Abstract
Objective
Anal sphincter complex consists of anatomically overlapping internal anal sphincter (IAS), external anal sphincter (EAS) & puborectalis muscle (PRM). We determined the functional morphology of anal sphincter muscles using high definition manometery (HDAM), 3D-ultrasound (US) and Magnetic resonance (MR) imaging.
Patients
We studied 15 nulliparous women.
Interventions
HDAM probe equipped with 256 pressure transducers was used to measure the anal canal pressures at rest and squeeze. Lengths of IAS, PRM and EAS were determined from the 3D-US images and superimposed on the HDAM plots. Movements of anorectal angle with squeeze were determined from the dynamic MR images.
Results
HDAM plots reveal that anal canal pressures are highly asymmetric in the axial and circumferential direction. Anal canal length determined by the 3D-US images is slightly smaller than measured by HDAM. The EAS (1.9 ± 0.5 cm long) and PRM (1.7 ± 0.4 cm long) surround distal and proximal parts of the anal canal respectively. With voluntary contraction, anal canal pressures increase in the proximal (PRM) and distal (EAS zone) parts of anal canal. Posterior peak pressure in the anal canal moves cranially in relationship to the anterior peak pressure, with squeeze. Similar to the movement of peak posterior pressure, MR images show cranial movement of anorectal angle with squeeze.
Conclusion
Our study proves that the PRM is responsible for the closure of the cranial part of anal canal. HDAM, in addition to measuring constrictor function can also record the elevator function of levator ani/pelvic floor muscles.
Keywords: Puborectalis muscle, Anal Sphincter anatomy, Anal pressure topography, pelvic floor muscles, Transverse perinei muscles
INTRODUCTION
In humans, anal sphincter complex consists of overlapping internal anal sphincter (IAS), external anal sphincter (EAS) and puborectalis muscle (PRM), which makes determination of the contribution of individual muscles to anal canal pressure difficult. It is suggested that the PRM contributes to the anal continence mechanism by forming the anorectal angle (1–3). The latter can be seen on barium defecography (X ray) and magnetic resonance (MR) defecography. Using 3D–US imaging and station pull-through pressure measurements of the anal canal, we observed that the PRM contraction increases pressure in the cranial part of anal canal (4, 5).
Several investigators have observed that the anal canal pressures are asymmetric, the reason for which is suggested to be the unique triple looped anatomy of the EAS (6, 7). A novel probe developed by Sierra Scientific measures pressures from 256, closely spaced micro-transducers and for the first time allows detailed assessment of the pressure distribution in the anal canal. Transperineal 3D-US imaging allows precise quantification of the individual components of anal sphincter complex (IAS, EAS and PRM) (8, 9). Goal of our study was to reassess the functional anatomy of anal canal using HDAM, 3D-US imaging and dynamic MR imaging. We were especially interested in assessing the relationship between anatomy and pressure distribution in the anal canal.
Our study provides novel insight into the functional anatomy of anal canal and proves that in addition to the formation of anorectal angle the PRM is actually responsible for the closure of the proximal anal canal. Furthermore, besides measuring the constrictor function of PRM, HDAM can also determine the elevator function of pelvic floor muscles.
METHODS
Ethical Approval
The UCSD Institutional Review Board approved the study protocol and each subject signed an informed consent prior to enrollment in the study protocol. These subjects responded to an advertisement and were reimbursed nominal amount of money for their participation. Study was conducted in 15 nulliparous women with a mean age of 37 years (range: 21–61 years). Of these, 10 women underwent HDAM and 3D-US imaging while 5 women underwent MR imaging only. Each subject completed medical history and previously validated urinary incontinence and anal incontinence score questionnaires to confirm the absence of urinary and anal incontinence symptoms. Prior to starting the study, each subject was instructed to contract the pelvic floor by the prompt “squeeze as if you were trying to stop the stream of your urine.” A simultaneous anal/vaginal examination performed by the investigator confirmed the ensured contraction of anal and pelvic floor muscles.
High Definition Anal Manometry (HDAM)
Anal canal pressures were recorded using a newly developed HDAM probe by Sierra Scientific Instruments Inc, Los Angles, CA., (ManoScan 360 HD™) that has following features; 1) the probe is 10 mm in diameter with a pressure sensitive length of 64 millimeters, 2) 256 micro-transducers are located on the surface of the HDAM probe that form a continuous grid in the axial and circumferential direction and 3) each transducer is 4 mm long and 2 mm wide. HDAM probe has following functional characteristics: 1) pressure from each transducer is recorded digitally and displayed on the computer screen as a pseudo color plot (ManoView HD beta™, Sierra Scientific Instruments Inc), 2) in–vitro testing prove that an externally applied pressure on each transducer does not affect the output from the adjacent transducer; 3) pressure recordings are accurate to within 4 to 5 mmHg, which was tested with a pressure gage. HDAM probe was placed in the anal canal in such a fashion that the entire anal canal high-pressure zone (HPZ) was captured with some clearance on the cranial (rectal pressures) and caudal (atmospheric pressure) ends. The circular orientation of the probe in relation to the anterior midline, posterior midline, left lateral and right lateral orientation of anal canal was maintained by the investigator during rest and squeeze with the aid of markings on the probe. Three separate measurements were obtained at rest and sustained maximal anal sphincter/pelvic floor contractions (squeeze) respectively.
Three Dimensional (3D) Transperineal Ultrasound Imaging
A 3D-US imaging system (Philips HD11; Phillips Medical Systems, Bothell, WA) was used to image the anatomy of anal canal and its muscles. With subject in the lithotomy position, ultrasound volumes were obtained by placing a 3–9 MHz trans-vaginal US transducer on the perineum. In order to increase the field of view and to capture images of the structures close to the skin, a custom-built standoff made of agar was placed on the US transducer. Images were captured with the US probe pointing in the cranial direction in order to include the anal canal and surrounding structures. Another set of US volumes were captured with a custom built, non compressible, 10 cm long and 10 mm diameter bag when fully distended with water in the anal canal. This was done for the following reasons, 1; to determine if HDAM probe alters the anatomy of anal canal in relationship to the other structures of pelvis, 2; to improve imaging of the most distal part of anal canal i.e., caudal to the lower end of the internal anal sphincter, and 3; to allow comparison of anatomy and function with an equally distended anal canal, i.e., either a 10 mm diameter bag or a 10 mm diameter HDAM probe. Ultrasound volumes were captured at rest and at the peak of anal sphincter contraction. All ultrasound volumes were archived on a compact disk (CD) and viewed off line with the Q lab, 4.2 software program (Phillips Medical Systems, Bothell, WA).
Magnetic Resonance Imaging
Dynamic MR Images of pelvis were recorded in mid sagittal plane using 1.5 T, super conducting magnet (Symphony; Siemens Medical Systems, Erlangen, Germany) machine. Following MR parameters were used for imaging: echo time of 2.52 ms, repetition time of 5.03 ms, slice thickness of 4 mm with no slice gap, yielding an image matrix of 256 × 100. Images were recorded at rest and during pelvic floor contraction. MR sequences captured 2D images in the mid sagittal plane at a temporal resolution of 1Hz. Images were recorded at rest and continuously during sustained and maximal pelvic floor contraction. The MR images were viewed with the Syngo Fast view software program (Siemens AG 2004, Munich Germany).
DATA ANALYSIS
High Definition Anal Canal Manometry
Pressures were viewed as 2 dimensional topographical color plots of all pressure transducers and revealed 3 distinct pressure zones. Upper zone represents rectal or intra-abdominal pressure; middle zone represents anal canal pressures and the lower zone shows the atmospheric pressures (Figure 1). In order to define the upper and lower borders of anal HPZ, raw data from 256 sensors was exported to an Excel sheet and viewed as a 16 × 16 table. Two sets of rest (just prior to contraction) and squeeze (at the peak of contraction) pressures were averaged in each subject. Pressures from the anal HPZ of all 10 subjects were averaged in order to obtain the composite HDAM profile of anal canal. Mean pressure calculation at each transducer was performed by optimally aligning the pressure profile in each subject and it was achieved by calculating the correlation coefficient between pressure values of two subjects and then sliding the transducer position axially (±3 transducer position) and circumferentially (±1 transducer position) (Figure 2). The optimal pressure transducer alignment was taken as the offset that yielded maximal correlation coefficient, which allowed correction for variations in the probe depth insertion and slight, if any, misalignment in the circumferential direction.
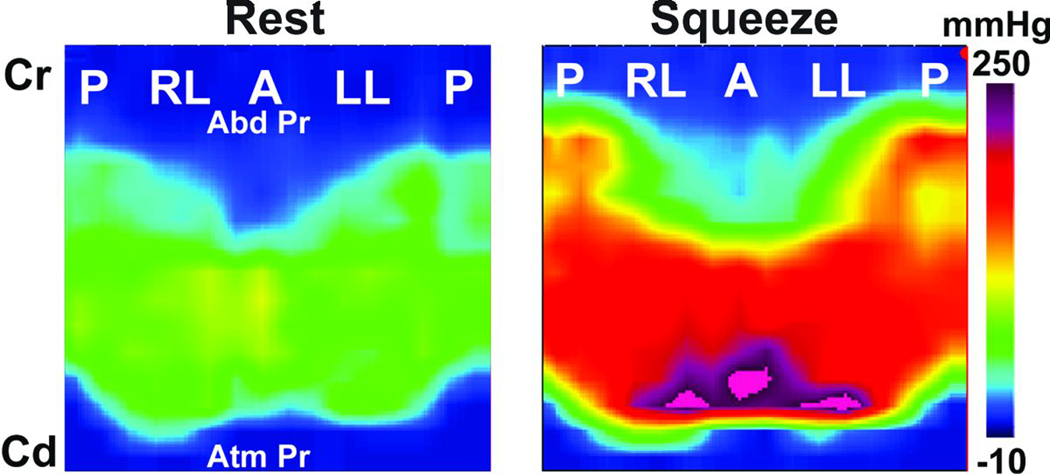
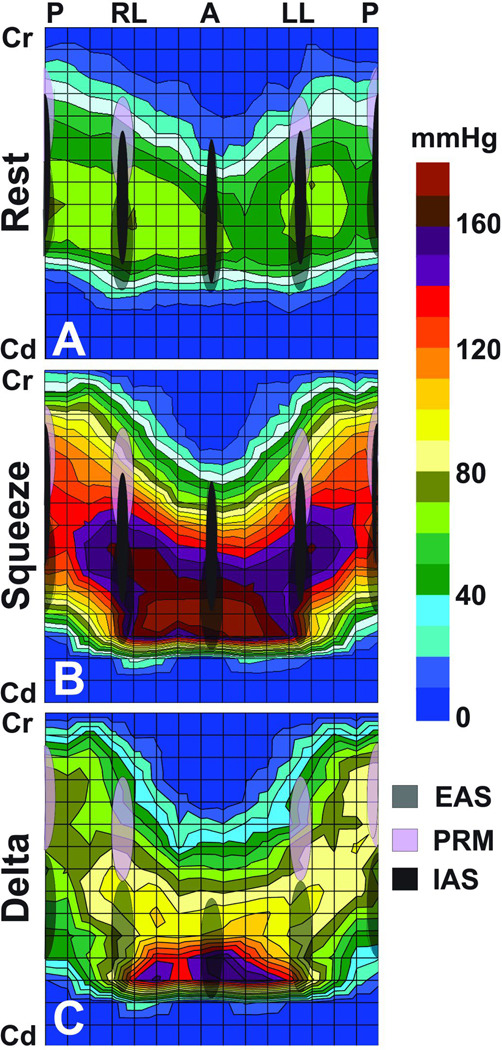
Figure 1. High definition Anal Manometery (HDAM) Pressures at Rest and Squeeze.
Circular anal canal is split at the posterior midline to show surface plot view of the anal canal pressure. Note the asymmetry of pressures along the axial and circumferential directions. The lower edge of anal canal begins at a more cranial level and extends more cranially in the posterior midline as compared to the anterior midline.
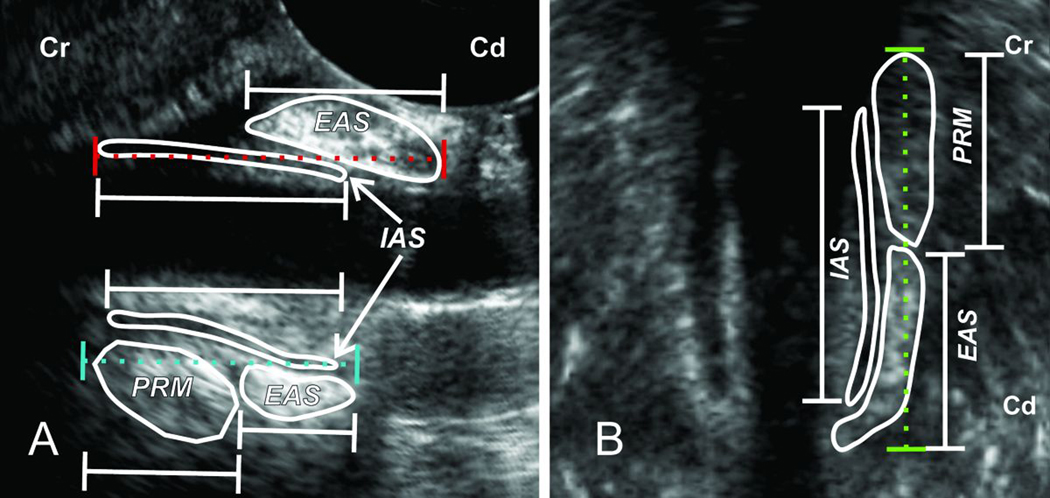
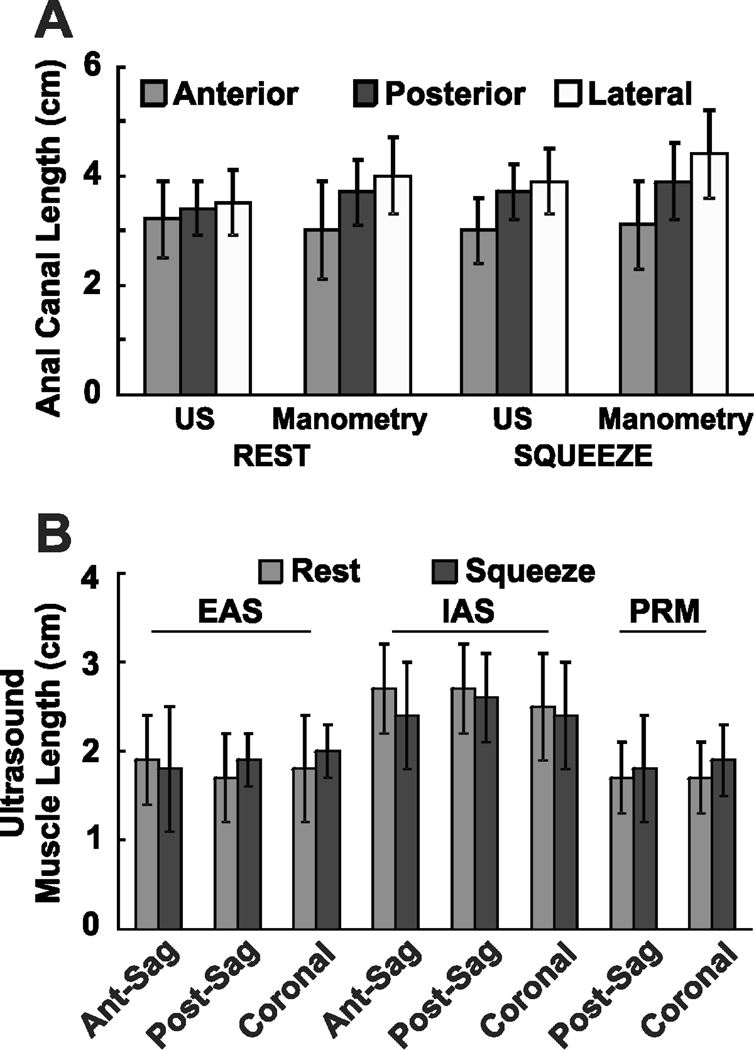
Figure 2. Measuring Cranio-caudal Length of Anal Canal, Internal Anal Sphincter (IAS), External Anal Sphincter (EAS) and Puborectalis Muscle (PRM) from the 3D-US Images.
Panel A, on the left, and Panel B on the right show US image of the anal canal in the mid sagittal and mid coronal planes respectively. The length of anal canal was measured from the lower edge of the EAS to the upper edge of PRM in the posterior midline and lateral directions. On the other hand, in the anterior midline anal canal length extends from the lower edge of EAS to the upper edge of IAS.
Pressure from the first and last two rows of sensors was averaged and standard deviation was computed separately to determine rectal and atmospheric pressures respectively. These were later used to define the cranial and caudal edges of anal HPZ. Cranial and caudal edges were defined as a location where pressure was noted to be 2 standard deviations higher than the abdominal and the atmospheric pressures respectively. Measurements of the length, peak pressures and location of peak pressures in the anal HPZ were determined from the reconstructed anal HPZ (Figure 2). Area of the anal HPZ was determined by multiplying number of pressure transducers within HPZ and the surface area of one transducer (8 mm2),
Three Dimensional Transperineal Ultrasound Imaging
Anal canal anatomy was displayed and viewed in the 3 orthogonal planes, i.e., mid sagittal, mid coronal and transverse planes. Measurements (cranio-caudal length) of IAS, EAS and PRM were made in the anterior, posterior and lateral directions (Figure 2). For the anterior and posterior measurements, midline sagittal view was used. Right and left lateral measurements were made from the mid coronal view of anal canal. IAS is seen clearly in the US images as an echo lucent (black) structure. The proximal and distal extents of the EAS and PRM were measured in the coronal and sagittal planes by confirming their extent in the axial plane. Two US volumes at rest and two US volumes at squeeze were analyzed in each subject to determine the reproducibility of measurements. Mean value was obtained in each subject for the comparison of the total length of the anal canal and individual muscles, i.e., EAS, IAS and PRM.
Magnetic Resonance Images
MR images were analyzed at rest and at the peak of contraction. Anorectal angle was identified and a line was drawn from the lower edge of pubic symphysis to the apex of anorectal angle. Angle between the above line and a horizontal line drawn from the lower edge of pubic symphsis was determined and changes in this angle were determined between rest and squeeze as shown in figure 3.
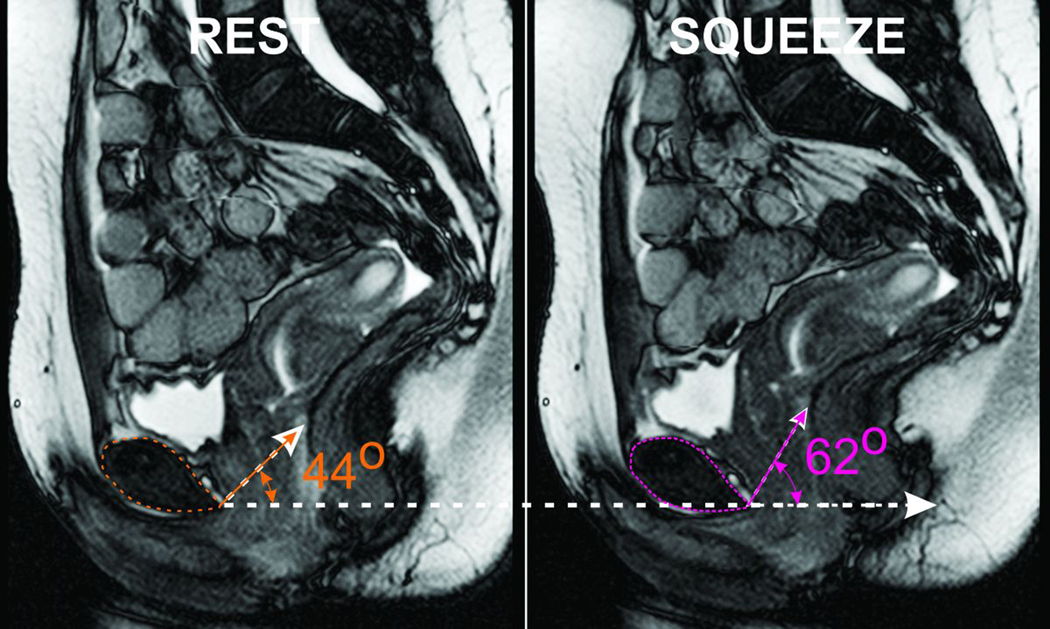
Figure 3. MR mid-sagittal Image of the Pelvis at Rest and Squeeze.
Note that the anorectal angle moves in the anterior and cranial direction with squeeze resulting in an increase in the angle formed with a horizontal line drawn from the lower edge of the pubic-symphsis.
Statistical Analysis
Anal HPZ length, pressures and location of peak pressure in the HPZ obtained from each quadrant was compared between rest and squeeze. Two tailed, paired t-test were used for statistical comparisons and significance was defined as P < 0.05. Data are reported as mean ± SEM, unless stated otherwise.
RESULTS
Anatomy of the Anal Canal: 3 Dimensional Ultrasound Imaging
Review of US volumes visualized anal canal anatomy and its component muscles in the 3 orthogonal planes simultaneously, i.e., sagittal, coronal and axial (transverse) planes, along the entire anal canal length. EAS surrounds the caudal part of the IAS and extends further caudal to it. Anterior part of the EAS merges into the perineal body (seen as an oval structure in the mid sagittal plane) with contribution from the right and left transverse perinei muscles towards the midline (seen in the transverse planes). Only the inner fibers of EAS traverse around the entire circumference of anal canal (Figure 4). Lateral fibers of EAS merge with the right and left transverse perinei muscles. In the posterior plane, PRM extends from the anorectal angle to the cranial end of the EAS. In fact, for a short distance the PRM and EAS appear to overlap each other. The mean cranio-caudal lengths of anal canal, IAS, EAS and PRM in the anterior midline, posterior midline, right lateral and left lateral directions are shown in the figure 5.
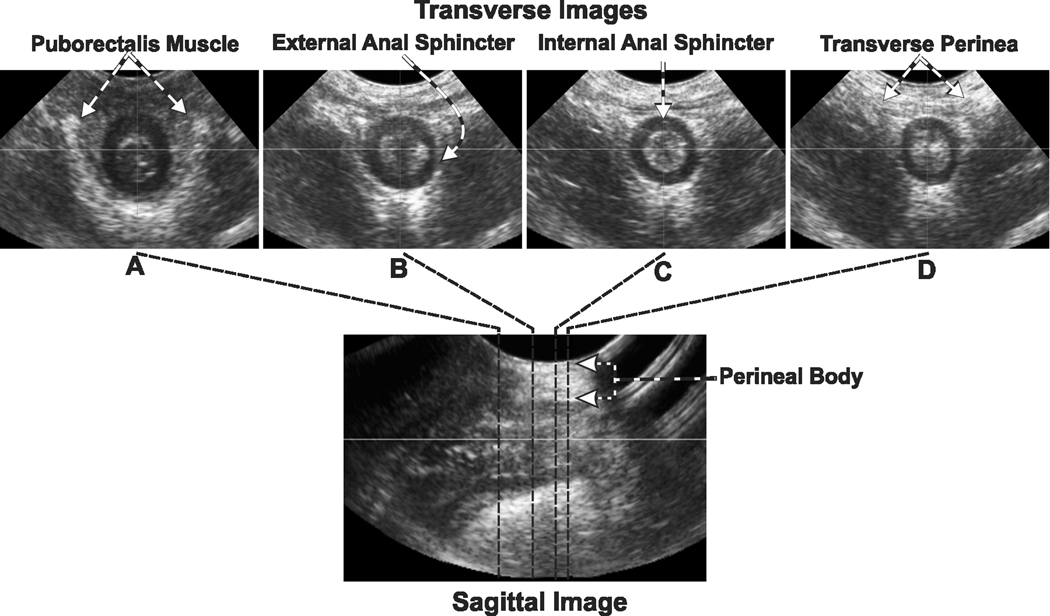
Figure 4. Transverse & Sagittal Image of the Anal Canal.
Lower panel shows the ultrasound image of the anal canal in the mid-sagittal plane. The images in the upper row show transverse or tomographic axial images at different levels along the cranio-caudal extent of the anal canal. Note the appearance and location of internal anal sphincter (IAS), external anal sphincter (EAS) and puborectalis muscle (PRM) along the length of the anal canal. Also note that the inner fibers of the EAS surround the whole circumference of the anal canal but the outer fibers merge into the right and left transverse perinei muscles anteriorly.
Figure 5. A & B: Length of Anal Canal and Muscle Lengths.
Panel A shows the anal canal lengths measured by high definition anal manometry and 3D ultrasound in the anterior, posterior and lateral orientation both at rest and squeeze. Panel B shows the lengths of external anal sphincter (EAS), internal anal sphincter (IAS) and puborectalis muscle (PRM) in the anterior, posterior and lateral directions measured from 3D ultrasound. Ant-sag = anterior muscle length measured from the sagittal projection, post-sag = posterior muscle length measured from the sagittal projection and coronal = muscle lengths measured from the coronal projections. Data in this figure are shown as mean ± SD
Anal Canal Pressures: High Definition Manometry
HDAM plots were visualized as cut (posterior midline) and unfolded cylinder, as a 2 dimensional surface color plot of the anal canal (Figures 1 & 6). Different colors represent different pressures (shown as color scale). These color plots reveal several important features; 1, pressures are markedly asymmetric in the axial and circumferential directions, 2; anal canal extends inferiorly in the anterior midline than the posterior midline, 3; squeeze pressures are greater in the lower anterior part of anal canal, as compared to the remainder of anal canal (p = 0.017). Figure 6B shows the anal canal pressures with squeeze in 10 subjects. Length of the anal canal at 0°, 90°, 180° and 270° around the circumference (Figure 5) as well as the surface area of anal HPZ increases with squeeze.
Figure 6. A, B & C: Rest, Squeeze & Delta HDAM pressures.
These surface pressure plots of the anal canal are developed from the average data obtained in 10 normal subjects. The length of the internal anal sphincter (IAS), external anal sphincter (EAS) & puborectalis muscle (PRM) were measured from the US images and mean values from 10 subjects are graphed on the surface pressure plot to show the possible contributions from individual muscles to the anal canal pressure. Note that with squeeze the pressures increase in the caudal as well as the cranial part of the anal canal surrounded by the EAS and PRM respectively. Delta pressure plot is the difference between the rest and squeeze pressure and reflects the contribution of the muscles under volitional control, i.e., EAS and PRM. The area of the pressure surface plots in the delta plot is not different from the one at squeeze.
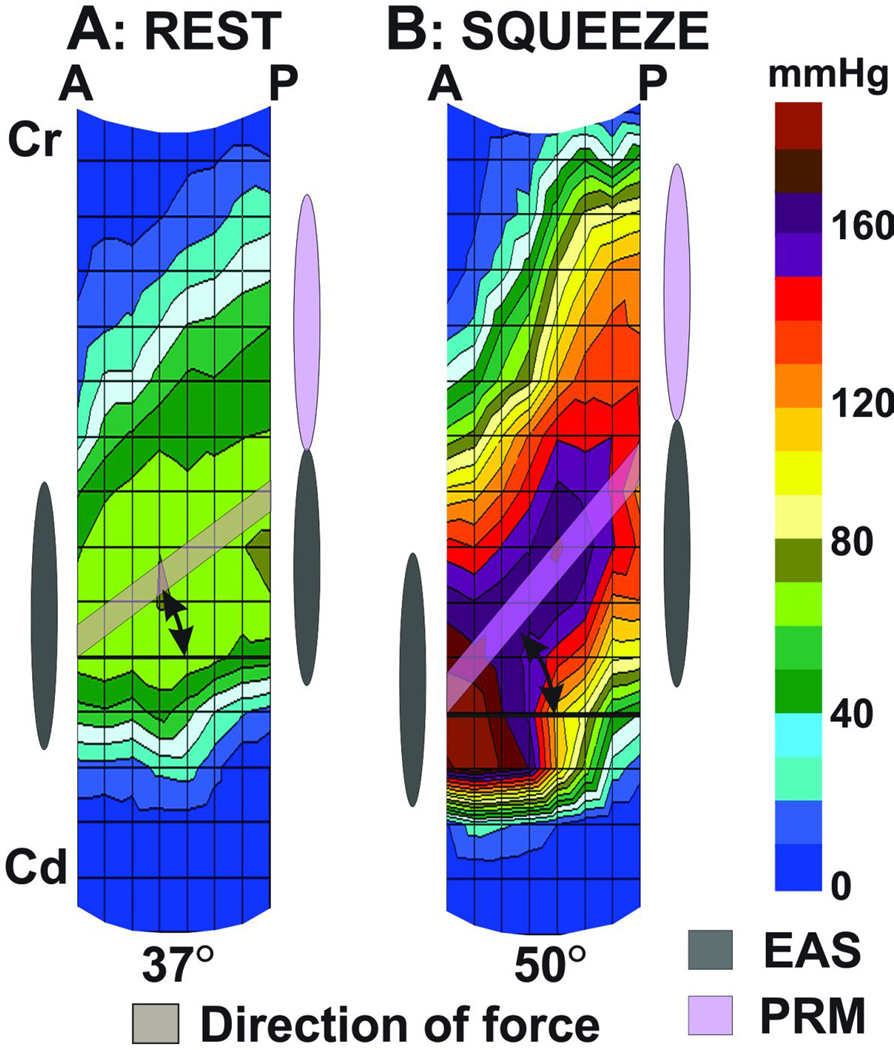
At rest, a line connecting the peak pressure point in the anterior and posterior mid line planes makes an angle of 37° (from the mean plot of 10 subjects) with the horizontal line; with posterior peak pressure point located more cranially than the anterior peak pressure (Figure 7). With squeeze there is an increase in the above angle to 50°, suggesting a lift of the peak posterior point of the anal HPZ in the cranial direction. The above mean angles at rest and squeeze measured in each subject were 23 ± 8° and 59 ± 5° respectively.
Figure 7. Sagittal image of the HDAM pressure plot at Rest & Squeeze.
A line connecting the peak pressure in the anterior and posterior midline shows movements of the peak posterior pressure in the cranial direction with squeeze. The change in the angle of the above line is similar to the change in angle measured in the MR images and reflects the elevator function of the pelvic floor muscle.
Correlation between Anal Canal Anatomy and Function
Anal canal length measured by ultrasound images is slightly smaller (3 to 4 mm) than the HDAM length and these differences are significant for the anterior (p < 0.01), posterior (p < 0.05) and lateral (p < 0.025) directions. Mean US measurements of the IAS, EAS & PRM were superimposed on the mean HDAM plot (from 10 subjects), as shown in figure 6A, B & C. With squeeze, pressures increase in the entire area of anal canal, i.e., both in the EAS as well as the PRM zones; the increase is significantly larger in the anterior EAS zone as compared to the remainder of anal canal. Difference between rest and squeeze pressure (delta surface plot) eliminates contribution from the IAS and shows contribution from only those muscles that are under volitional control, i.e., EAS and PRM (figure 6C). The delta surface area is not significantly different from the rest or the squeeze, which suggests that the cranial anal canal pressure are generated by the PRM contraction, (rest area 12.3 ± 0.2 cm2 vs squeeze area 14.0 ± 0.1 cm2 p < 0.05, delta area 13.4 ± 0.1 cm2).
Dynamic MR Imaging & High Definition Manometry
Dynamic MR Images of pelvis show that the anorectal angle moves in the cranial and ventral directions with squeeze (figure 3). A line connecting the lower edge of pubic symphysis with anorectal angle makes an angle of 27 ±12° with the horizontal line. With squeeze the above angle increases to 37 ± 8°. Change in the angle observed on dynamic MR images is not significantly different from the change in the angle observed on HDAM (10° vs 13°).
Reproducibility of Measurements
Reproducibility of HDAM, Ultrasound images and MR image measurements were also assessed from 2 separate measurements in each subject using linear regression analysis. For the rest and squeeze HDAM data the respective r values were 0.74 (y=0.86x + 12) and 0.92 (y=0.99x+2). For the ultrasound images the rest and squeeze r values were 0.86 (y=0.93x + 0.26) and 0.83 (y=0.82x + 0.33). Since the area measurements were derived from the HDAM data that were fairly reproducible, one would expect the same for the area measurement as well. Since MRI measurements were made on high quality images with the well defined landmarks these were also high reproducible.
DISCUSSION
In summary, our data show following, 1) HDAM reveals axial and circumferential asymmetry of anal canal pressures that is related to the unique anatomy of anal canal. 2) On the 3D-US images, cranial and caudal edges of the anal canal were defined to correspond with cranial and caudal edges of the PRM and the EAS respectively. The length of anal canal determined by HDAM is slightly but significantly longer than the one measured by US images. 3) Superimposition of anatomy and anal HPZ signature recorded by HDAM reveal the role of PRM in the closure of the cranial part of the anal canal. 4) Similar to the cranial movement of anorectal angle on the MR images, peak posterior pressure in the HDAM plot moves in the cranial direction.
Controversy regarding the anatomy of anal canal dates back to 1897 (10) when Holls, a German anatomist identified that some muscle fibers of the “pubococcygeus”, instead of inserting into the coccyx, loop around the rectum to continue on to the opposite side, and coined the term puborectalis muscle (PRM) (10). Cadaver dissection and imaging studies continue to argue back and forth whether EAS is 2 or 3 parts structure (11–14). In the Milligan and Morgan 1934 paper (15) the EAS is shown as 3 parts and it appears that the PRM was assumed to be the part of levator ani muscle. In the Goligher 1967 paper PRM is located just below the levator ani and EAS extends down without subdivisions (16). In the OH and Clark (1974) study, the deep parts of the EAS and PRM are considered to be one muscle (17). Using endoanal MRI, Hussenin et al (18) found that the deep part of the EAS is actually the PRM. Our 3D-US images are quite clear in this regard, the proximal half of the anal canal is indeed surrounded by the PRM. In the mid sagittal plane, proximal edge of PRM corresponds to the anorectal angle and the entire vertical extent of PRM is located below the anorectal angle. Anteriorly, the upper half of anal canal is formed by the IAS only, because PRM being a sling shaped muscle is deficient anteriorly, which can be clearly seen in the transverse/axial sections of the cranial half of anal canal. Another interesting aspect of the EAS, even though mentioned in the literature but not widely appreciated, is that the EAS is not a circular muscle (19, 20). US images show that only the most medial or inner fibers of the EAS continue around the whole circumference of anal canal. Majority of the EAS muscle mass actually merges into the right and left transverse perinei muscles. In fact, more than 25% of anterior circumference of EAS, along its entire cranio-caudal extent is formed by the transverse perinei muscles, which suggests that the transverse perinei muscles must be important in the EAS function and damage to these muscles may cause anal sphincter dysfunction. The latter is highly relevant because episiotomy involves sectioning of transverse perinea muscles and obstetrical injury often involves the anterior part of the EAS. US images of anal canal in patients with fecal incontinence reveal that the most common site of injury of the EAS is anterior (21)..
Vertical extents of anal canal measurements by HDAM in 4 quadrants are slightly greater than the one made by the 3D ultrasound images. The reason for the above is that each HDAM transducer is 4 mm long and does not discriminate between 1 mm long versus 4 mm long squeeze. On the other hand, US measurements are more precise. Therefore, various lengths measurement by HDAM can be over estimated by up to 2 transducers length (8 mm), most proximal and most distal one. Our finding that HDAM measurements of anal canal are 3–4 mm longer than the US measurement is consistent with the average potential error by the HDAM.
HDAM, for the first time provides exquisite details of surface pressure distribution in the anal canal and show that the pressures are quite asymmetrical, both axially and circumferentially. Several aspects of anal canal pressure asymmetry can be explained on the basis of unique anal canal anatomy. For instance the lower edge of the anal canal is more cranial in the posterior midline when compared to the anterior midline. This results in a unique pressure profile showing a cranial location of posterior midline pressures as compared to the anterior pressures. Similarly, anatomy and pressure plots reveal that the upper end of anal canal extends more cranially in the posterior midline as compared to anterior midline. Since vertical extent of IAS, EAS and PRM are somewhat different in the four quadrants the direction of muscle fibers must be at an angle to the transverse plane of HDAM probe and pressure transducers (placed at right angle to the length of probe). We believe that the difference in direction of muscle fibers and direction of transducers could be another reason for circumferential asymmetry of anal canal pressures. Furthermore, in the upper part of anal canal where posterior pressures are greater than the anterior pressures, the asymmetry is likely related to a “horse shoe” shape anatomy of the PRM.
Most investigators agree that the PRM is a key component of anal continence mechanism. It is generally felt that the PRM contraction and relaxation result in acute and obtuse angulations at anorectal angles respectively and as a result the angle between rectum and anal canal contributes to the continence mechanism. In support of the above, during normal defection, as the PRM relaxes, anorectal angle becomes obtuse and in some patients with fecal incontinence anorectal angle is obtuse even at rest suggesting a dysfunctional PRM (22). Our study shows that with voluntary contraction, pressures increase significantly in the cranial part of anal canal that is surrounded by the PRM. The above suggests that in addition to the anorectal angle formation, PRM is actually responsible for the closure of the cranial half of the anal canal. This is likely to be an important mechanism by which PRM contributes to the anal continence mechanism.
MR imaging of pelvis show that the anorectal angle moves in the ventral and cranial direction during voluntary anal sphincter contraction (23, 24). Anorectal angle movement is thought to be related to the contraction of pelvic floor or levator ani muscles of which PRM is one of the components, other components are pubococcygeus, ileococcygeus and ischiococcygeus (also called coccygeus). Levator function of pelvic floor muscle has been measured by several investigators using special techniques and is considered to be an important component of anal continence mechanism (3, 25, 26). Fraga et al found that elevator function of anal canal was impaired in patients with fecal incontinence. Furthermore, they demonstrated that improvement in incontinence symptoms with biofeedback therapy correlates most with the improvement in elevator function of anal canal (25). The HDAM plots reveal that the cranial movement of the posterior pressure point is similar to the cranial movement of anorectal angle (seen on MR images). We propose that the relative movement between peak anterior and posterior pressure point in the anal canal is related to the elevator function of pelvic floor muscles. Future studies should investigate if HDAM, besides measuring anal sphincter pressures is also useful in measuring the elevator function of pelvic floor muscle.
Acknowledgments
Grant: This research was supported by an NIH RO-1 grant (DK60733).
ABBREVIATIONS
- 3D
three dimensional
- CD
compact disk
- EAS
external anal sphincter
- HDAM
high definition anal manometry
- HPZ
high-pressure zone
- IAS
internal anal sphincter
- MR
Magnetic resonance
- PRM
puborectalis muscle
- US
3D-ultrasound
Footnotes
Authors Contributions:
Varuna Raizada: enroll subjects, acquire data, data analysis, and helped with writing of the manuscript.
Valmik Bhargava: design of experiment, develop data analysis programs, image and physiological data analysis, drawing figures and write the manuscript.
Anna Karstens: enroll subjects, acquire data and data analysis.
Ravinder Mittal: design of experiment, supervise data analysis and write manuscript.
Disclosures: No conflicts of interest exists
REFERENCES
- 1.Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399–411. doi: 10.1111/j.1572-0241.2003.07235.x. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE. Outcome measures for fecal incontinence: anorectal structure and function. Gastroenterology. 2004;126:S90–S98. doi: 10.1053/j.gastro.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Diamant NE, Kamm MA, Wald A, Whitehead WE. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Guaderrama N, Nager CW, Pretorius DH, Master S, Mittal RK. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol. 2006;101:1092–1097. doi: 10.1111/j.1572-0241.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 5.Jung SA, Pretorius DH, Weinstein M, Nager CW, Den-Boer D, Mittal RK. Closure Mechanism of the Anal Canal in Women: Assessed by Three-Dimensional Ultrasound Imaging. Dis Colon Rectum. 2008 doi: 10.1007/s10350-008-9221-8. [DOI] [PubMed] [Google Scholar]
- 6.McHugh SM, Diamant NE. Anal canal pressure profile: a reappraisal as determined by rapid pullthrough technique. Gut. 1987;28:1234–1241. doi: 10.1136/gut.28.10.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor BM, Beart RW, Jr, Phillips SF. Longitudinal and radial variations of pressure in the human anal sphincter. Gastroenterology. 1984;86:693–697. [PubMed] [Google Scholar]
- 8.Dietz HP. Ultrasound imaging of the pelvic floor. Part I: two-dimensional aspects. Ultrasound Obstet Gynecol. 2004;23:80–92. doi: 10.1002/uog.939. [DOI] [PubMed] [Google Scholar]
- 9.Dietz HP. Ultrasound imaging of the pelvic floor. Part II: three-dimensional or volume imaging. Ultrasound Obstet Gynecol. 2004;23:615–625. doi: 10.1002/uog.1072. [DOI] [PubMed] [Google Scholar]
- 10.Holl M. Handbuch des Anatomie. Jena: Fischer; 1897. [Google Scholar]
- 11.Peschers UM, DeLancey JO, Fritsch H, Quint LE, Prince MR. Cross-sectional imaging anatomy of the anal sphincters. Obstet Gynecol. 1997;90:839–844. doi: 10.1016/S0029-7844(97)00406-7. [DOI] [PubMed] [Google Scholar]
- 12.Bogduk N. Issues in anatomy: the external anal sphincter revisited. Aust N Z J Surg. 1996;66:626–629. doi: 10.1111/j.1445-2197.1996.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 13.Rociu E, Stoker J, Eijkemans MJ, Lameris JS. Normal anal sphincter anatomy and age- and sex-related variations at high-spatial-resolution endoanal MR imaging. Radiology. 2000;217:395–401. doi: 10.1148/radiology.217.2.r00nv13395. [DOI] [PubMed] [Google Scholar]
- 14.Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation: mass contraction of the pelvic floor muscles. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:28–32. doi: 10.1007/BF01900538. [DOI] [PubMed] [Google Scholar]
- 15.Milligan ETC, Morgan CN. Surgical anatomy of the analcanal with special reference to ano-rectal fistulae. Lancet. 1934;2:1213–1217. [Google Scholar]
- 16.J. G. Surgery of the anus, rectum and colon. 2nd ed. London, England: Bailhere Tindall; 1967. [Google Scholar]
- 17.Oh C, Kark AE. Anatomy of the perineal body. Dis Colon Rectum. 1973;16:444–454. doi: 10.1007/BF02588867. [DOI] [PubMed] [Google Scholar]
- 18.Hussain SM, Stoker J, Lameris JS. Anal sphincter complex: endoanal MR imaging of normal anatomy. Radiology. 1995;197:671–677. doi: 10.1148/radiology.197.3.7480737. [DOI] [PubMed] [Google Scholar]
- 19.Lawson JO. Pelvic anatomy. I. Pelvic floor muscles. Ann R Coll Surg Engl. 1974;54:244–252. [PMC free article] [PubMed] [Google Scholar]
- 20.Fritsch H, Brenner E, Lienemann A, Ludwikowski B. Anal sphincter complex: reinterpreted morphology and its clinical relevance. Dis Colon Rectum. 2002;45:188–194. doi: 10.1007/s10350-004-6144-x. [DOI] [PubMed] [Google Scholar]
- 21.Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329:1905–1911. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- 22.Bharucha AE. Fecal incontinence. Gastroenterology. 2003;124:1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 23.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Fraga X, Azpiroz F, Malagelada JR. Significance of pelvic floor muscles in anal incontinence. Gastroenterology. 2002;123:1441–1450. doi: 10.1053/gast.2002.36586. [DOI] [PubMed] [Google Scholar]
- 26.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]