognized for SWD expression (Kelly, 2004). Inbred Wistar-unrelated strains that display generalized SWDs include Fischer 344 (F344), Brown Norway, and dark agouti (Coenen et al., 1992; van Luijtelaar and Coenen, 1986; Willoughby and Mackenzie, 1992). Outbred strains include Sprague-Dawley, Wistar, and Long-Evans (Buzsaki et al., 1990; Semba et al., 1980; Vergnes et al., 1982; Willoughby and Mackenzie, 1992).
Previous studies in rats have demonstrated that SWDs occur more frequently as the animals age (Buzsaki et al., 1990; Coenen et al, 1987; Vergnes et al., 1986; Willoughby and Mackenzie, 1992), up to hundreds of times a day in aged animals (van Luijtelaar et al., 1995). The cause of this increased rate of SWDs over an animal's lifespan is not known but suggests aging-related changes in the regulation of the corticothalamic mechanisms generating the SWD (Buzsaki, 1991). Based on the convergence of three lines of experimental evidence: 1) a nonlinear measure of brain dynamics suggests that age-related changes in SWDs may be associated with resetting mechanisms of brain dynamics (Nair et al., 2008); 2) a cortical focus drives widespread corticothalamic networks during SWDs (Meeren et al., 2002; Polack et al., 2007); and 3) decreased incidence (Kelly et al., 2006; Kharlamov et al., 2003) and shorter duration (Kelly et al., 2006) of SWDs occurs in infarcted young rats compared to age-matched controls, we sought to determine whether the pairing of advanced age and cortical infarction in rats would alter the form of resetting mechanisms of brain dynamics previously reported for aged animals (Nair et al., 2008).
Brain dynamics, the neurophysiological changes of brain that occur in time and in space, can be assessed by the changes of quantitative EEG (qEEG) properties such as signal energy, frequency, or complexity. These qEEG measures can be applied to the study of resetting mechanisms of brain dynamics, i.e., how the brain “resets” itself to a more normal functional state following a transient period of abnormal function, e.g., a SWD. Resetting related to SWD occurrence can be evaluated by comparing qEEG measurements in the periods before (preictal) to those after (postictal) the SWD. In this study, we estimated: 1) signal energy using amplitude variation (AV); 2) signal frequency using mean zero-crossings per second (ZC); and 3) complexity using the nonlinear statistical measures Pattern Match Regularity Statistic (PMRS) (Kelly et al., 2010; Shiau 2001; Shiau et al., 2004; 2010) and the Short-Term Maximum Lyapunov Exponent (STLmax) (Iasemidis et al., 1990; Iasemidis and Sackellares, 1996). Signal energy and frequency are conventional qEEG measures that are related to power spectrum (Fast Fourier Transform) analysis. Signal complexity measures are related to nonlinear dynamic statistics, which primarily characterize how ordered a time series is based on the temporal structure of the signal.
We applied these qEEG measures in this study to determine whether any or all of the measures could provide insight to specific brain (EEG) dynamics that are influential in brain resetting following SWDs. Based on the known increased frequency of SWDs in aged animals, we hypothesized that brain resetting mechanisms were impaired in aged animals and that photothrombotic cortical infarction (Kelly et al., 2001; Kharlamov et al., 2003) would further affect these resetting mechanisms. The main goals of this study were to determine the effects of aging, infarction, and their potential interaction on the resetting of EEG dynamics as measured by AV, ZC, PMRS, and STLmax.
Materials and Methods
Animals
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Allegheny-Singer Research Institute and were carried out according to NIH guidelines and regulations. Animals were housed individually, maintained in a 12 hr light/12 hr dark cycle environment with controlled temperature (23 ± 2C°), and provided food and water ad libitum. Sixteen F344 rats were divided into four groups for evaluation: 1) 4-month-old (n=4); 2) 4-month-old with infarction (n=4); 3) 20-month-old (n=4); and 4) 20-month-old with infarction (n=4).
Photothrombosis
Photothrombosis was performed according to Watson et al. (1985) with modifications (Kelly, 2006). Rats were anesthetized with ketamine and xylazine (9:1) and placed in a stereotaxic frame. A midline scalp incision was made and the scalp was retracted laterally. Rose bengal (20 mg/kg; Sigma) was injected through a catheter into the left femoral vein over 2 min, as the brain was stimulated through intact skull for 10 min by an argon laser-activated light beam (Lexel model 75, class IV, 514.5 nm, 15 amp power supply, 150 mW output). The incident beam was ~5 mm wide and focused 1.8 mm posterior to the bregma and 2.8 mm lateral to the midline corresponding to the area of the left sensorimotor cortex (Fig. 1A), creating ~25 mm3 cortical infarcts in both 4- and 20-month-old animals (Fig. 1B). The skull was cooled by continuous airflow from a fan to prevent heat-mediated tissue injury. Body temperature was monitored and maintained at 37°C using a thermo-regulated pad. After stimulation, the catheter was removed and incisions were sutured. Animals received a subcutaneous injection (5 ml) of lactated Ringer's solution immediately after surgery and each day postoperatively until adequate hydration and nutrition were reestablished.
Figure 1.
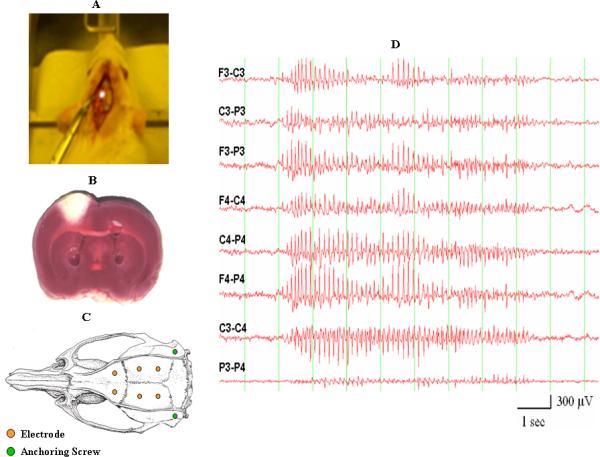
(A) Placement of an argon laser beam on the exposed skull of a rat. The argon laser beam is used to create the infarct in the lesioned groups of animals. (B) A cortical infarct generated by laser stimulation as assessed by TTC staining. (C) Placement of recording and anchoring screws on the rat's skull. (D) Sample of a generalized SWD recorded from a Fischer 344 rat.
Electrode placement
Electrodes were placed at least one week after animal lesioning. Six screws were placed in the skull and used as recording electrodes; two additional screws were placed laterally for anchoring the headset (Fig. 1C). The abbreviations (F3, C3, and P3) refer to skull screw electrodes that are placed on the left frontal, central, and parietal regions of the animal's brain, respectively; F4, C4, and P4 refer to the areas on the right. An exposed end of an insulated stainless steel wire was wrapped tightly around each recording screw; the other end was soldered into a pin contact. The six pin contacts were aligned in a plastic connector, which was secured to the skull with dental acrylic. The scalp was sutured and the animal recovered from anesthesia in a temperature-regulated chamber before return to the vivarium.
Video-EEG Recordings
Video-EEG recordings were obtained by serial connections from the animal's headset cable to the commutator of the recording chamber (Dragonfly) and the input box of a Stellate Systems EEG recording system. EEG amplifier outputs were cabled to a Compaq computer and processed by software (Harmonie, Stellate Systems) for EEG display. Video recordings were input to the computer and merged with EEG traces by compatible software (Diva, Stellate Systems). Eight EEG channels were generated for each animal (F3-C3, C3-P3, F3-P3, F4-C4, C4-P4, F4-P4, C3-C4, and P3-P4), sampling electrocerebral activity from both hemispheres; an “F3-C3” designation corresponds to the EEG channel produced by the output of one differential amplifier with inputs from the F3 and C3 electrodes. Because the F4 electrode was used as a linked common reference electrode for each set of four simultaneously recorded animals - the Stellate recording system utilized a single common reference - derivations including the F4 electrode (i.e., F4-C4, F4-P4) were excluded from analysis. Video-EEG data were stored on computer hard drives during acquisition and transferred to external hard drives for offline analysis.
EEG samples with SWD activity
One hundred SWD-containing EEG epochs were sampled initially from each rat within several months of electrode implantation. Each epoch included: 1) the 2-min period of baseline EEG activity that occurred immediately before (preictal) the SWD; 2) the SWD itself; and 3) the EEG activity immediately after (postictal) the SWD up to a total epoch time of 4 min. In order to have clear preictal and postictal periods for assessing resetting of EEG dynamics, epochs with SWDs that did not have a clear offset were excluded from analysis. In addition, SWDs that lasted less than 3 sec - a duration that was considered to be potentially too short to affect resetting mechanisms - were also excluded from analysis. Of note is that post-sampling review of the EEG epochs from 20-month-old animals revealed far more SWDs without clear offsets or with durations less than 3 sec compared with those sampled from 4-month-old animals. These findings accounted for most of the overall reduction in analyzed EEG epochs from aged animals. Therefore, fewer “aged” SWDs included in the analysis was due to the properties of the SWDs, not the frequency of their occurrences. As a result, a total of 545 EEG epochs were included in this study: 4-month-old (n = 183); 4-month-old with infarction (n = 188); 20-month-old (n = 92); 20-month-old with infarction (n = 82). Figure 1D shows an example of a generalized SWD.
Data analysis and statistics
Estimation of EEG dynamics: For each of the 4-min EEG epochs, qEEG measurements (AV, ZC, PMRS, and STLmax) were calculated for each sequential 5-sec non-overlapping EEG segment for each of the six recording channels (excluding F4-C4, F4-P4). Amplitude variation (AV), a measure that reflects signal energy, is the standard deviation of the EEG signal within the calculation window; it is a statistical measure that increases with larger signal peaks (amplitude changes). Mean zero-crossings (ZC) is estimated by the average number of occurrences of positive zero crossings (after 0-mean shifted) within the calculation window. ZC increases when the frequency of the main waveform becomes larger. PMRS measures the complexity of the EEG signal based on the repeatability of the signal patterns. Mathematically, PMRS estimates the probability of pattern similarity (i.e., stationary parts) for a given time series. PMRS has been applied for detecting EEG state changes, especially seizures (Shiau, 2001; Shiau et al., 2004; 2010; Kelly et al., 2010). Major advantages of PMRS are its ability to be interpreted in both stochastic and chaotic models, along with quick computation. STLmax measures the stability of the EEG signal by estimating the largest Lyapunov exponent of a state space attractor reconstructed from the original EEG time series. Mathematically, it measures how fast two neighboring trajectories diverge (or converge) over a short period of time (Iasemidis et al., 1990; Iasemidis and Sackellares, 1996).
Figure 2 demonstrates the four qEEG measurements used in this study calculated over a 4-min EEG (channel C3-C4) containing a 10-sec SWD recorded in the middle. Each qEEG value represents the signal characteristic of a 5-sec epoch. Except for STLmax, signals during the SWD caused significant changes in qEEG values, and also affected the values afterwards. This observation constituted the basis of the “dynamic resetting” investigated in this study, which was defined as the change of qEEG measures calculated in a postictal period compared to the corresponding preictal period. That is, for each SWD, its dynamic resetting (DR) was quantified as: DR = average of (qEEGpostictal – qEEGpreictal) over 6 channels. For each of the qEEG measures, this study tested the null hypotheses that: 1) there was no effect of the “age” factor on the mean DR; 2) there was no effect of the “infarction” factor on the mean DR; and 3) there was no interaction between “age” and “infarction” factors on the mean DR. The hypotheses were tested using a nested two-factor ANOVA test, in which rats represent a nested factor. Effects with a p-value < 0.05 were considered significant.
Figure 2.
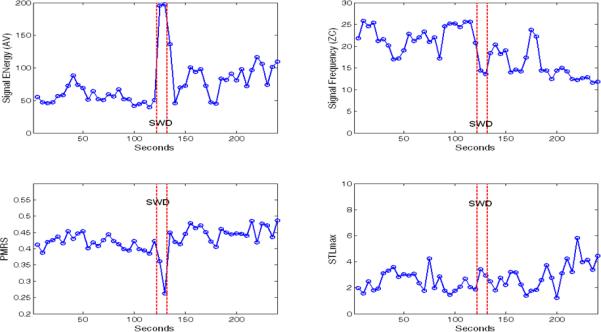
Quantitative EEG (qEEG) measures, AV (μV), ZC (Hz), PMRS (-ln of probability), and STLmax (bits/sec), calculated over a 4-minute EEG (channel C3-C4) containing a 10-second SWD recorded in the middle. Each qEEG value represented the signal characteristic of a 5-second EEG epoch.
Results
General Considerations
qEEG Analysis and Statistical Test
Signal Energy
Overall, AV values increased from the preictal to the postictal period (~18.6% increase), indicating that the signal after the SWD contained higher energy compared to that in the preictal period. The mean AV increase (over all SWDs) in each rat is shown in Fig. 3A; 4-month-old rats had larger AV increases than 20-month-old rats, especially for the non-infarction groups (blue circles). Distinctions between with and without infarction groups are less clear (except the two 4-month-old rats with infarction). These observations were further suggested by the statistical tests: the AV increase was significantly affected by the “age” factor (4-month-old / 20-month-old = 2.76; p = 0.006), but was not significantly affected by the “infarction” factor (no infarction / infarction = 1.28; p = 0.123). There was insufficient evidence to reject the null hypothesis that there was no interaction effect between “age” and “infarction” (p = 0.260).
Figure 3.
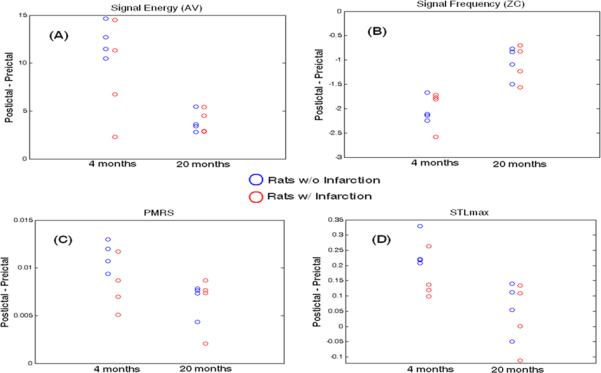
Resetting (postictal – preictal) quantification for each rat (mean over all 6 channels) with respect to each of the studied qEEGs. The closer the value is to zero, the smaller is the degree of the resetting effect from the SWDs. Non-infarcted animals (blue circles); infarcted animals (red circles).
Signal Frequency
Overall, ZC values decreased from the preictal to the postictal period (~ 8.3% reduction), indicating that the signal after the SWD was slower compared to that in the preictal period. The mean ZC decrease (over all SWDs) in each rat is shown in Fig. 3B; 4-month-old rats had larger ZC reductions than 20-month-old rats, for both infarction and non-infarction groups (blue circles). Distinctions between with and without infarction groups are not obvious. These observations were further suggested by the statistical tests: the ZC decrease was significantly affected by the “age” factor (4-month-old / 20-month-old = 1.92; p < 0.001), but was not significantly affected by the “infarction” factor (no infarction / infarction = 1.02; p = 0.662). The interaction effect between “age” and “infarction” was not significant (p = 0.567).
PMRS
Overall, PMRS values increased from the preictal to the postictal period (~ 2% increase), indicating that the signal after SWD was more complex compared to that in the preictal period. The mean PMRS increase (over all SWDs) in each rat is shown in Fig. 3C; 4-month-old rats had larger PMRS increases than 20-month-old rats; however, the distinction became smaller in infarction rat groups. This was mainly due to the smaller “PMRS resetting” in 4-month-old rats with infarction. These observations were further suggested by the statistical tests: the PMRS increase was significantly affected by the “age” factor (4-month-old / 20-month-old = 1.45; p = 0.03) and was also significantly affected by the “infarction” factor (no infarction / infarction = 1.25; p = 0.031). Similarly, the data did not suggest significant interaction effect between “age” and “infarction” (p = 0.219).
STLmax
Observations in STLmax analysis were similar to those for PMRS. Overall, STLmax values increased from the preictal to the postictal period (~ 6% increase). The mean STLmax increase (over all SWDs) in each rat is shown in Fig. 3D; 4-month-old rats had larger STLmax increases than 20-month-old rats; however, the distinction became smaller in infarction rat groups (also due to the smaller “STLmax resetting” in 4-month-old rats with infarction). These observations were further suggested by the statistical tests: the STLmax increase was significantly affected by the “age” factor (4-month-old / 20-month-old = 4.12; p = 0.005), but was not significantly affected by the “infarction” factor (no infarction / infarction = 1.66; p = 0.062). There was no significant interaction effect between “age” and “infarction” (p = 0.219).
P-values for all the ANOVA tests are summarized in the Table.
Table.
Significance (p-value) of Factor Effect on Dynamic Resetting
| Interaction | Age | Infarction | |
|---|---|---|---|
| Signal Energy (AV) | 0.260 | 0.006* | 0.123 |
| Signal Frequency (ZC) | 0.567 | < 0.001* | 0.662 |
| PMRS | 0.219 | 0.030* | 0.031* |
| STLmax | 0.272 | 0.005* | 0.062 |
statistically significant at 0.05 significance level
Discussion
Using a spontaneous SWD rat model, the main findings of this study were: 1) dynamics of EEG characteristics (signal energy, frequency, and complexity) were influenced following the occurrences of SWDs, referred to as “dynamic resetting”; 2) animal age significantly affected the degree of dynamic resetting as measured by all four qEEG measures studied: SWDs in older rats exhibited a lower degree of dynamic resetting; 3) infarction significantly affected the degree of dynamic resetting only in terms of EEG signal complexity: SWDs in infarcted rats exhibited a lower degree of dynamic resetting; and 4) in all four qEEG measures, there was no significant interaction effect between age and infarction on dynamic resetting, i.e., there was insufficient evidence that the difference in dynamic resetting between the two age groups was affected by the infarction condition of the animals, or vice versa.
Results of this study raise several issues regarding the relationships of animal age and/or cortical injury to the alteration of brain dynamics that affects the occurrence of SWDs, as well as the quantitative measures used in assessing this alteration. Our findings confirmed a preliminary study that found a lower degree of STLmax resetting in 20-month-old compared to 4-month-old animals, as well as significant differences in postictal STLmax values between the animal groups but not in preictal STLmax values (data not shown) (Nair et al., 2008). Importantly, the present study demonstrated that all of the applied dynamical measures, both linear (signal energy and frequency) and nonlinear (signal complexity), indicated less dynamic resetting following SWDs in aged F344 rats. This alteration in dynamic resetting may have less to do with animal senescence per se - and its associated increase in SWD frequency - than a common mechanism operative at any age when there is a clear acceleration of SWD occurrence. This would apply to the genetically-driven, aging-related increased SWD frequency phenotype observed at much earlier ages in WAG/Rij and GAERS rat strains; dynamic resetting has not been tested over early animal ages in these strains when SWD frequency increases dramatically.
In a study by Meeren et al. (2002), a consistent focus within the peri-oral region of the somatosensory cortex was found to drive widespread corticothalamic networks during SWDs in WAG/Rij rats, a finding contrary to the conventional theory that generalized seizures begin in midline brain structures and spread simultaneously to bilateral cerebral hemispheres. This finding has been supported subsequently by studies in the GAERS strain (Polack et al., 2007) and in children with typical absence seizures based on changes in fMRI blood oxygen level-dependent (BOLD) signal in parietal cortex preceding SWDs (Carney et al., 2010). Based on the finding of Meeren et al. (2002), we used the photothrombosis model to determine whether infarction that involved the somatosensory cortex would result in an alteration of SWDs (Kharlamov et al., 2003). We found that lesioned young adult animals demonstrated fewer SWDs - typical absence discharges associated with motor arrest - than age-matched controls, but more “generalized pseudoperiodic spike discharges,” i.e., intermittent solitary spike discharges of 0.5-1.0 Hz that could last up to a minute or longer associated with altered animal behavior. The spike morphology and polarity of these latter discharges consistently matched those of typical SWDs and could occur between or after SWDs, suggesting the discharges might represent non-propagating absence events and raised the possibility that cortical lesions could injure or ablate focal cortical generators of SWDs and disrupt their network propagation. Importantly, cortical photothrombosis has been used recently to study the survival and reorganization of neurons of the inhibitory reticular thalamic nucleus, which regulate thalamocortical transmission and generate cerebral rhythms, including those involved in thalamocortical epilepsies (Paz et al., 2010).
We sought to duplicate our finding of decreased SWDs in young adult animals lesioned by cortical photothrombosis (Kharlamov et al., 2003) in a subsequent study of cortical infarction generated by transient unilateral middle cerebral artery and common carotid artery occlusion (MCA/CCAo) (Kelly et al., 2006). MCA/CCAo resulted in markedly decreased SWD frequency (discharges/hour) and duration compared to age-matched control animals; however, there was no significant change in the frequency or duration of generalized pseudoperiodic spike discharges (Kelly et al., 2006).
Although the effects of infarction on SWD frequency were clearly evident in these previous studies, the mechanisms by which they occurred are not known. The possibility that dynamic resetting mechanisms are altered by brain injury prompted the present study, which tested the effects of brain injury on dynamic resetting in both young adult and aged animals. The results of the present study suggest that a nonlinear measure of brain complexity, the PMRS, may be the most sensitive among the qEEG measures used to assess dynamic resetting, given that it demonstrated independent main effects of age and infarction on dynamic resetting. The reason for this is not clear but may be due to its ability to quantify signal complexity in either a deterministic or stochastic process, even in a nonstationary time series. However, given that PMRS reflects signal properties similar to that of STLmax, which only suggested a moderate effect (p = 0.06) of infarction on dynamic resetting, there may be only a modest effect of infarction on resetting mechanisms in these studies. This would suggest that the effects of infarction on reducing seizure frequency (Kelly et al., 2006; Kharlamov et al., 2003) may be due to a different mechanism, e.g., disruption of the network that triggers SWDs, which has a stronger effect than resetting on SWD occurrence. Interestingly, our results did not provide evidence of interactive effects of age and infarction, including the PMRS measure, a result possibly related to the relatively small volume of infarction that resulted from the photothrombosis method.
Studies of intracranial EEG recordings obtained from patients with temporal lobe epilepsy demonstrated that seizures occur following a transition of “preictal entrainment” and are followed by a “postictal disentrainment,” where “entrainment” was basically defined and quantified by the convergence of STLmax values among recording brain regions (Sackellares et al., 2002; Iasemidis et al., 2004). The combined phenomenon of preictal entrainment and postictal disentrainment was termed “dynamical resetting of the epileptic brain.” Based on these results, the authors postulated that after a brain sustained massive dynamical entrainment of its electrical activity, a seizure needed to occur in order to reset the brain back to a more normal interictal state, similar to the theory of electroconvulsive therapy (ECT), a treatment modality used for several neurological disorders (Fink, 2000). If this is indeed the case, then it is reasonable to further hypothesize that when the resetting ability of a brain is altered (weakened) by either an internal (e.g., aging) or an external (e.g., injury) factor, the brain might evolve into a more severe pathological condition that results in an increase of seizure frequency and/or duration. An extreme case under such a condition would be “status epilepticus,” in which seizures occur continuously or successively, at brief intervals, without recovery of consciousness between seizures.
In this study using several measures of EEG dynamics, we observed significant differences in “brain resetting” between young adult and aged rat groups (young > old), as well as between age-matched control and infarction-injured rat groups (control > injured). Based on the findings of previous studies (Sackellares et al., 2002; Iasemidis et al., 2004), we interpret the present set of results to indicate that the recovery of the brain back to its normal interictal state following SWDs was better in young adult animals compared with aged animals, as well as in age-matched controls compared with infarction-injured animal groups. In addition, the resetting difference due to age seemed to be more obvious than that due to infarction, which might be associated with the large difference in ages (4-month-old vs. 20 month-old animal groups) vs. a relatively modest photothrombotic brain injury in the infarction animal groups. Another interesting observation was that infarction had more effect on brain resetting in younger animals. This could be due to older animals having a more abnormal baseline condition of brain function that potentially would not be worsened substantially by a small cortical infarct. A study with a larger sample size that also takes into account the duration and frequency of SWDs in each rat will further verify these hypotheses and address the unexplored issue of whether SWD duration could have an impact on the speed of resetting mechanisms and SWD frequency. These investigations could eventually lead to a better understanding of the mechanism of absence seizures, as well as to the design of improved treatments for controlling these events in patients.
In conclusion, this study provides new, relevant, and applicable results in an underappreciated field borrowing from epileptology, stroke studies, and brain aging electrophysiology models. As it is becoming increasingly clear that rodent models of aging can mirror human statistics on the increased incidence of epilepsy with age, the results of this study raise the possibility that brain aging mechanisms and affected networks should be considered in the context of an epileptic background.
Research Highlights.
EEG dynamics were altered after SWDs, referred to as “dynamic resetting”
SWDs in aged rats had a lower degree of dynamic resetting across all qEEG measures
SWDs in infarcted rats had a lower degree of dynamic resetting by signal complexity
There was no interaction effect between age and infarction on dynamic resetting
Acknowledgments
Epilepsy Foundation Research Initiative for Seniors Award (SPN), Pennsylvania Department of Health Research for the Formula Fund RFA 04-07-09 SAP 4100031268 (KMK), and NINDS R01 NS046015 (KMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure / Conflict of Interest
The authors have no duality of interest to declare.
References
- Buzsáki G, Laszlovszky I, Lajthma A, Vadász C. Spike-and-wave neocortical patterns in rats: Genetic and aminergic control. Neuroscience. 1990;38:323–333. doi: 10.1016/0306-4522(90)90031-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Smith A, Berger S, Fisher LJ, Gage FH. Petit mal epilepsy and parkinsonian tremor: hypothesis of a common pacemaker. Neuroscience. 1990;36(1):1–14. doi: 10.1016/0306-4522(90)90345-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41:351–364. doi: 10.1016/0306-4522(91)90332-i. [DOI] [PubMed] [Google Scholar]
- Carney PW, Masterton RAJ, Harvey AS, Scheffer IE, Berkovic SF, Jackson GD. The core network of absence epilepsy: differences in cortical and thalamic BOLD response. Neurology. 2010;75:904–911. doi: 10.1212/WNL.0b013e3181f11c06. [DOI] [PubMed] [Google Scholar]
- Coenen AM, van Luijtelaar EL. The WAG/Rij rat model for absence epilepsy: Age and sex factors. Epilepsy Res. 1987;1:297–301. doi: 10.1016/0920-1211(87)90005-2. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Drinkenburg WH, Inoue M, van Luijtelaar EL. Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats. Epi. Res. 1992;12(2):75–86. doi: 10.1016/0920-1211(92)90029-s. [DOI] [PubMed] [Google Scholar]
- Fink M. Electroshock revisited. Am. Sci. 2000;88:162–167. [Google Scholar]
- Iasemidis LD, Sackellares JC, Zaveri HP, Williams WJ. Phase space topography and the Lyapunov exponent of the electrocorticogram in partial seizures. Brain Topogr. 1990;2(3):187–201. doi: 10.1007/BF01140588. [DOI] [PubMed] [Google Scholar]
- Iasemidis LD, Sackellares JC. Chaos theory and epilepsy. Neuroscientist. 1996;2:118–126. [Google Scholar]
- Iasemidis LD, Shiau DS, Sackellares JC, Pardalos PM, Prasad A. Dynamical resetting of the human brain at epileptic seizures: application of nonlinear dynamics and global optimization techniques. IEEE Trans. Biomed. Eng. 2004;51(3):493–506. doi: 10.1109/TBME.2003.821013. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Kharlamov A, Hentosz TM, Kharlamov EA, Williamson JM, Bertram EH, Kapur J, Armstrong DM. Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res. 2001;47:189–203. doi: 10.1016/s0920-1211(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Kelly KM. Spike-wave discharges: absence or not, a common finding in common laboratory rats. Epilepsy Curr. 2004;4(5):176–177. doi: 10.1111/j.1535-7597.2004.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM. Stroke. In: Pitkanen A, Moshe S, Schwartzkroin PA, editors. Models of Seizures and Epilepsy. Elsevier; San Diego: 2006. pp. 501–519. [Google Scholar]
- Kelly KM, Jukkola PI, Kharlamov EA, Downey KL, McBride JW, Strong R, Aronowski J. Long-term video-EEG recordings following transient unilateral middle cerebral and common carotid artery occlusion in Long-Evans rats. Exp. Neurol. 2006;201:495–506. doi: 10.1016/j.expneurol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Shiau DS, Kern RT, Chien JH, Yang MCK, Yandora KA, Valeriano JP, Halford JJ, Sackellares JC. Assessment of a scalp EEG-based automated seizure detection system. Clin. Neurophys. 2010;121(11):1832–1843. doi: 10.1016/j.clinph.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharlamov EA, Jukkola PI, Schmitt KL, Kelly KM. Electrobehavioral characteristics of epileptic rats following photothrombotic brain infarction. Epilepsy Res. 2003;56:185–203. doi: 10.1016/j.eplepsyres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J. Neurosci. 2002;22(4):1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SP, Jukkola PI, Quigley M, Wilberger A, Shiau DS, Sackellares JC, Pardalos PM, Kelly KM. Absence seizures as resetting mechanisms of brain dynamics. Cybern. Syst. Anal. 2008;44:664–672. doi: 10.1007/s10559-008-9051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Christian CA, Parada I, Prince DA, Huguenard JR. Focal cortical infarcts alter intrinsic excitability and synaptic excitation in the reticular thalamic nucleus. J. Neurosci. 2010;30(15):5465–5479. doi: 10.1523/JNEUROSCI.5083-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack P, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep Layer Somatosensory Cortical Neurons Initiate Spike-and-Wave Discharges in a Genetic Model of Absence Seizures. J. Neurosci. 2007;27(24):6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackellares JC, Iasemidis LD, Pardalos PM, Shiau DS. Combined Application of Global Optimization and Nonlinear Dynamics to Detect State Resetting in Human Epilepsy. In: Pardalos PM, Principe JC, editors. Biocomputing. Kluwer Academic Publishers; 2002. [Google Scholar]
- Semba K, Szechtman H, Komisaruk BR. Synchrony among rhythmical facial tremor, neocortical ‘alpha’ waves, and thalamic non-sensory neuronal bursts in intact awake rats. Brain Res. 1980;195(2):281–298. doi: 10.1016/0006-8993(80)90065-7. [DOI] [PubMed] [Google Scholar]
- Shiau DS. Ph.D. Dissertation. University of Florida; 2001. Signal identification and forecasting in nonstationary time series data. [Google Scholar]
- Shiau DS, Iasemidis LD, Yang MCK, Carney PR, Pardalos PM, Suharitdamrong W, Nair SP, Sackellares JC. Pattern-match regularity statistic - A measure quantifying the characteristics of epileptic seizures. Epilepsia. 2004;45(S7):85–86. [Google Scholar]
- Shiau DS, Halford JJ, Kelly KM, Kern RT, Inman M, Chien JH, Pardalos PM, Yang MCK, Sackellares JC. Signal regularity-based automated seizure detection system for scalp EEG monitoring. Cybern. Syst. Anal. 2010;46(6):922–935. doi: 10.1007/s10559-010-9273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Luijtelaar EL, Coenen AM. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci. Lett. 1986;70(3):393–397. doi: 10.1016/0304-3940(86)90586-0. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar EL, Ates N, Coenen AM. Role of L-Type Calcium Channel Modulation in Nonconvulsive Epilepsy in Rats. Epilepsia. 1995;36(1):86–92. doi: 10.1111/j.1528-1157.1995.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Micheletti G, Reis J, Depaulis A, Rumbach L, Warter JM. Spontaneous paroxysmal electroclinical patterns in rat: a model of generalized non-convulsive epilepsy. Neurosci. Lett. 1982;33(1):97–101. doi: 10.1016/0304-3940(82)90136-7. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Depaulis A, Micheletti G, Warter JM. Ontogeny of spontaneous petit-mal like seizures in Wistar rats. Dev. Brain Res. 1986;30:85–87. doi: 10.1016/s0006-8993(86)80011-7. [DOI] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, Wachte MS, Ginsburg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L. Nonconvulsive electrocorticographic paroxysms (absence epilepsy) in rat strains. Lab. Anim. Sci. 1992;42(6):551–554. [PubMed] [Google Scholar]


