Abstract
Objective: To assess the clinical outcomes of fertility-sparing treatments in young patients with epithelial ovarian carcinoma (EOC). Methods: A retrospective study of young EOC inpatients (≤40 years old) was performed during January 1994 and December 2010 in eight institutions. Results: Data were analyzed from 94 patients treated with fertility-sparing surgery with a median follow-up time of 58.7 months. As histologic grade increased, overall survival (OS) and disease-free survival (DFS) of patients receiving fertility-sparing surgery declined. Neither staging surgery nor laparoscopy of early stage EOC with conservative surgery had a significant effect on OS or DFS. Normal menstruation recommenced after chemotherapy in 89% of the fertility-sparing group. Seventeen pregnancies among twelve patients were achieved by the end of the follow-ups. Conclusions: Fertility-sparing treatment for patients with EOC Stage I Grade 1 could be cautiously considered for young patients. The surgical procedure and surgical route might not significantly influence the prognosis. Standard chemotherapy is not likely to have an evident impact on ovarian function or fertility in young patients.
Keywords: Epithelial ovarian carcinoma, Fertility-sparing treatment, Overall survival, Disease-free survival, Pregnancy
1. Introduction
Epithelial ovarian carcinoma (EOC) is the most common cause of death in gynecological malignancy, comprising 75%–90% of ovarian malignant tumors (Greenlee et al., 2001). However, 5-year survival trends for patients with early stage EOC disease have been shown to be as high as 62%–85% (90%–95% for Stage Ia) (Kottmeier et al., 1968). Nowadays, as lifestyles change, the onset age of EOC occurs at a much younger age. Several reports have estimated that women under 40 years of age comprise 3%–17% of all EOCs (Duska et al., 1999; Greenlee et al., 2001). As diagnostic and treatment techniques have improved over the past decade, there has been a gradual abandonment of radical surgical procedures in favor of more conservative treatments in an effort to decrease morbidity and preserve fertility, without affecting overall survival (OS) and disease-free survival (DFS) (Berman, 2003).
Literature on the conservative management of EOC in selected patients is limited (DiSaia, 1989). Only four studies involving relatively large numbers of patients have been published (Colombo et al., 1994; Schilder et al., 2002; Park et al., 2008; Kajiyama et al., 2010). However, as the results of such studies remain controversial, we designed a large retrospective multicenter analysis of conservative management of EOC in China, to evaluate the clinical outcomes and fertility of young EOC patients who underwent fertility-sparing treatment.
2. Materials and methods
2.1. Patients
A multicenter retrospective study was conducted by members of several hospitals affiliated to universities, involving the collection and analysis of cases of conservative treatment of EOC. Data were recorded from medical documents. Only women under 40 years of age and with more than 12 months of post-surgical follow-up were included. Ninety-four patients were enrolled during January 1994 and December 2010.
Disease was staged as recommended by the International Federation of Gynecology and Obstetrics (FIGO, 1989) classification. Two experienced pathologists were assigned for the pathological diagnosis of invasive ovarian cancer. Histological diagnosis was performed according to the World Health Organization (WHO) criteria (Scrov et al., 1973). Adjuvant therapy was given to patients at stages higher than Ic and early-stage patients with high-risk factors, including high tumor grade, clear cell histologic type, etc. Adjuvant platinum-based chemotherapeutic regimens included TP (taxol and platinum), CP (cyclophosphamide and platinum), or CAP (cyclophosphamide, adriamycin and platinum). Follow-up after primary treatment consisted of clinical visits every three months for two years, then every six months during the next three years, and yearly thereafter. All patients were interviewed by telephone to assess their obstetrical outcomes.
Fertility-sparing surgery (FSS) is a surgical treatment in which one ovary and the uterus are conserved. A complete staging operation included the following procedures: peritoneal washing, intracolonic or partial omentectomy, and retroperitoneal/para-aortic lymph node dissection or biopsy. All other cases were considered to have incomplete surgical staging.
2.2. Statistical analysis
OS, DFS, and menstruation or fertility were evaluated as clinical outcomes. Specific prognostic factors, such as stage, histology, grade, staging procedures, and surgery route, were analyzed. Survival distributions were calculated by the Kaplan-Meier method and statistical significance was determined using the log-rank test. Risk factors for DFS were analyzed using Cox proportional hazard survival regression. All variables were categorized and included in the analysis. The significance level was set at P<0.05. SPSS software Version 11.5.0 (SPSS, Institution Inc., Somers, NY, USA) was used.
3. Results
3.1. Patient characteristics
From the medical records of the multicentric institutions between January 1994 and December 2010, a total of 110 patients’ data were reviewed. Sixteen patients were excluded from this study because of incomplete clinical data or for being lost to follow up. Ninety-four records satisfied all inclusion criteria. The characteristics of the patients enrolled are shown in Table 1.
Table 1.
Characteristics of patients (n=94) undergoing conservative treatment
| Characteristics | Value* |
| Age (year) | 28.3 (21.0–40.0) |
| Gravidity times | 1 (0–3) |
| Parity times | 0.4 (0.0–2.0) |
| Nulliparous | 40.4% (38/94) |
| Previous history of surgery | |
| Cystectomy | 18.1% (17/94) |
| Salpingo-oophorectomy | 3.2% (3/94) |
| Preoperative serum levels | |
| CA125>35 U/ml | 27.7% (26/94) |
| CA125 (U/ml) | 291.7 (15.0–2 500.0) |
| Tumor size (cm) | 11.5 (3.0–20.0) |
| Residual tumor size (cm) | 0.8 (0.0–3.0) |
| Chemotherapy | |
| Standard chemotherapy | 68.1% (64/94) |
| No chemotherapy | 20.2% (19/94) |
| Non-standard chemotherapy | 11.7% (11/94) |
Values are presented as mean (range) or percentage (number of subjects)
Table 2 illustrates the detailed oncological data (including stage, grade, and histology) of patients receiving FSS. The median follow-up time was 58.7 months (range 12–196 months). In China, the indications for FSS of EOC are quite strict. Most practices converge to early stage and highly differentiated cases. In our study there were only a few cases at stages higher than Ic or at grades higher than 2, and some patients were not required even to receive subsequent chemotherapy. These factors were taken into account in our analysis of the results.
Table 2.
Patient demographics and tumor characteristics in cases (n=94) studied
| Characteristics | Number of patients |
| FIGO stage | |
| Ia | 46 (48.9%) |
| Ib | 8 (8.5%) |
| Ic | 28 (29.8%) |
| II | 1 (1.1%) |
| III | 11 (11.7%) |
| IV | 0 (0.0%) |
| Histological type | |
| Serous | 34 (36.2%) |
| Mucinous | 34 (36.2%) |
| Endometrioid | 9 (9.6%) |
| Clear cell | 4 (4.3%) |
| Mixed | 6 (6.4%) |
| Others | 7 (7.4%) |
| Grade | |
| 1 | 64 (68.1%) |
| 2 | 13 (13.8%) |
| 3 | 1 (1.1%) |
| NG | 16 (17.0%) |
NG: not graded
3.2. Prognostic outcomes
Among the patients enrolled, there were four cases of tumor-related death, with a mean survival of 132 months (95% CI 116–147 months). Disease recurred in nine patients after primary surgery, with a recurrence rate of 9.6% (9/94). Most recurrences occurred in Stages II and III disease. One case of Stage II and eleven cases of Stage III were included in this study. Five patients presented with apparent Stages II and III before the operation, and seven patients were upstaged after staging, having initially been diagnosed with early stage disease (Table 3).
Table 3.
Clinical characteristics of FSS patients with Stage II or III
| No. | Age (year) | Initial surgery | Stage | HT | Grade | PBC | tr (month) | RS | tf (month) | Status |
| 1 | 29 | USO+OM | IIIc | Serous | NG | Yes | 22 | Rectum | 23 | DOD |
| 2 | 33 | UC | IIIa | Clear cell | NG | Yes* | 7 | Ovary | 9 | DOD |
| 3 | 37 | BC | IIIc | Mucinous | 1 | Yes* | 26 | PC | 31 | AWD |
| 4 | 28 | USO+OM | IIIc | Endometrioid | 1 | Yes | NR | NR | 56 | AWD |
| 5 | 29 | USO+CC | IIIa | Serous | 1 | Yes* | 24 | PC | 58 | NED |
| 6 | 24 | USO | IIIc | Mixed | 1 | Yes | NR | NR | 15 | AWD |
| 7 | 21 | USO+OM+PLND/PALND | IIIa | Mucinous | 2 | Yes | NR | NR | 53 | DOD |
| 8 | 30 | UC+BO+OM | IIIc | Endometrioid | 2 | Yes | NR | NR | 35 | NED |
| 9 | 37 | USO+OM+PLND/PALND | IIIc | Endometrioid | 2 | Yes | 24 | PC | 36 | DOD |
| 10 | 33 | USO+BO | II | Serous | 1 | Yes | NR | NR | 50 | NED |
| 11 | 29 | USO+OM | IIIa | Endometrioid | 1 | Yes | NR | NR | 48 | NED |
| 12 | 31 | USO+OM+PLND/PALND | IIIc | Mixed | 1 | Yes | NR | NR | 52 | NED |
HT: histological type; PBC: platinum-based chemotherapy; t r: time to recurrence; RS: recurrence site; t f: follow-up time; USO: unilateral salpingo-oophorectomy; OM: omentectomy; UC: unilateral cystectomy; BC: bilateral cystectomy; CC: contralateral cystectomy; PLND/PALND: pelvic/para-aortic lymph nodes dissection or biopsy; BO: biopsy from the opposite ovarian; NG: not graded; NR: no recurrence; PC: peritoneal cavity recurrence; DOD: died of the disease; AWD: alive with the disease; NED: no evidence of the disease
Non-standard platinum-based chemotherapy
3.2.1. Stage, grade and histology
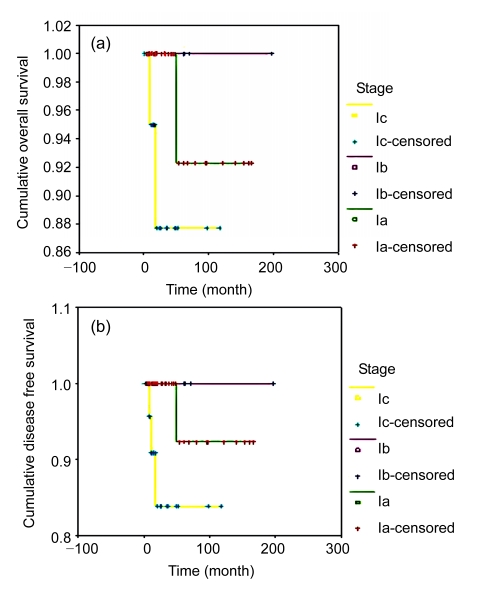
Estimates of OS and DFS were obtained by Kaplan-Meier analysis of data from Stages Ia–Ic patients who underwent FSS (Fig. 1). For patients with Stage Ia, Ib, or Ic disease, the 5-year OS rates were 92.3%, 100.0%, and 88.0%, respectively (P=0.7640), and the 5-year DFS rates were 92.3%, 100.0%, and 83.9%, respectively (P=0.0944). Although the survival duration of Stage Ic patients was shorter than that of Stage Ia patients, the difference was not significant. Differences between the DFS of Stages Ia, Ib and Ic patients were not statistically significant. Among the 28 cases of Stage Ic, 6 had surface excrescence, 9 had intra-operative rupture, and 13 had cytology positive sub-groups. There were no significant differences in OS (P=0.4675) or DFS (P=0.0591) among these sub-groups.
Fig. 1.
Survival analyses of Stages Ia–Ic patients undergoing FSS
(a) Overall survival; (b) Disease free survival
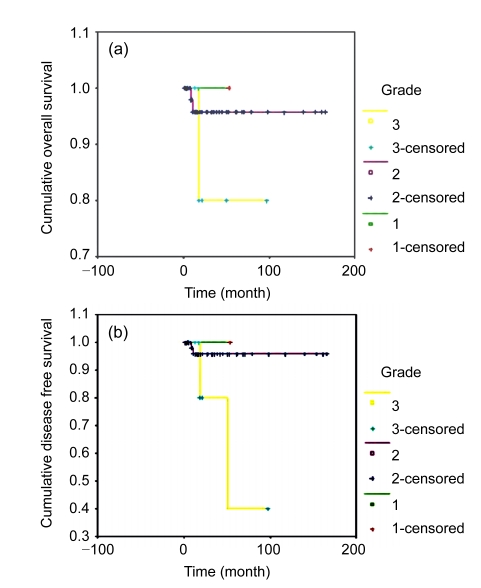
The relationships between OS and DFS and tumor grade are shown in Fig. 2. For patients with Grade 1, 2, or 3 disease, the 5-year OS rates were 95.6%, 85.7%, and 55.0%, respectively (P=0.0001), and the 5-year DFS rates were 88.9%, 83.0%, and 35.7%, respectively (P=0.0188). These results indicate that patients with higher tumor grades would have a poorer prognosis. Even in patients with Stage Ia disease, the OS and DFS rates of Grade 3 disease were significantly poorer than those of patients with Grade 1 disease (P=0.001).
Fig. 2.
Survival analyses by tumor grade of patients undergoing FSS
(a) Overall survival; (b) Disease free survival
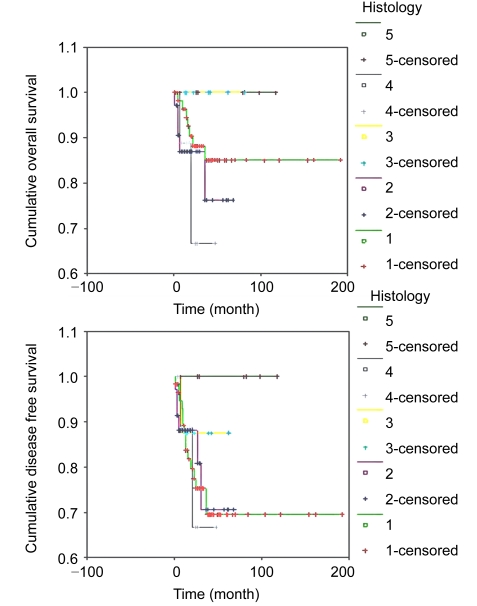
For patients with serous, mucinous, endometrioid, clear cell, or mixed histology, the 5-year DFS rates were 85.1%, 76.2%, 100.0%, 66.7%, and 88.1%, respectively (P=0.3315), and the 5-year OS rates were 69.5%, 70.7%, 87.5%, 66.7%, and 80.1%, respectively (P=0.6475). Differences in prognosis among histological types were not statistically significant (Fig. 3). If we divide the cases into serous and non-serous histology groups, the differences in OS (P=0.4650) and DFS (P=0.5256) were not significant. Likewise, if we divide the cases into high risk and usual histology groups, the differences in OS (P=0.1168) and DFS (P=0.8001) were not significant.
Fig. 3.
Survival analyses by histological type of patients undergoing FSS
(a) Overall survival; (b) Disease free survival. Histology: 1, serous; 2, mucinous; 3, endometrioid; 4, clear cell; 5, mixed
3.2.2. Staging procedure and surgical route
Ninety-four cases of conservative surgery comprised cases of complete staging surgery (SS) and surgery without complete staging procedure (non-SS) (Table 4). Of the 94 patients, 22 had laparoscopies and 72 had laparotomies, including 5 cases in which laparoscopy had to be changed into laparotomy intraoperatively. Many patients underwent unilateral cystectomy and six patients underwent bilateral cystectomy. This differs from the outcome of other case series, because most of our cases converged to Stage Ia with only one ovary involved. Therefore, most of these patients underwent unilateral cystectomy with or without biopsy. The prognosis of these patients was quite promising, since in only two cases disease recurred in the contralateral ovary and in only one case the patient died of the disease. Moreover, our series had eight cases of Stage Ib, six of which underwent bilateral cystectomy, and two of which underwent unilateral salpingo-oophorectomy and biopsy. The diagnosis and staging of the two cases were not confirmed until the post-operational pathology was completed. The outcomes are shown in Table 5.
Table 4.
Types of surgery in initial treatment
| Type of surgery | Number of patients |
| SS group | 23 |
| USO+OM+RLND | 15 |
| USO+OM+RLND+BO | 4 |
| USO+OM+RLND+CC | 1 |
| UC+OM+RLND | 2 |
| UC+OM+RLND+BO | 1 |
| Non-SS group | 71 |
| USO | 28 |
| USO+CC | 6 |
| USO+BO | 4 |
| UC | 20 |
| BC | 6 |
| UC+BO | 1 |
| UC+OM+BO | 1 |
| USO+OM | 4 |
| USO+OM+BO | 1 |
Descriptive statistics was used for data process. USO: unilateral salpingo-oophorectomy; OM: omentectomy; RLND: retroperitoneal lymph nodes dissection or biopsy; BO: biopsy from the opposite ovarian; CC: contralateral cystectomy; UC: unilateral cystectomy; BC: bilateral cystectomy
Table 5.
Clinical characteristics of Stage Ib patients
| No. | Age (year) | Initial surgery | Surgical route | Histological type | Grade | Platinum-based chemotherapy | Follow-up time (month) | Status |
| 1 | 33 | USO+BO+OM | Lap | Serous | 2 | Yes | 61 | NED |
| 2 | 28 | BC | Exp | Endometrioid | 2 | Yes | 62 | NED |
| 3 | 28 | BC | Exp | Serous | 2 | Yes | 70 | NED |
| 4 | 34 | BC | Exp | Serous | 2 | Yes* | 45 | NED |
| 5 | 38 | BC | Lap | Serous | 2 | Yes | 28 | NED |
| 6 | 21 | BC | Exp | Mucinous | 1 | Yes | 15 | NED |
| 7 | 20 | USO+BO+OM+PLND/PALND | Lap | Mixed | NG | Yes | 13 | NED |
| 8 | 21 | BC | Exp | Endometrioid | NG | Yes | 197 | NED |
USO: unilateral salpingo-oophorectomy; BO: biopsy from the opposite ovarian; OM: omentectomy; BC: bilateral cystectomy; PLND/PALND: pelvic/para-aortic lymph nodes dissection or biopsy; Lap: laparoscopy; Exp: exploration; NG: not graded; NED: no evidence of the disease
Non-standard platinum-based chemotherapy
Kaplan-Meier analysis was used to estimate OS and DFS of those patients receiving SS/non-SS and laparoscopy/laparotomy classified by FIGO stage (Ia–Ic). The results showed that there was no significant difference between the SS and non-SS groups (OS: P=0.0813; DFS: P=0.4009). Also, the differences in DFS and OS between laparoscopy and laparotomy were not statistically significant (OS: P=0.5454; DFS: P=0.8213).
3.2.3. Risk factors for recurrence
The DFS of patients with conservative treatment in our study was analyzed in order to look into the risk factors for recurrence. Results were analyzed using the Cox proportional hazards model. We modeled all categories of each variable and controlled for all variables that may predict the event. We selected variables based on previous studies (Wright et al., 2009; Satoh et al., 2010), and all variables were included in the analysis. There were no significant predictors of DFS from the multivariate analysis (Table 6).
Table 6.
Prognostic factors of DFS of EOC
| Factor | P | OR | 95% CI |
| Age | |||
| <25 years | 1 | 1 | 1 |
| 25–40 years | 0.349 | 0.86 | 0.63–1.17 |
| Tumor size | |||
| ≤5 cm | 1 | 1 | 1 |
| >5 cm | 0.285 | 1.18 | 0.87–1.59 |
| Preoperational CA125 | |||
| ≤35 U/L | 1 | 1 | 1 |
| >35 U/L | 0.114 | 0.98 | 0.96–1.00 |
| Residual tumor size | |||
| ≤1 cm | 1 | 1 | 1 |
| >1 cm | 0.225 | 0.11 | 0.00–3.86 |
| Stage | |||
| I | 1 | 1 | 1 |
| II | 0.063 | 7.01 | 0.86–90.17 |
| III | 0.051 | 8.93 | 0.99–80.32 |
| Histology | |||
| Serous | 1 | 1 | 1 |
| Non-serous | 0.408 | 0.63 | 0.21–1.88 |
| Tumor grade | |||
| 1 | 1 | 1 | 1 |
| 2 | 0.104 | 10.45 | 0.84–119.62 |
| 3 | 0.086 | 12.25 | 0.70–213.59 |
| Peritoneal cytology | |||
| Negative | 1 | 1 | 1 |
| Positive | 0.258 | 1.71 | 0.99–2.18 |
| Ascites | |||
| Absent | 1 | 1 | 1 |
| Present | 0.167 | 0.92 | 0.13–4.68 |
| Capsular integrity | |||
| Intact | 1 | 1 | 1 |
| Invaded/ruptured | 0.380 | 1.36 | 1.10–3.09 |
| Chemotherapy | |||
| Standard | 1 | 1 | 1 |
| Non-standard/no chemotherapy | 0.088 | 14.08 | 0.67–294.76 |
| Staging procedure | |||
| SS | 1 | 1 | 1 |
| Non-SS | 0.417 | 1.09 | 0.35–3.87 |
| Surgical route | |||
| Lap | 1 | 1 | 1 |
| Exp | 0.563 | 2.19 | 0.87–5.47 |
OR: odds ratio; 95% CI: 95% confidence interval; SS: complete staging surgery; non-SS: surgery without complete staging procedure; Lap: laparoscopy; Exp: exploration. Upper references are normalized to 1
3.3. Reproductive outcomes
In our current study, normal menstruation recommenced three months after operation or two months after chemotherapy in 89% of patients who underwent FSS. Sixty-nine patients were menstruating regularly, except for six cases of polymenorrhea, and 36 out of 45 patients who had the desire for pregnancy had chemotherapy perioperatively. Pregnancy outcomes are shown in Table 7. The stages of three women requiring fertility treatment after conservative surgery were Ia, Ic, and IIIc. No recurrence was seen among the 12 pregnant patients, and the offsprings were all normal.
Table 7.
Pregnancy outcomes after conservative treatment for EOC
| Characteristics | Value* |
| Known desire for pregnancy | 45 |
| Patients who conceived | 12 |
| Total pregnancies | 17 |
| Age at conception (year) | 29.2 (24.0–35.0) |
| Time to conceive (month) | 28.6 (4.0–37.0) |
| Spontaneous pregnancies | 14 |
| Induction of ovulation+intrauterine insemination | 1 |
| In vitro fertilization | 2 |
| Spontaneous abortion | 3 |
| Elective abortion | 5 |
| Term deliveries | 7 |
| Live newborns | 7 |
| Vaginal delivery | 3 |
| Cesarean section | 4 |
| Ongoing pregnancy | 2 |
Descriptive statistics was used for data process
Values are presented as mean (range) or number of subjects
4. Discussion
4.1. Surgical indication of FSS in EOC
In 2005, the National Comprehensive Cancer Network (NCCN) defines the surgical indication as Stage I EOC patients who desire to preserve their fertility function. Recently, conservative treatment of EOC has become a focus in this field. Most researchers consider that it is possible for young women with Stage Ia EOC to maintain their fertility and receive successful and safe treatment of their disease (Zanetta et al., 1997; Borgfeldt et al., 2007). A French multicentric study [Groupe des Chirurgiens de Centre de Lutte Contre le Cancer (GCCLCC) and Société Française d′Oncologie Gynécologique (SFOG)] recommended that conservative surgery is acceptable in young patients with Stage Ia Grade 1 disease, but not suitable for stages higher than Ia (Morice et al., 2005). Such patients should always commit to a close follow-up and complete hysterectomy and contralateral adnexectomy or oophorectomy, if necessary, after giving birth (Ding and Xie, 2006).
Recently, some researchers have extended the surgical indication to Stage Ic, Grade 3 and even to Stages II and III. However, their series of patients were too limited to draw a definite conclusion. In China, FSS on advanced stage EOC patients is not usually acceptable, except for young patients with a great desire to preserve their fertility. In our study, most cases converged into the early stage of disease, and most did not have complete SS. So, although most recurrence occurred in patients in advanced stages of disease, we cannot confirm the relationship between the stage and outcome of EOC patients. Our multivariate analysis showed that the stage is not a significant predictor of DFS. Therefore, surgical indication for higher stages needs further investigation in large series. We should always bear it in mind that the main objective of conservative treatment for women with EOC is to spare fertility without negatively affecting OS and DFS.
4.2. Clinical factors influencing the prognosis of FSS in EOC
Nowadays, it is generally accepted that the main factors influencing the prognosis of fertility-sparing treatment are the FIGO stage, pathological grade, and pathological type. Patients with high-risk factors (Stage over Ic, Grade 3, or clear cell carcinoma, etc.) would have significantly higher death and recurrence rates after FSS (Shimada et al., 2005; Zeng et al., 2005).
EOC patients with higher tumor grades would have a poorer prognosis. Vergote et al. (2001) carried out a retrospective study of 1 545 Stage Ia EOC patients. Multivariate analyses identified the degree of differentiation as the most powerful prognostic indicator of DFS. They recommended that the degree of differentiation should be used in making decisions on therapy in clinical practice and in the FIGO classification of Stage I ovarian cancer. We reached the same conclusion from our study. Even in patients with Stage Ia disease, the prognosis of Grade 3 disease was significantly poorer.
Most researchers have found that conservative treatment is suitable for patients with serous, mucinous, or endometrioid carcinoma but not for patients with high-risk factors, such as clear cell or anaplastic carcinoma (Morice et al., 2005). However, in our study, although patients with clear cell histological type showed a trend of lower survival and higher recurrence, there was no significant difference compared with other types of histology. The small number of clear cell samples might account for the result, and verification of the hypothesis needs larger samples.
There has been debate over the use of tumor stage as a prognostic factor. Some researchers do not agree with the inclusion of Stage Ic in the high-risk group (Seli and Tangir, 2005; Satoh et al., 2010). Kajiyama et al. (2010) analyzed 60 cases of Stage I EOC patients with FSS, including 30 cases of Stage Ia, 1 case of Stage Ib, and 29 cases of Stage Ic. The results indicated that differences in DFS and OS rates between Stages Ia and Ic were not significant. Our data showed a similar result. So it seems reasonable to revise the criteria for high-risk classification. However, the main limitation of our study was that most patients did not undergo complete SS procedures. Therefore, we cannot confirm that they had true Stage Ia or Ic disease. So data modification and staging procedure identification in large populations are required for further investigation.
There are few published studies of conservative treatment of Stage Ib patients. In our study, the eight cases of Stage Ib were all alive with no evidence of disease. Although we are unable to draw a definite conclusion from these few cases, it is still reasonable to say that conservative surgery of this stage may not have a considerable risk of tumor recurrence.
As for the choice of surgical range and surgical route in early stage patients, a Korean study including 264 Stage I epithelial ovarian tumors (62 complete SS and 202 non-SS) indicated that differences in OS and DFS rates between the two groups were not statistically significant and complete SS was not necessary for patients with Stage I mucinous epithelial tumors (Cho et al., 2006). A Chinese study of 89 patients indicated that lymph node excision could not improve the prognosis of macroscopic Stage I EOC (Ma et al., 2010). Our survival analysis showed no significant difference in OS or DFS between SS and non-SS or between laparoscopy and laparotomy groups for conservative treatment, which may lead to a reconsideration of SS and laparoscopy in clinical practice.
A multivariate analysis of 164 early stage EOC patients revealed that there were three factors related to occult metastasis: CA125 levels over 500 U/ml, positive peritoneal cytology, and Grade 3 disease (Ayhan et al., 2007). Multivariate analyses (Vergote et al., 2001) identified that, except for histological grade, none of the following were of prognostic value: histological type, dense adhesion, extracapsular growth, ascites, FIGO stage, and size of tumour. Our study, which included multivariate analysis of several other clinical factors, indicated that there were no individual prognostic factors for conservative treatment of EOC. So it is reasonable to conclude that the recurrence of disease might be affected by complicated situations.
4.3. Effect of chemotherapy on ovarian function and fertility
There is still controversy over the use of chemotherapy after FSS in EOC patients. The key point lies in the effect of chemotherapy on the prognosis and ovarian function, especially on the fertility function and offspring. It is generally accepted that EOC patients of Stage Ia Grade 1 do not need subsequent chemotherapy. However, patients with stages higher than Ic or other high-risk factors should be given auxiliary chemotherapy (Zeng et al., 2005). Chemotherapy would lead to temporary menstrual disorder, and 65%–70% patients would regain normal ovarian function after the completion of chemotherapy, while the congenital malformation rate would not be raised.
Previous studies have shown promising reproductive outcomes after chemotherapy for malignant disease (Morice et al., 2001; Schilder et al., 2002; Park et al., 2008). Maltaris et al. (2006) reported 282 cases of early stage EOC patients under FSS. A total of 113 (40.07%) patients managed to become pregnant, among whom 87 reached full term. Our data showed similar results suggesting that standard chemotherapy is unlikely to have an evident impact on ovarian function and fertility, despite a high frequency of adjuvant chemotherapy.
Although patients of subsequent chemotherapy should try to become pregnant as early as possible during the disease-free-period, pregnancy should not begin until at least 6–12 months after the end of chemotherapy in order to reduce the negative effect of chemotherapy on the oocytes (Maltaris et al., 2007). It may be advisable to establish a limit to the amount of time during which pregnancy may be attempted. Once the predetermined period has elapsed, whether patients have been successful or not, a strong consideration should be given to perform complete radical surgery for ovarian cancer.
5. Conclusions
FSS in the management of EOC is an efficient alternative from an oncologic point of view and could be cautiously considered in young patients who desire to preserve fertility with Stage I Grade 1 disease. However, careful follow-up and patient compliance will be required to detect early ovarian recurrence of disease. Staging procedure and surgical route might not significantly influence the OS or DFS. Standard chemotherapy is unlikely to have an evident impact on ovarian function and fertility.
The main limitation of our current study was the small amount of retrospective data. A larger scale investigation in EOC patients with FSS is expected in future.
References
- 1.Ayhan A, Gultekin M, Celik NY, Dursun P, Taskiran C, Aksan G, Yuce K. Occult metastasis in early ovarian cancers: risk factors and associated prognosis. Am J Obstet Gynecol. 2007;196(1):81.e1–81.e6. doi: 10.1016/j.ajog.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 2.Berman ML. Future directions in the surgical management of ovarian cancer. Gynecol Oncol. 2003;90(2):S33–S39. doi: 10.1016/S0090-8258(03)00342-1. [DOI] [PubMed] [Google Scholar]
- 3.Borgfeldt C, Iosif C, Måsbäck A. Fertility-sparing surgery and outcome in fertile women with ovarian borderline tumors and epithelial invasive ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2007;134(1):110–114. doi: 10.1016/j.ejogrb.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Cho YH, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, Nam JH. Is complete surgical staging necessary in patients with stage I mucinous epithelial ovarian tumors? Gynecol Oncol. 2006;103(3):878–882. doi: 10.1016/j.ygyno.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Colombo N, Chiari S, Maggioni A, Bocciolone L, Torri V, Mangioni C. Controversial issues in the management of early epithelial ovarian cancer: conservative surgery and role of adjuvant therapy. Gynecol Oncol. 1994;55(3):S47–S51. doi: 10.1006/gyno.1994.1341. [DOI] [PubMed] [Google Scholar]
- 6.Ding ZM, Xie X. Conservation of the reproductive capability in treating the ovarian epithelium carcinoma. Chin J Pract Gynecol Obstet. 2006;22(4):247–249. (in Chinese) [Google Scholar]
- 7.DiSaia PJ. Conservative management of the patient with early gynecologic cancer. CA Cancer J Clin. 1989;39(3):135–154. doi: 10.3322/canjclin.39.3.135. [DOI] [PubMed] [Google Scholar]
- 8.Duska LR, Chang YC, Flynn CE, Chen AH, Goodman A, Fuller AF, Nikrui N. Epithelial ovarian carcinoma in the reproductive age group. Cancer. 1999;85(12):2623–2629. doi: 10.1002/(SICI)1097-0142(19990615)85:12<2623::AID-CNCR19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.FIGO. Annual report on the results of treatment in gynecologic cancer. Int J Gynaecol Obstet. 1989;28(2):189–193. doi: 10.1016/0020-7292(89)90482-7. [DOI] [Google Scholar]
- 10.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2001;51(1):15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Kajiyama H, Shibata K, Suzuki S, Ino K, Nawa A, Kawai M, Nagasaka T, Kikkawa F. Fertility-sparing surgery in young women with invasive epithelial ovarian cancer. Eur J Surg Oncol. 2010;36(4):404–408. doi: 10.1016/j.ejso.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Kottmeier HL, Gentil F, Junqueira A. UICC Monograph Series No. 11. New York: Springer Verlag; 1968. Surgical Management: Conservative Surgery; pp. 443–459. [Google Scholar]
- 13.Ma K, Wen HW, Liao QP. Significance of systematic lymphadenectomy in patients with macroscopic stage I epithelial ovarian carcinoma. Chin J Clin Obstet Gynecol. 2010;11(2):105–108. (in Chinese) [Google Scholar]
- 14.Maltaris T, Boehm D, Dittrich R, Seufert R, Koelbl H. Reproduction beyond cancer: a message of hope for young women. Gynecol Oncol. 2006;103(3):1109–1121. doi: 10.1016/j.ygyno.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Maltaris T, Seufert R, Fischl F, Schaffrath M, Pollow K, Koelbl H, Dittrich R. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007;130(2):148–155. doi: 10.1016/j.ejogrb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Morice P, Wicart-Poque F, Rey A, El-Hassan J, Pautier P, Lhommé C, Crevoisier R, Haie-Meder C, Duvillard P, Castaigne D. Results of conservative treatment in epithelial ovarian carcinoma. Cancer. 2001;92(9):2412–2418. doi: 10.1002/1097-0142(20011101)92:9<2412::AID-CNCR1590>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Morice P, Leblanc E, Rey A, Baron M, Querleu D, Blanchot J, Duvillard P, Lhommé C, Castaigne D, Classe JM, et al. Conservative treatment in epithelial ovarian cancer: results of a multicentre study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer) and SFOG (Société Française d′Oncologie Gynécologique) Hum Reprod. 2005;20(5):1379–1385. doi: 10.1093/humrep/deh777. [DOI] [PubMed] [Google Scholar]
- 18.Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: oncologic safety and reproductive outcomes. Gynecol Oncol. 2008;110(3):345–353. doi: 10.1016/j.ygyno.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Satoh T, Hatae M, Watanabe Y, Yaegashi N, Ishiko O, Kodama S, Yamaguci S, Ochiai K, Takano M, Yokota H, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol. 2010;28(10):1727–1732. doi: 10.1200/JCO.2009.24.8617. [DOI] [PubMed] [Google Scholar]
- 20.Schilder JM, Thompson AM, DePriest PD, Ueland FR, Cibull ML, Kryscio RJ, Modesitt SC, Lu KH, Geisler JP, Higgins RV, et al. Outcome of reproductive age women with stage Ia or Ic invasive epithelial ovrian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87(1):1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- 21.Scrov SF, Scully RE, Sobin LH. Histological Typing of Ovarian Tumors. Geneva, Switzerland: World Health Organization; 1973. [Google Scholar]
- 22.Seli E, Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005;17(3):299–308. doi: 10.1097/01.gco.0000169108.15623.34. [DOI] [PubMed] [Google Scholar]
- 23.Shimada M, Kigawa J, Kanamori Y, Itamochi H, Oishi T, Minagawa Y, Ishihara K, Takeuchi Y, Okada M, Terakawa N. Outcome of patients with early ovarian cancer undergoing three courses of adjuvant chemotherapy following complete surgical staging. Int J Gynecol Cancer. 2005;15(4):601–605. doi: 10.1111/j.1525-1438.2005.00115.x. [DOI] [PubMed] [Google Scholar]
- 24.Vergote I, de Brabanter J, Fyles A, Bertelsen K, Einhorn N, Sevelda P, Gore ME, Kærn J, Verrelst H, Sjövall K, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357(9251):176–182. doi: 10.1016/S0140-6736(00)03590-X. [DOI] [PubMed] [Google Scholar]
- 25.Wright JD, Shah M, Mathew L, Burke WM, Culhane J, Goldman N, Schiff PB, Herzog TJ. Fertility preservation in young women with epithelial ovarian cancer. Cancer. 2009;115(18):4118–4126. doi: 10.1002/cncr.24461. [DOI] [PubMed] [Google Scholar]
- 26.Zanetta G, Chiari S, Rota S, Bratina G, Maneo A, Torri V, Mangioni C. Conservative surgery for stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol. 1997;104(9):1030–1035. doi: 10.1111/j.1471-0528.1997.tb12062.x. [DOI] [PubMed] [Google Scholar]
- 27.Zeng DY, Shen K, Huang HF, Wu M, Pan LY, Yang JX, Lang JH. Analysis of prognostic factors of malignant ovarian tumor after fertility-preserving treatment. Natl Med J China. 2005;85(36):2562–2565. (in Chinese) [PubMed] [Google Scholar]