Abstract
Given the poor immunogenicity of current H5N1 influenza vaccines, additives and adjuvants remain a viable solution for increasing efficacy. Here, we demonstrate that a 20-amino acid peptide (EB) possessing influenza antiviral activity also enhances the immune response to H5N1 vaccination in mice. The addition of EB to formalin-inactivated whole-virus vaccine induced virion aggregation and these aggregates were readily engulfed by phagocytic cells in vitro. In vivo, mice vaccinated with a suboptimal dose of inactivated vaccine containing EB peptide had reduced morbidity, improved viral clearance, and faster recovery than mice receiving vaccine alone. This phenomenon was not accompanied by an increase in virus-specific antibodies. Instead, cell-mediated immunity was enhanced as demonstrated by increased interferon-γ production from splenocytes. This data demonstrates that the EB peptide may a useful adjuvant for boosting the efficacy of poorly immunogenic influenza vaccines.
Keywords: H5N1 influenza virus, inactivated influenza vaccine, adjuvant, cell-mediated immunity
1. INTRODUCTION
The 2009 H1N1 influenza virus pandemic clearly demonstrated the importance of influenza vaccination in reducing infections rates and controlling virus dissemination in large populations [1, 2]. Despite the fact that influenza vaccination programs have existed for over 40 years [3], several limitations exist in both the production of vaccine stocks, and the immunological response to influenza virus. First, influenza viruses undergo constant mutation as they circulate in human and animal populations, allowing them to successfully evade host immune responses to previously encountered virus variants [4, 5]. Second, influenza vaccines are most commonly produced in embryonated chicken eggs; a process which can be severely affected by contamination of eggs, poor replication of seed viruses, and egg supply shortages [6–9]. Vaccine seed viruses chosen for production and distribution may be mismatched to the circulating strain, providing inadequate protection [10]. Additionally, influenza vaccines are often less effective in high risk groups such as the very young, elderly or immunocompromised [11]. Finally, vaccines against certain influenza subtypes, such as H5N1 viruses, are poorly immunogenic [12–14]. These drawbacks highlight the need to continually evaluate and improve upon our existing vaccines.
Highly pathogenic avian H5N1 viruses have been at the forefront of the influenza vaccine discussion since they crossed the avian to human species barrier in 1997. Over the past decade, H5N1 viruses have become endemic to poultry populations in many parts of the world, broadened their host range, and are associated with a 60% mortality rate in humans [15–20]. Formulation of an effective H5N1 virus vaccine is a crucial component in control strategies aimed to limit spread and severity of potential pandemic viruses. However, several challenges exist when producing H5N1 vaccines, including their continual evolution and poor immunogenicity [12–14, 21–24]. One method to improve vaccine efficacy is through inclusion of vaccine additives or adjuvants. Adjuvants boost vaccine efficacy by inducing a stronger protective immune response and/or lowering the dose of antigen required to induce a response (dose sparing). Adjuvants exert their effect through a variety of mechanism including concentration and retention of antigen at the injection site, and/or promoting uptake and subsequent effector functions of phagocytic cells such as macrophages and dendritic cells [22, 25–29].
We previously demonstrated that a 20-amino acid peptide derived from fibroblast growth factor-4, designated “EB”, displayed antiviral activity against multiple subtypes of influenza viruses [30]. The EB peptide inhibited virus binding to host cells in vitro, and prevented influenza virus-induced morbidity and mortality in mice. Here, we demonstrate that the EB peptide aggregates influenza virions while keeping them structurally intact. We hypothesized that the EB-induced aggregation would enhance uptake by phagocytic cells and boost downstream immune responses to influenza virus. These studies support that hypothesis and show that EB increases the protective immune response against poorly immunogenic H5N1 virus vaccines. Surprisingly, EB did not increase humoral immunity to H5N1 vaccines, but instead enhanced cell- mediated responses to formalin-inactivated whole virus.
2. MATERIALS & METHODS
2.1. Ethics Statement
All procedures involving animals were approved by the University of Wisconsin-Madison and St. Jude Children’s Research Hospital IACUC committees and were in compliance with the Guide for the Care and Use of Laboratory Animals.
2.2. Viruses and Cells
A/Puerto Rico/8/34 (PR/8, H1N1) and A/Vietnam/1203/04 (VN/1203, H5N1) viruses were propagated in 10-day-of-age embryonated chicken eggs (Sunnyside Farms, Beaver Dam, WI) at 37°C. Allantoic fluid was harvested, centrifuged for clarification, and stored at −70°C. VN/1203 virus for animal challenge was propagated in Madin Darby canine kidney (MDCK, ATCC, Manassas, VA) cells. Supernatants were harvested 72 hr post infection (hpi), centrifuged for clarification, and stored at −70°C. Viral titers were determined either by hemagglutination (HA) assay as described previously [30] and reported as hemagglutination units (HAU) or by fifty percent tissue culture infectious dose (TCID50) analysis in MDCK cells and calculated by the method of Reed and Muench [31]. MDCK cells were cultured in Modified Eagle’s Medium (MEM, CellGro, Herndon, VA) supplemented with 4.5 g of glucose per liter, 2 mM L-glutamine, and 10% fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, CA) at 37°C, 5.5% CO2. RAW 264.7 cells (ATCC, Manassus, VA) were cultured in RPMI 1640 medium (CellGro) supplemented with 4.5 g of glucose per liter, 1 mM L-glutamine, 1 mM sodium pyruvate, and 10% heat inactivated FBS.
2.3. Laboratory Facilities
All H5N1 virus experiments were conducted in a Select Agent-approved bio-safety level-3 enhanced laboratory. Investigators were required to wear appropriate respirator equipment (RACAL, Health and Safety Inc., Frederick, MD). Mice were housed in HEPA filtered negative pressure cages (M.I.C.E. racks, Animal Care Systems, Littleton, CO).
2.4. Virus Inactivation and Purification
Allantoic fluid containing influenza virus was treated with 0.1% in formalin (v/v) at 4°C for 5 days. To verify inactivation, MDCK cells and 10-day-of-age embryonated chicken eggs were inoculated with a neat dilution of formalin-treated virus and viability was assessed after 72 hpi. All viruses, both inactivated VN/1203 and PR/8 and live PR/8 virus were purified by overlaying allantoic fluid onto a 30–60% discontinuous sucrose gradient. The samples were centrifuged for 90 min at 26,000 RPM in an SW-28 rotor. The virus layer was extracted from the 30–60% interface and pelleted by another round of centrifugation for 60 min. Virus pellets were resuspended in phosphate buffered saline (PBS), refluxed through an 18g needle to break up aggregates, and titered by HA assay.
2.5. Density Ultracentrifugation
Purified PR/8 virus (512 HAU) was treated with PBS alone (mock) or EB peptide (30 µM) for 1 hr at 37°C and layered on continuous 20-60% sucrose gradients. Samples were subjected to ultracentrifugation 90,000 ×g for 60 min. Fractions (500 µl) were collected from the bottom of the tube and 7 µl of each was dotted to nitrocellulose, blocked with 3% milk in Tris-buffered saline containing 0.1% Tween-20 (TTBS), and probed with goat anti-H5 hemagglutinin (1:1000 in TTBS) for 1 hr at room temp, followed by donkey anti-goat IgG (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA). Immune complexes were detected by enhanced chemiluminescence (Pierce, Rockford, IL). Results were quantitated by densitometry (Image J, NIH, Bethesda, MD) and reported as relative pixel intensity (RPI). The density of each sucrose fraction was determined by measuring refractive index in a Bausch and Lomb 334610 Refractometer (Rochester, NY). The attachment activity from each fraction was determined by HA assay and is represented as HAU/50 µl.
2.6. Electron Microscopy
Purified PR/8 (512 HAU) or inactivated VN/1203 viruses (1 µg total protein) were treated with PBS alone (mock) or EB peptide (30 µM) for 1 hr at 37°C. Samples were adsorbed to poly-L-lysine coated grids, stained with 2% phosphotungstic acid (pH 7) and air dried as described [32]. Grids were examined in a JEOL JEM-1200EX electron microscope. Results are indicative of 3 independent experiments.
2.7. Virus Labeling and Flow Cytometry
Purified PR/8 virus was labeled with fluorescein isothiocyanate (FITC-PR/8) using the EZ-Label FITC protein labeling (Pierce, Rockford, IL) according to manufacturer’s instructions. Infectivity of labeled virus was confirmed by TCID50 analysis and was re-titered by HA assay for use in flow cytometry experiments. The FITC-PR/8 (512 HAU) virus was treated with increasing concentrations of EB peptide for 1 hr at 37°C and then added to RAW 264.7 macrophages (2×105) in 1 ml of RPMI 1640 containing 1% heat inactivated FBS. Samples were incubated with gentle rocking for 1 hr at 37°C, followed by 2 washes with PBS. Cells were fixed with 1% paraformaldehyde and virus-cell association measured on a LSR-II Benchtop flow cytometer (BD Biosciences, Franklin Labs, NJ). Single cell populations of mock infected cells were gated using forward and side scatter properties. At least five-thousand events from these gates were recorded from each experimental group in duplicate or triplicate. Results are indicative of 2 independent experiments.
2.8. Vaccine Formulation
The vaccine was composed of 1 µg of formalin inactivated and sucrose purified VN/1203 or PR/8 virus diluted into sterile PBS. Adjuvant groups were supplemented with 0.5 mg/ml aluminum ammonium sulfate or 200 µM EB peptide. All groups were incubated for 1 hr at 37°C prior to administration.
2.9. Animals, Vaccination and Challenge
Blood was drawn from the tail vein from 4- 6 week old female BALB/C mice (n=5-6/group) followed by hind-limb intramuscular injection with 50 µl of vaccine preparation. All mice were pre-bled (Day 0) and vaccinated, followed by a boost of vaccine 15 days after the initial injection. Mice were bled again at day 28 post-initial vaccination. Mice were given one day to recover after bleeding, lightly anesthetized with isoflurane, and intransally inoculated with 10x the 50% mouse lethal dose (MLD50) of VN/1203 or PR/8 virus as indicated. Mice were monitored for weight and clinical scores every 48 hours. Clinical scores were recorded on the following criteria: 0 = no signs of infection, 1 = ruffled coat, hunched posture, 2 = slowed movement, shivering, 3 = labored breathing, anorexia, little to no movement, 4 = moribund [33]. Mice reaching 25% weight loss or clinical scores of 4 were euthanized. At days 3 and 7 post-infection, 3 mice from each group were sacrificed and the lungs extracted for titering on MDCK cells as described previously. Combined data from three independent experiments are presented.
2.10. Serology
All serum was treated with receptor destroying enzyme (RDE, Denka Seiken, Tokoyo, Japan) as per manufacturer’s instructions. H5 hemagglutinin-specific IgG and IgG subclass ELISAs were performed as described with slight modifications [34, 35]. Briefly, microtiter wells were coated with 100 µl of PBS containing 10 ng of recombinant H5 hemagglutinin (HA, Protein Sciences, Meridian, CT) overnight at 4°C. Non-specific binding sites were blocked with 4% BSA in PBS plus 0.5% Tween-20 (PBST) for 1 hr at room temp. Log dilutions of sera in 1% BSA/ PBST were added to wells and incubated for 1 hr at room temp. Bound antibody was detected by anti-mouse IgG, IgG1, or IgG2a conjugated streptavidin (1:3000 Invitrogen, Carlsbad, CA) diluted in 1% BSA/PBST for 1 hr at room temp, followed by quantification using tetramethylbenzidine (R&D Systems, Minneapolis, MN). Absorbance was measured on a SpectraMax 250 Spectrophotometer (Molecular Devices, Sunnyvale, CA) at A405 nm with an A605 nm correction after 8 min of incubation. Virus neutralizing titers were determined by method of hemagglutination inhibition (HI) by standard method [36] against homologous virus.
2.11. IFN-γ ELISpot Assay
At day 28 post-prime, spleens were isolated from 2 to 4 mice from each group and single cell populations prepared by passing the spleens through cell strainers followed by washing with 5 ml of R10 medium (RPMI 1640, 10% heat-inactivated FBS, 1 mM sodium pyruvate, 1× penicillin/streptomycin). Cells were then treated with AKC red blood cell lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA) for 5 min and counted on a hemacytometer. All ELISpot assay components were purchased from Mabtech (Cincinnati, OH) and developed per manufacturer’s instructions. Freshly isolated splenocytes (1×106 per well) were added to each well of a 96-well ELISpot plate in 100 µl R10 medium. The cells were stimulated for 24 hr with either R10 medium alone, or formalin inactivated VN/1203 or PR/8 virus (1 µg). The number of IFN-γ secreting splenocytes was determined on an ELISpot Reader (AID, Strassberg, Germany). CD3 antibody was used as a positive control and yielded > 100 spots in each assay (data not shown). Combined data from two independent experiments is presented.
2.12. Statistics
All experiments are representative of, or combined data (as indicated) from 2 to 4 independent tests. The results represent the means ± standard deviations of at least triplicate determinations. Statistical significance of the data was determined by ANOVA with appropriate subtest using GraphPad Prism software (Graphpad Software Inc., La Jolla, CA).
3. RESULTS
3.1. EB peptide aggregates influenza virions
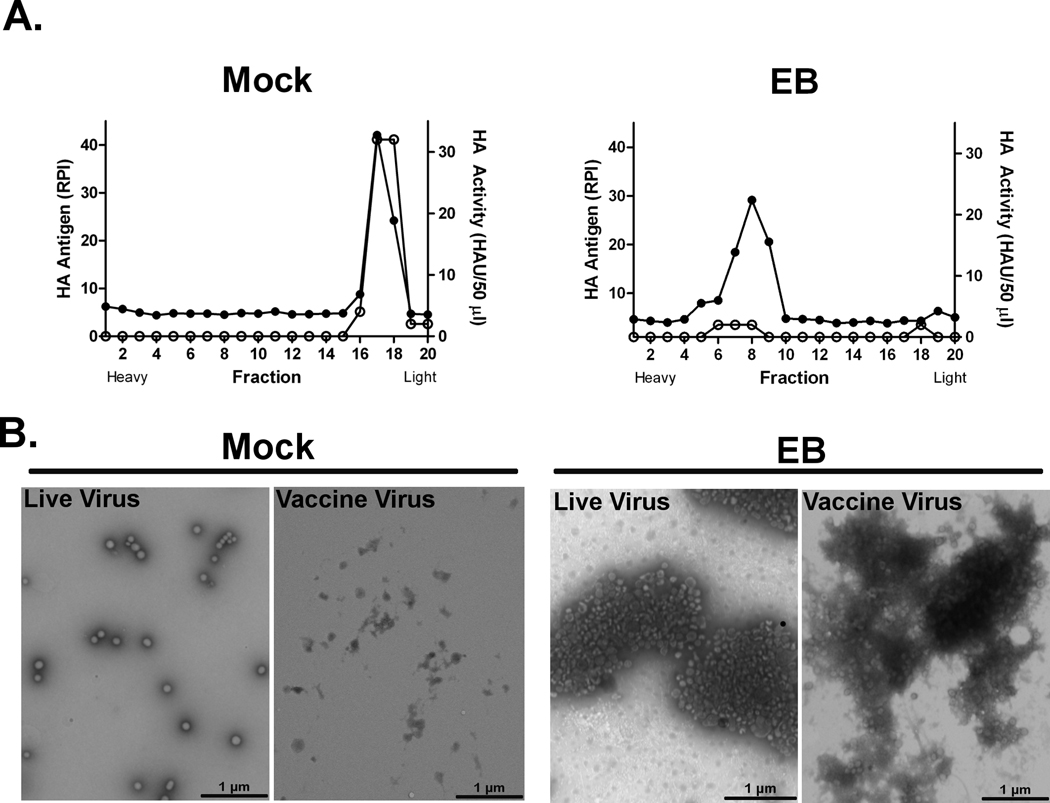
We previously demonstrated that EB, a 20 amino acid peptide derived from fibroblast growth factor-4, displayed antiviral activity against influenza viruses through inhibition of attachment to host cells [30]. We hypothesized that EB may aggregate virions leading to reduced attachment to cellular receptors. To test this hypothesis, purified PR/8 virus was mock (0 µM) or treated with 30 µM EB peptide for 1 hr at 37°C and the resulting particle size was determined by sucrose gradient ultracentrifugation. Gradient fractions were tested for the presence of the hemagglutinin (HA) protein by dot immunoblotting and binding activity of the protein was determined by HA assay. Mock-treated virus was collected in low-density fractions composed of ~ 30% sucrose, which were positive for both HA protein (Fig. 1A, closed circles) and HA activity (Fig. 1A, open circles). In contrast, the EB-treated virions eluted in denser fractions of ~41–53% sucrose and the fractions lacked HA binding activity (Fig. 1A). The change in the elution profile suggested that EB was inducing aggregation. To confirm this, mock or EB-treated PR/8 virus was visualized by electron microscopy (EM). In mock-treated samples, individual virions were uniformly scattered across the field, with sporadic aggregates (~2 to 4 particles) observed (Fig. 1B). In contrast, nearly all of the virions in the EB-treated sample were aggregated in large clusters of 25 to 100 particles (Fig. 1B). Similar results were obtained when inactivated virus vaccine preparations were tested (Fig. 1B). Collectively, these data demonstrate that EB induced influenza virus aggregation.
Figure 1. EB peptide induces influenza virus aggregation.
A) PR/8 virus (512 HAU) was mock treated (0 µM) or treated with 30 µM EB peptide for 1 hr at 37°C, overlaid onto continuous sucrose gradients, and subjected to ultracentrifugation at 90,000 × g for 1 hr. Fractions (500 µl) were collected from the bottom of the gradient and HA antigen (●) and HA activity (○) was determined by immunoblot and hemagglutination assay respectively. B) PR/8 (512 HAU) or formalin-inactivated PR/8 (1 µg) virus was mock treated or EB-treated as described and samples were adsorbed to grids, stained with phosphotungsic acid, and visualized by electron microscopy. Inset scale bars = 1 micrometer. Results are representative of 3 independent experiments.
3.2. EB peptide enhances uptake of virus antigen
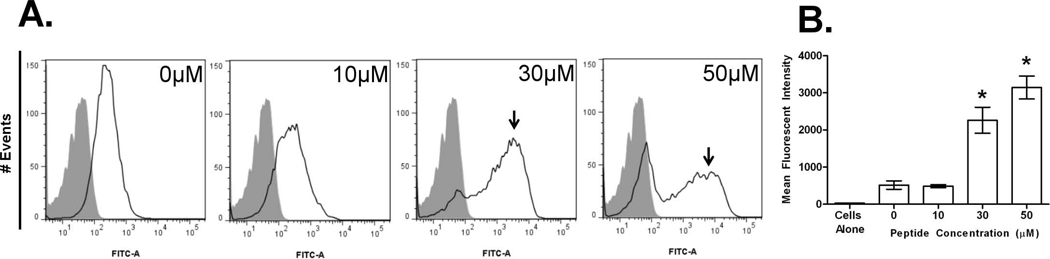
Aggregation can increase uptake by antigen presenting cells [37, 38]. Thus, we hypothesized that EB-induced viral aggregates would be more efficiently ingested by phagocytes. To test this, FITC-labeled purified-PR/8 virus was incubated with macrophages and cell-associated virus measured by flow cytometry. When fluorescently labeled virus (Fig. 2A, open peaks), was mock-treated (0 µM EB), minimal association with macrophages was observed as compared to the signal for cells alone (Fig. 2A, shaded peaks), and likely represented virus binding to extracellular receptors. In contrast, treatment of virus with either 30 or 50 µM EB led to significant shift in the cell associated peak (indicated by arrowheads). The association peak is lower in overall number of cells but higher in fluorescence intensity representing macrophages that have engulfed large fluorescent virus aggregates. At 30 or 50 µM EB treatment, samples had 4 or 6-fold higher mean fluorescent intensity as compared to mock treated samples (Fig. 2B). In these same samples, the appearance of a corresponding peak void of fluorescent signal was observed. The peak lacking fluorescence consists of cells with no virus associated and likely results from a decrease in available virus particles to ingest as higher concentrations of peptide induce larger aggregates, or quenching of the FITC signal upon entering an acidic phagosome. These data suggest that EB-aggregated virus is more readily associated with phagocytic cells.
Figure 2. EB peptide induces influenza uptake by macrophages.
FITC-labeled PR/8 virus (512 HAU) was mock (0 µM) or treated with increasing concentrations of EB peptide for 1 hr at 37°C. Samples were added to 2×105 RAW264.7 macrophages for 1 hr at 37°C and analyzed for virus uptake by flow cytometry. A) Histograms are presented as FITC signal of cells alone (shaded peak) or cells associated with FITC-PR/8 virus (non-shaded peak, arrows). Cells were gated by forward and side scatter. B) Median fluorescent intensity of each sample is indicated. Triplicate measurements of at least 5000 events per experimental sample and are presented as the mean ± standard deviation, and results are indicative of two independent experiments. * (p < 0.05)
3.3. Inclusion of EB peptide in H5N1 vaccine enhances protection
We hypothesized that EB-induced viral aggregation and subsequent phagocytosis by antigen presenting cells may promote downstream immune responses, leading to enhanced vaccine immunogenicity. To test this, we used a suboptimal inoculum (1 µg) of formalin-inactivated H5N1 virus vaccine; a dose in which mice receiving vaccine alone were not protected from virus-induced morbidity. Inactivated virions were mock treated (0 µM, naïve) or treated with EB (200 µM) or alum (0.5 mg/ml) and administered intramuscularly in the hind-limb of mice. All animals were boosted 15 days post-prime. Twenty-nine days post-prime, mice were intranasally challenged with 10× the 50% mouse lethal dose (MLD50) of homologous H5N1 virus and monitored for morbidity. Naïve mice lost significant weight and 100% succumbed to infection or had to be euthanized by 7 dpi. All the vaccinated mice survived infection. However, only the mice administered EB pre-treated inactivated virus regained all their initial body weight within 6 dpi (Fig. 3A), and overall, lost significantly less weight than mice receiving inactivated virus alone or virus plus alum. In addition, mice given EB pre-treated virus had only minimal clinical signs of infection; primarily only ruffled fur (score of 1–2) early in the challenge (Fig 3B). All other mice displayed more severe clinical scores (2 or higher) throughout the study (Fig 3B). Similar results were generated when mice were vaccinated with inactivated PR/8 (H1N1) virus with EB as an adjuvant (data not shown). The reduced morbidity in mice administered EB pre-treated-vaccine was accompanied by reduced virus burden in the lungs at 3 and 7 dpi (Fig. 3C&D). By 3 dpi only 2 of 6 animals had virus in their lungs. This was further reduced to 1 of 9 animals by 7 dpi. The rest of the animals were below the limit of detection. Overall, these data indicate that EB-supplemented vaccine decreased morbidity associated with influenza infection and enhanced viral clearance compared to the no adjuvant and alum adjuvant groups.
Figure 3. H5N1 vaccine containing EB peptide reduces virus induced morbidity and lung titers.
Mice were vaccinated and challenged with H5N1 virus and A) weighed and B) scored for clinical signs every 48 hrs as an indicator of virus induced morbidity. At days C) 3 and D) 7 post-infection, 3 animals from each group were sacrificed, and the lungs were collected for virus titration by TCID50 analysis. Combined data of 2 to 3 independent experiments are presented. Data are presented as the mean or mean ± standard deviation. * (p < 0.05), ** (p < 0.001), *** (p < 0.0001). ND = not determined.
3.4. EB peptide enhances a cell mediated, but not humoral response
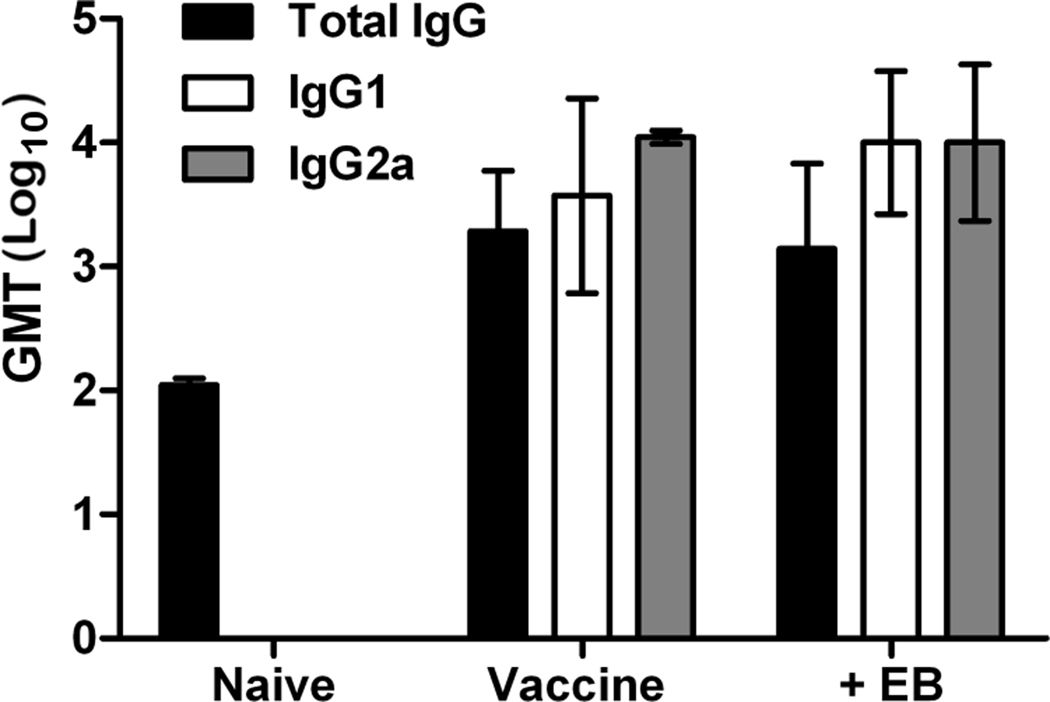
Influenza vaccines typically protect by eliciting virus neutralizing antibody responses [4, 5]. Thus, neutralizing antibody responses to EB supplemented vaccine were measured via hemagglutination inhibition assay. None of the VN/1203 H5N1 vaccinated mice produced neutralizing antibody (Table 1). This is not unusual for H5N1 vaccines when administered at suboptimal doses [39–41]. In contrast, mice receiving PR/8 (H1N1) vaccine did produce neutralizing antibody titers upon vaccination and the addition of EB induced a modest increase (1-doubling dilution) over vaccine alone. However, titers were well below that induced by alum adjuvant (Table 1). H5-specific IgG subtypes were also measured by ELISA on days 28 post-prime sera. There was no difference in the levels of HA-specific total IgG or IgG subtypes between groups with or without EB (Fig. 4). Thus, in the context of H5N1 vaccination, EB-enhanced protection was independent of increased antibody responses though enhancement of humoral responses may be observed with other influenza subtypes (i.e. H1N1).
Table 1.
Neutralizing titers generated by EB supplemented H5N1 or H1N1 vaccinea
| Vaccine | H5N1 Vaccination | H1N1 Vaccination | ||
|---|---|---|---|---|
| Pre-bleed | Pre-challenge | Pre-bleed | Pre-challenge | |
| Naïve | <20 | <20 | <20 | <20 |
| Vaccine Alone | <20 | <20 | <20 | 40 |
| + Alum | <20 | <20 | <20 | 160 |
| + EB | <20 | <20 | <20 | 80 |
| Anti-H5 serumb | >400 | |||
Neutralizing titers were determined by Hemagglutination Inhibition (HI) assay. HI titer is presented as triplicate determinants of reciprocal of the highest dilution inhibiting 4 HA units of homologous vaccine virus (H5N1, VN/1203 or H1N1, PR/8).
Goat anti-H5 serum was included as a control to validate the HI assay with H5N1 mouse sera.
Figure 4. Inclusion of EB peptide in H5N1 vaccine does not influence levels of virus specific serum IgG.
Mice (n=7) were bled 28 days post-prime (1 day pre-challenge) and sera was subjected to ELISA analysis of H5 specific total IgG, IgG1 or IgG2a as indicated. Data are presented as geometric mean titer ± standard deviation.
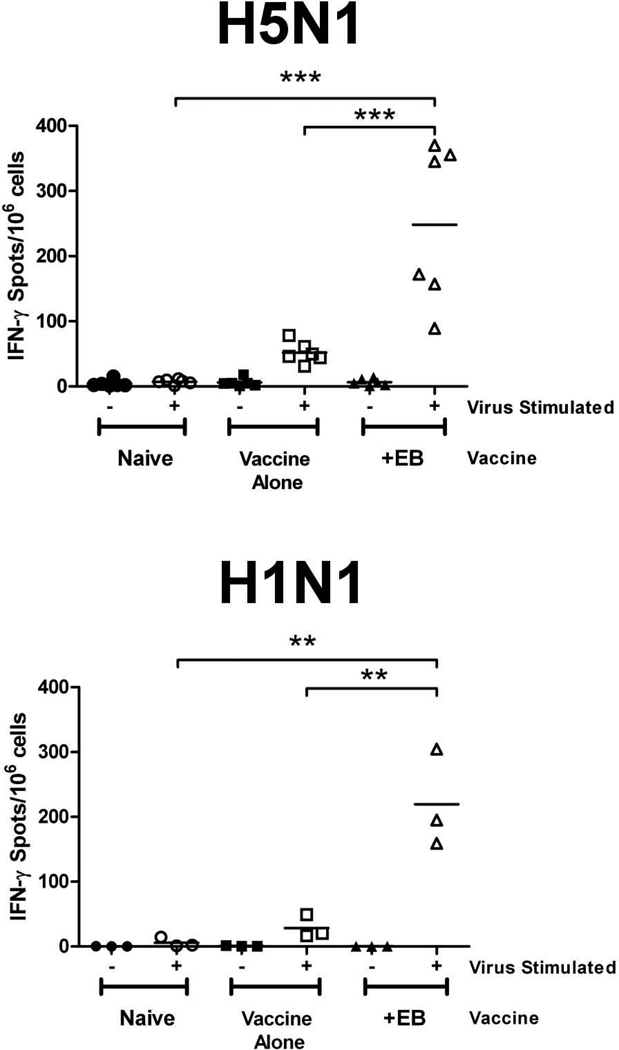
Given that the EB peptide enhanced protection from H5N1 viral challenge in the absence of neutralizing antibodies or changes in virus specific IgG, we hypothesized that enhanced cell-mediated immunity was responsible. IFN-γ ELISpot assays were performed using splenocytes harvested from VN/1203 or PR/8 vaccinated mice (n=2-4 per group) 28 days post-prime. Regardless of the subtype, mice receiving the EB-treated vaccine had a 2.5-fold increase in the number of IFN-γ secreting cells as compared to the naïve animals or animals receiving vaccine alone (Fig. 5). Similar results were found at days 15 post-prime and when mice were given vaccine plus EB peptide in a prime only regimen (data not shown). These data suggest that the viral clearance observed in the groups receiving EB-treated vaccine may result from enhanced cell-mediated immune responses. This same response may also contribute to the decrease in morbidity and viral titers observed in challenge experiments.
Figure 5. H5N1 vaccine containing EB peptide increases IFN-γ secreting splenocytes.
28 days post-prime, splenocytes were isolated from individual mice (n = 2 to 4) and 1×106 cells were stimulated with media alone (−) or media containing 1 µg inactivated H5N1 VN/1203 (+) or H1N1 PR/8 virus (+) for 24 hr. IFN-γ secreting splenocytes were determined by ELISpot assay. Mean values of triplicate measurements from 2 independent experiments are presented. ** (p < 0.001), *** (p < 0.0001).
4. DISCUSSION
Although originally identified as an anti-influenza compound, the data presented in this manuscript suggest that the EB peptide has potential use as a vaccine adjuvant. During the initial characterization of the peptide’s antiviral activity, we demonstrated that EB inhibited the attachment of virus to host cells and hypothesized this could be due to aggregation [30]. In these studies we demonstrate that EB induced large viral aggregates that could reduce interaction with host receptors and/or mediate loss of valence associated with each virion as receptor binding proteins are sequestered within aggregates, explaining the antiviral activity. In vivo, aggregation could limit the total number of individual viruses that could infect cells of the respiratory tract, decreasing infection rate and allowing more time to initiate antiviral responses. Similar actions are mediated by components of the innate and adaptive immune system including mucins, defensins, and secretory IgA antibody that sequester virus and prevent interaction with respiratory epithelia [38, 42–47].
Given that aggregation of influenza virus by human collectins and defensins leads to increased phagocytosis by neutrophils [37, 38] we hypothesized that EB-induced viral aggregation may concentrate the antigen at the injection site and provide a larger target for phagocytic cell uptake and processing. Our current studies show that EB-induced viral aggregation leads to enhanced association with macrophages. EM demonstrated that incubation of virus with EB led to aggregates at or greater than 1–5 µM in size. Although these particles were measured in one plane and not in solution, their size exceeds the lower limit particle size for active phagocytosis (approximately 0.50.75 µM [48, 49]) suggesting that the size of the EB-induced particle, rather than specific pattern recognition receptors, may be sufficient to induce uptake by sentinel cells. The charge of the target particle has also been implicated in non-receptor-mediated phagocytosis, with cationic particles more likely to be engulfed [50–53]. The EB peptide possesses a solubility tetrapeptide attached to the N-terminus of the EB peptide (RRKK). An overall positive charge on the EB-virus aggregates may contribute to uptake.
Inclusion of the EB peptide in the whole virus inactivated vaccine had a significant effect on disease progression during severe H5N1 viral challenge. Mice receiving EB-pre-treated vaccine showed less severe clinical signs of infection, recovered more quickly and cleared virus faster than vaccine alone or even vaccine plus alum adjuvant. The EB pre-treated vaccine group cleared virus by 3 and 7 dpi, while the other vaccine groups had 2 to 4-log higher viral titers suggesting a more robust immune response was induced by inclusion of EB. However, neither H5N1 vaccine alone nor EB–treated vaccine induced virus neutralizing titers as measured by HI or microneutralization assay. This is similar to other H5N1 vaccine studies in mice where a vaccine was protective in the absence of detectable neutralizing antibodies [38–40]. In such instances, specific IgG subclasses may correlate with outcomes of infection [49]. Yet we observed no differences in H5-specific antibody titers by ELISA regardless of the subtype tested (IgG, IgG1, IgG2a) between any vaccine group. Thus, the dominance of one antibody subclass over the other cannot explain the enhanced protection elicited by the EB-adjuvanted vaccine. However, H1N1 vaccines supplemented with EB or alum showed increased neutralizing titers over vaccine alone suggesting that the lack of detectable neutralizing antibodies may be specific to H5N1 viruses.
To explain the enhanced protection and increased viral clearance in mice vaccinated with EB adjuvant, cellular responses were examined. Mice vaccinated with the EB-pre-treated vaccine had significantly higher levels of IFN-γ secreting splenocytes as compared to other vaccine groups. These cytokine secreting cells may represent effector T cells of either the Th1 or Th2 type immunity. Th1 responses are characterized by inflammatory cytokine profiles and activation of CD8+ cytotoxic T lymphocytes (CTLs) that function to eliminate virus infected cells [54]. Influenza vaccine containing viral aggregates showed enhanced immunogenicity over detergent- split virus [48]. However, the response generated by such a vaccine was primarily Th2-biased and is often associated with activation of CD4+ T-helper cells and induction of antibody production. Additionally, natural killer (NK) cells may contribute to the enhanced IFN-γ secretion observed. However, their contribution to this effect is likely minimal in our ELISpot assays where whole inactivated virus was the stimulating antigen. In such a scenario, virus is non-infectious and would not mediate down-regulation of surface MHC I in infected cells; a common NK cell activating determinant. Additionally, He et al. demonstrated that NK cell production of IFN-γ in response to inactivated influenza vaccine was dependent upon cytokine stimulation by CD3+ lymphocytes presumably responding to the vaccine antigens. Thus, NK cell produced IFN-γ, which may contribute to the increased levels observed, is likely a secondary response to the activation of virus specific T-lymphocytes. Nevertheless, activated NK cells are induced by influenza vaccination and their resulting effector functions could contribute to virus clearance [55].
There are a number of mechanisms whereby the EB adjuvant could be promoting an altered immune response to vaccination. Our observation that EB enhanced the association of virus with macrophages in vitro might indicate that the peptide increases the efficiency of uptake allowing the presentation of more antigen to the immune system. This is unlikely to be the entire explanation, however, since aggregates are formed with alum and viral aggregates have been shown to primarily drive antibody mediated responses [48]. It is possible that the EB peptide alters antigen presentation allowing for processing of diverse viral epitopes or mediates cross-presentation to class I molecules as has been demonstrated with several adjuvanted influenza vaccines [56–58]. The EB peptide might also alter the cytokine profiles secreted by APCs and this altered cytokine profile drives an altered immune response. Another possibility is that the EB peptide adjuvant has an effect on the activation or differentiation of APCs. Finally, these mechanisms are not mutually exclusive and EB may be affecting multiple components of the afferent immune response. Ongoing studies are focusing on understanding how the EB adjuvant enhances a cellular immune response to a formalin-inactivated influenza vaccine and the precise response that is generated.
In conclusion, these studies demonstrate that in addition to antiviral activity, EB peptide is a potential vaccine additive or adjuvant. Unlike many adjuvants, EB primarily enhances cell-mediated immunity, a phenomenon often deficient in non-replicating vaccines. While the identity of the IFN-γ producing cells in the ELISpot assays is still unknown, cells of the adaptive response such as CTLs often display this phenotype and are implicated in virus clearance. CTLs may also provide cross-subtype protection, which is a highly desirable outcome for influenza vaccination. Finally, the cell mediated enhancement by EB could be combined with adjuvants that boost humoral immunity (alum, MF-59, etc.) to provide a robust immune response that stimulate both arms of adaptive immunity and provide improved protection.
Highlights.
EB peptide induces influenza virus aggregation and enhanced uptake by macrophages
Addition of the EB peptide to inactivated influenza vaccine enhances protection
EB adjuvant enhances IFN-γ production and cell-mediated immune responses
ACKNOWLEDGMENTS
We wish to thank Drs. Robert Webster, Ronald Schultz, Yoshi Kawaoka, Randy Massey, Sharon Frase, Jenny Gumperz, David Watkins, Jonah Sacha and Nick Negovetich for advice and/or use of equipment. We gratefully acknowledge the members of the Schultz-Cherry Lab for experimental assistance and/or critical reviews of this manuscript. This work was supported by funds from the Sigma Xi Grants-In-Aid of Research (JCJ), University of Wisconsin Foundation (CRB), University of Wisconsin Medical Education and Research Committee (SSC) and in part by the NIH NIAID contract number HHSN266200700005C and the American Lebanese Syrian Associated Charities (SSC).
ABBREVIATIONS
- EB
Entry blocker peptide adjuvant
- hpi
hours post-infection
- TCID50
50% tissue culture infectious dose
- PR/8
influenza virus A/PuertoRico/8/34 H1N1
- VN/1203
influenza virus A/Vietnam/1203/04 H5N1
- HA
hemagglutinin protein/assay
- HAU
hemagglutinating units
- HI
hemagglutination inhibition
- dpi
days post-infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Joshi SR, Shaw AC, Quagliarello VJ. Pandemic influenza H1N1 2009, innate immunity, and the impact of immunosenescence on influenza vaccine. The Yale journal of biology and medicine. 2009;82(4):143–151. [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson AL, Budd AP, Perl TM. Control of influenza in healthcare settings: early lessons from the 2009 pandemic. Current opinion in infectious diseases. 2010;23(4):293–299. doi: 10.1097/QCO.0b013e32833bb804. [DOI] [PubMed] [Google Scholar]
- 3.Wareing MD, Tannock GA. Live attenuated vaccines against influenza; an historical review. Vaccine. 2001;19(25–26):3320–3330. doi: 10.1016/s0264-410x(01)00045-7. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson I, Nicholson KG. Influenza: vaccination and treatment. Eur Respir J. 2001;17(6):1282–1293. doi: 10.1183/09031936.01.00084301. [DOI] [PubMed] [Google Scholar]
- 5.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 6.Girard MP, et al. A review of vaccine research and development: human acute respiratory infections. Vaccine. 2005;23(50):5708–5724. doi: 10.1016/j.vaccine.2005.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz-Cherry S, Jones JC. Influenza vaccines: the good, the bad, and the eggs. Adv Virus Res. 77:63–84. doi: 10.1016/B978-0-12-385034-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich HJ, et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med. 2008;358(24):2573–2584. doi: 10.1056/NEJMoa073121. [DOI] [PubMed] [Google Scholar]
- 9.Wright PF. Vaccine preparedness--are we ready for the next influenza pandemic? N Engl J Med. 2008;358(24):2540–2543. doi: 10.1056/NEJMp0803650. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Flu Season Summary, 2007–2008 [Google Scholar]
- 11.Fichera E, et al. New strategies to overcome the drawbacks of currently available flu vaccines. Adv Exp Med Biol. 2009;655:243–252. doi: 10.1007/978-1-4419-1132-2_15. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368(9540):991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 13.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194 Suppl 2:S111–S118. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 14.Leroux-Roels I, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370(9587):580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 15.Trampuz A, et al. Avian influenza: a new pandemic threat? Mayo Clin Proc. 2004;79(4):523–530. doi: 10.4065/79.4.523. quiz 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiken T, et al. Avian H5N1 influenza in cats. Science. 2004;306(5694):241. doi: 10.1126/science.1102287. [DOI] [PubMed] [Google Scholar]
- 17.Keawcharoen J, et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis. 2004;10(12):2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Geographical spread of H5N1 avian influenza in birds - update 28: Situation assessment and implications for human health. 2005
- 19.WHO. Cumulative number of confirmed cases of avian influenza A/(H5N1) Reported to WHO. 2011
- 20.WHO. Avian influenza: H5N1 detected in pigs in China. 2004
- 21.Guan Y, et al. Molecular epidemiology of H5N1 avian influenza. Rev Sci Tech. 2009;28(1):39–47. doi: 10.20506/rst.28.1.1868. [DOI] [PubMed] [Google Scholar]
- 22.Prieto-Lara E, Llanos-Mendez A. Safety and immunogenicity of prepandemic H5N1 influenza vaccines: a systematic review of the literature. Vaccine. 28(26):4328–4334. doi: 10.1016/j.vaccine.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 23.Smith GJ, et al. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A. 2009;106(28):11709–11712. doi: 10.1073/pnas.0904991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen HL, Webster RG. Pandemic influenza as a current threat. Curr Top Microbiol Immunol. 2009;333:3–24. doi: 10.1007/978-3-540-92165-3_1. [DOI] [PubMed] [Google Scholar]
- 25.Barchfeld GL, et al. The adjuvants MF59 and LT-K63 enhance the mucosal and systemic immunogenicity of subunit influenza vaccine administered intranasally in mice. Vaccine. 1999;17(7–8):695–704. doi: 10.1016/s0264-410x(98)00252-7. [DOI] [PubMed] [Google Scholar]
- 26.Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine. 2009;27 Suppl 4:D65–D68. doi: 10.1016/j.vaccine.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins DA, Carlson JR, Van Nest G. MF59 adjuvant enhances the immunogenicity of influenza vaccine in both young and old mice. Vaccine. 1996;14(6):478–484. doi: 10.1016/0264-410x(95)00240-2. [DOI] [PubMed] [Google Scholar]
- 28.Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19(17–19):2673–2680. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 29.Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27(25–26):3331–3334. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 30.Jones JC, et al. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J Virol. 2006;80(24):11960–11967. doi: 10.1128/JVI.01678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed LJM, H A simple method of estimating fifty percent endpoints. The American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- 32.Bultmann H, Busse JS, Brandt CR. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J Virol. 2001;75(6):2634–2645. doi: 10.1128/JVI.75.6.2634-2645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morton DB. A systematic approach for establishing humane endpoints. ILAR J. 2000;41(2):80–86. doi: 10.1093/ilar.41.2.80. [DOI] [PubMed] [Google Scholar]
- 34.Katz JM, et al. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J Infect Dis. 1997;175(2):352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 35.Lu X, et al. Immunity to influenza A H9N2 viruses induced by infection and vaccination. J Virol. 2001;75(10):4896–4901. doi: 10.1128/JVI.75.10.4896-4901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer DF, DD, Coleman MT, Schild GC. C.f.D.C.a. Prevention, Editor. Atlanta, GA: 1975. Advanced laboratory techniques for influenza diagnosis. [Google Scholar]
- 37.Hartshorn KL, et al. Conglutinin acts as an opsonin for influenza A viruses. J Immunol. 1993;151(11):6265–6273. [PubMed] [Google Scholar]
- 38.Tecle T, et al. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol. 2007;178(12):8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- 39.Lu X, et al. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine. 2006;24(44–46):6588–6593. doi: 10.1016/j.vaccine.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 40.Ninomiya A, et al. Inactivated influenza H5N1 whole-virus vaccine with aluminum adjuvant induces homologous and heterologous protective immunities against lethal challenge with highly pathogenic H5N1 avian influenza viruses in a mouse model. Vaccine. 2007;25(18):3554–3560. doi: 10.1016/j.vaccine.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 41.Takada A, et al. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003;21(23):3212–3218. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 42.Hartshorn KL, et al. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol. 1997;273(6 Pt 1):L1156–L1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- 43.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81(7):3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83(Pt 9):2091–2108. doi: 10.1099/0022-1317-83-9-2091. [DOI] [PubMed] [Google Scholar]
- 45.Mason RJ, Greene K, Voelker DR. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol. 1998;275(1 Pt 1):L1–L13. doi: 10.1152/ajplung.1998.275.1.L1. [DOI] [PubMed] [Google Scholar]
- 46.Outlaw MC, Dimmock NJ. Mechanisms of neutralization of influenza virus on mouse tracheal epithelial cells by mouse monoclonal polymeric IgA and polyclonal IgM directed against the viral haemagglutinin. J Gen Virol. 1990;71(Pt 1):69–76. doi: 10.1099/0022-1317-71-1-69. [DOI] [PubMed] [Google Scholar]
- 47.Reading SA, Dimmock NJ. Neutralization of animal virus infectivity by antibody. Arch Virol. 2007;152(6):1047–1059. doi: 10.1007/s00705-006-0923-8. [DOI] [PubMed] [Google Scholar]
- 48.Xiang SD, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40(1):1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178(2):509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 145(3):182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao K, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 32(13):3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capo C, et al. Dimethylsulphoxide induction of the murine macrophage-like line P388D1: change of phagocytic ability and cell surface properties. J Cell Sci. 1983;64:281–293. doi: 10.1242/jcs.64.1.281. [DOI] [PubMed] [Google Scholar]
- 53.Stossel TP, et al. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babiuk S, et al. Aggregate content influences the Th1/Th2 immune response to influenza vaccine: evidence from a mouse model. J Med Virol. 2004;72(1):138–142. doi: 10.1002/jmv.10540. [DOI] [PubMed] [Google Scholar]
- 55.He XS, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. The Journal of clinical investigation. 2004;114(12):1812–1819. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guillonneau C, et al. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci U S A. 2009;106(9):3330–3335. doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haining WN, et al. pH-triggered microparticles for peptide vaccination. J Immunol. 2004;173(4):2578–2585. doi: 10.4049/jimmunol.173.4.2578. [DOI] [PubMed] [Google Scholar]
- 58.Jelinek I, et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol. 186(4):2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]