Abstract
Global DNA hypomethylation has been associated with increased risk for cancers of the colorectum, bladder, breast, head and neck, and testicular germ cells. The aim of this study was to examine whether global hypomethylation in blood leukocyte DNA is associated with the risk of hepatocellular carcinoma (HCC). A total of 315 HCC cases and 356 age-, sex- and HBsAg status-matched controls were included. Global methylation in blood leukocyte DNA was estimated by analyzing long interspersed element-1 (LINE-1) repeats using bisulfite-polymerase chain reaction (PCR) and pyrosequencing. We observed that the median methylation level in HCC cases (percentage of 5-methylcytosine (5mC)=77.7%) was significantly lower than that in controls (79.5% 5mC) (P=0.004, Wilcoxon rank-sum test). The odds ratios (ORs) of HCC for individuals in the third, second, and first (lowest) quartiles of LINE-1 methylation were 1.1 (95% confidence interval (CI) 0.7–1.8), 1.4 (95% CI 0.8–2.2), and 2.6 (95% CI 1.7–4.1) (P for trend <0.001), respectively, compared to individuals in the fourth (highest) quartile. A 1.9-fold (95% CI 1.4–2.6) increased risk of HCC was observed among individuals with LINE-1 methylation below the median compared to individuals with higher (>median) LINE-1 methylation. Our results demonstrate for the first time that individuals with global hypomethylation measured in LINE-1 repeats in blood leukocyte DNA have an increased risk for HCC. Our data provide the evidence that global hypomethylation detected in the easily obtainable DNA source of blood leukocytes may help identify individuals at risk of HCC.
Keywords: Cancer risk, Epigenetics, Global hypomethylation, Hepatocellular carcinoma, LINE-1 repetitive element
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, while ranks the third most common cause of cancer-related death (Parkin et al., 2005; Gomaa et al., 2008). Its overall incidence remains high especially in the developing world and is steadily rising across most of the developed world (Shariff et al., 2009). Chronic infection with hepatitis B or C virus (HBV or HCV), aflatoxin exposure, liver cirrhosis, chronical alcohol consumption, and genetic factors have been characterized to play a major role in the HCC etiology (Morgan et al., 2004; Kim and Lee, 2005; Parkin et al., 2005; Okuda, 2007; Gomaa et al., 2008; Shariff et al., 2009).
Epigenetic changes in methylation patterns are increasingly implicated in cancer development (Feinberg and Tycko, 2004). Global DNA hypomethylation seems to occur in early neoplasia and has been regarded as an important component of cancer development (Das and Singal, 2004; Zhu et al., 2011). Compared with normal tissue counterparts, lower global DNA methylation was observed in tumor tissues in a broad panel of cancers including HCC (Das and Singal, 2004; Formeister et al., 2010). Animal studies have shown that experimentally-induced hypomethylation can lead to cancer development at multiple sites (Wilson et al., 1984; Thomas and Williams, 1992; Gaudet et al., 2003), indicating the causal role of global DNA hypomethylation in cancer development. In addition, lower levels of global methylation were reported in leukocyte DNA from subjects with colorectal adenoma, the precursor of colorectal cancer (Pufulete et al., 2003; Lim et al., 2008). Further, in healthy populations, global hypomethylation in blood leukocyte DNA has been associated with exposure to environmental risk factors for carcinogenesis, such as benzene, tobacco smoke, persistent organic pollutants, and perfluorooctane sulfonate (Bollati et al., 2007; Rusiecki et al., 2008; Breton et al., 2009; Liu et al., 2010; Wan et al., 2010). Several case-control studies have shown that global hypomethylation measured in blood DNA was associated with an increased risk for cancers of the colorectum, bladder, breast, head and neck, and testicular germ cells (Pufulete et al., 2003; Hsiung et al., 2007; Moore et al., 2008; Choi et al., 2009; Cho et al., 2010; Mirabello et al., 2010), suggesting that global DNA hypomethylation is a potential biomarker of cancer susceptibility.
Global DNA methylation level derives from the overall 5-methylcytosine (5mC) dinucleotide CpG sites in the human genome, about 55% of which consists of repetitive elements (Lander et al., 2001), including approximately 500 000 long interspersed element-1 (LINE-1) repeats which represent appro-ximately 17% of the human genome (Cordaux and Batzer, 2009). Because of heavy methylation in normal tissue, high representation throughout the genome, and close correlation with genomic DNA methylation content, LINE-1 methylation status has been used as a surrogate marker for estimating the genomic DNA methylation level (Yang et al., 2004; Weisenberger et al., 2005). In the present case-control study based on 315 HCC cases and 356 controls from a Chinese population, we sought to determine the association between LINE-1 hypomethylation and the risk of HCC.
2. Materials and methods
2.1. Subjects
This is a hospital-based case-control study. A total of 315 newly diagnosed HCC patients were recruited from the Sixth People’s Hospital of Shanghai Jiao Tong University and Tongji Hospital of Tongji University, Shanghai, China from February 2003 to October 2009. All cases did not receive chemotherapy or radiation therapy before blood sample collection. Final diagnoses were pathologically confirmed from the specimens obtained by surgery. As a control, blood samples of 356 cancer-free subjects were randomly selected from the individuals who attended for the physical examination at the same hospitals during the period when the case patients were recruited. The controls were frequency-matched to the cases by age (±5 years), sex, and serum HBsAg status. For both case and control subjects, those with other cancers, diabetes, autoimmune disorders, or other major systemic diseases were excluded. Information on age, sex, habits of alcohol drinking and cigarette smoking, and HCC family history was obtained using structured questionnaire through in-person interviews. An ever-drinker was defined as a person who reported drinking alcoholic beverages at least once per week for at least six months. An ever-smoker was defined as a smoker of at least one cigarette per day for at least six months. Chronic infections of HBV and HCV were considered if the seropositivities of HBsAg and anti-HCV were detected, respectively. Tumor characteristics including tumor size, Edmondson-Steiner grade, and tumor stage (tumour-node-metastasis (TNM) staging, 6th Ed. (Lei et al., 2006)) were made on the basis of medical records. Written informed consent was obtained from each participant. The study protocol was approved by the ethics review committee of the Institutional Review Board of the two participant hospitals.
2.2. DNA extraction and bisulfite modification
Genomic DNA of blood leukocytes was extracted using the QIAamp DNA Blood Mini kit (Qiagen, Shanghai, China). Extracted DNA was treated with sodium bisulfite using the EZ DNA Methylation kit (Zymo, CA, USA) according to the manufacturer’s protocol.
2.3. LINE-1 polymerase chain reaction (PCR) and pyrosequencing
A modified method of PCR pyrosequencing originally described by Yang et al. (2004) was performed to quantitate methylation of the repetitive LINE-1 sequences. In brief, PCR was carried out in a 50-μl reaction volume containing 25 μl of GoTaq Green Master mix (Promega, WI, USA), 1 pmol of forward primer (TTT TGA GTT AGG TGT GGG ATA TA), 1 pmol of biotinylated reverse primer (biotin-AAA ATC AAA AAA TTC CCT TTC), and 50 ng of bisulphite-treated DNA. PCR conditions were 40 cycles of 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 30 s. PCR product was bound to streptavidin sepharose beads (Amersham Biosciences, Uppsala, Sweden), and then was purified, washed, denatured, and washed again. Then, 0.3 μmol/L pyrosequencing primer (AGT TAG GTG TGG GAT ATA GT) was annealed to the purified PCR product. Pyrosequencing actions were performed in the PSQ HS 96 Pyrosequencing System. The non-CpG cytosine residues in LINE-1 repetitive sequences, which had been documented to be rarely methylated (Burden et al., 2005), were used as built-in controls to verify bisulfite conversion, and complete conversion of cytosine at non-CpG sites ensured successful bisulfite conversion. The percentage of 5mC at each of three CpG dinucleotide positions in LINE-1 repetitive sequences, as described in detail by Tarantini et al. (2009), was measured. The degree of LINE-1 methylation was expressed as percentage of methylated cytosines divided by the sum of methylated and unmethylated cytosines (percentage of 5mC). To validate PCR-pyrosequencing assay, each CpG dinucleotide position was assayed in duplicate and their averages were used in final analysis. The within-sample coefficient of variation was 0.68%, which suggests good reliability in the measurement.
2.4. Statistical analysis
Differences between HCC cases and controls in age, sex, alcohol drinking, cigarette smoking, HBsAg status, anti-HCV status, and HCC family history were evaluated using the χ 2-test. The nonparametric comparisons of median LINE-1 methylation levels between HCC cases and controls were evaluated by Wilcoxon rank-sum test. The association of LINE-1 methylation with HCC risk was estimated using the odds ratios (ORs) and 95% confidence intervals (CIs) from multivariate logistic regression analyses, with adjustment for age, sex, alcohol drinking, cigarette smoking, HBsAg status, anti-HCV status, and HCC family history. Statistical analyses were conducted using the Stata 10.1 (Stata Corp., College Station, TX). All tests were two-sided and a P value of ≤0.05 was considered statistically significant.
3. Results
The general characteristics of the HCC cases and controls are shown in Table 1. No significant differences between HCC cases and controls were found in the distribution of age, sex, alcohol drinking, cigarette smoking, or HBsAg status. Compared with controls, more cases were anti-HCV positive (P=0.01) and had HCC family history (P=0.04).
Table 1.
Characteristics of HCC and control subjects
| Variable | Number of subjectsa |
Pb | |
| HCC cases (n=315) | Controls (n=356) | ||
| Age | |||
| ≤50 years | 143 (45.4%) | 167 (46.9%) | 0.70 |
| >50 years | 172 (54.6%) | 189 (53.1%) | |
| Sex | |||
| Female | 52 (16.5%) | 59 (16.6%) | 0.98 |
| Male | 263 (83.5%) | 297 (83.4%) | |
| Alcohol drinking | |||
| Never | 195 (61.9%) | 218 (61.2%) | 0.86 |
| Ever | 120 (38.1%) | 138 (38.8%) | |
| Cigarette smoking | |||
| Never | 171 (54.3%) | 206 (57.9%) | 0.35 |
| Ever | 144 (45.7%) | 150 (42.1%) | |
| HBsAg | |||
| Negative | 42 (13.3%) | 48 (13.5%) | 0.96 |
| Positive | 273 (86.7%) | 308 (86.5%) | |
| Anti-HCV | |||
| Negative | 298 (94.6%) | 350 (98.3%) | 0.01 |
| Positive | 17 (5.4%) | 6 (1.7%) | |
| HCC family historyc | |||
| No | 295 (93.7%) | 345 (96.9%) | 0.04 |
| Yes | 20 (6.3%) | 11 (3.1%) | |
| Tumor size | |||
| ≤5 cm | 155 (49.2%) | ||
| >5 cm | 160 (50.8%) | ||
| Edmondson-Steiner grade | |||
| I/II | 64 (20.3%) | ||
| III/IV | 251 (79.7%) | ||
| Tumor stage | |||
| I/II | 227 (72.1%) | ||
| III/IV | 88 (27.9%) | ||
Data are expressed as n (%)
χ 2-test
Family history of HCC in first-degree relatives
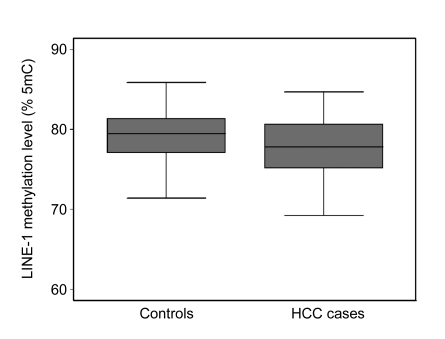
Fig. 1 depicts the distribution of the LINE-1 methylation levels in HCC cases and controls as box plots of the data. The median methylation level (77.7% 5mC) in cases was significantly lower than that (79.5% 5mC) in controls (P=0.004). Logistic regression analysis showed that the levels of LINE-1 methylation were inversely associated with the risk of HCC (Table 2). The ORs of HCC for individuals in the third, second and first (lowest) quartiles of LINE-1 methylation were 1.1 (95% CI 0.7–1.8), 1.4 (95% CI 0.8–2.2) and 2.6 (95% CI 1.7–4.1) (P for trend <0.001), respectively, compared to individuals in the fourth (highest) quartile. A 1.9-fold (95% CI 1.4–2.6) increased risk of HCC was observed among individuals with LINE-1 methylation below the median, compared to individuals with higher (>median) LINE-1 methylation.
Fig. 1.
Comparison of LINE-1 methylation levels measured in blood leukocyte DNA in HCC cases and controls (P=0.004)
Table 2.
Association of LINE-1 methylation with risk of HCC
| LINE-1 (% 5mC) | Number of subjectsa |
OR (95% CI)b | Pb | |
| HCC cases | Controls | |||
| Quartilec | ||||
| Q4 | 52 (16.5%) | 88 (24.7%) | 1.0d | |
| Q3 | 58 (18.4%) | 89 (25.0%) | 1.1 (0.7–1.8) | 0.68 |
| Q2 | 70 (22.2%) | 90 (25.3%) | 1.4 (0.8–2.2) | 0.22 |
| Q1 | 135 (42.9%) | 89 (25.0%) | 2.6 (1.7–4.1) | <0.001 |
| Medianc | ||||
| High | 110 (34.9%) | 177 (49.7%) | 1.0d | |
| Low | 205 (65.1%) | 179 (50.3%) | 1.9 (1.4–2.6) | <0.001 |
Data are expressed as n (%)
Adjusted for age, sex, alcohol drinking, cigarette smoking, HBsAg status, anti-HCV status, and HCC family history
The quartiles and the median of LINE-1 measures were based on values among control subjects
Reference
P for trend <0.001 for the ORs of HCC from the fourth, third, second to first quartiles of LINE-1 methylation
Stratification by HCC risk factors including age (≤50 years, >50 years), sex (female, male), alcohol drinking (never, ever), cigarette smoking (never, ever), HBsAg status (negative, positive), anti-HCV status (negative, positive), and HCC family history (no, yes), produced comparable results, regardless of quartile or median LINE-1 methylation evaluated (data not shown). Additional analyses showed that the interactions between LINE-1 methylation and these HCC risk factors in relation to HCC risk were not statistically significant (data not shown).
No significant associations were observed of age, alcohol drinking, cigarette smoking, HBsAg status, anti-HCV status or HCC family history with LINE-1 methylation, based on control subjects. However, males (79.8% 5mC) had higher LINE-1 methylation than females (78.2% 5mC; P=0.02) among controls, consistent with previous findings (El-Maarri et al., 2007; Hsiung et al., 2007). No significant correlation was found between LINE-1 methylation and tumor size, Edmondson-Steiner grade, or tumor stage (data not shown).
4. Discussion
Global hypomethylation in blood leukocyte DNA has been reported to correlate with increased risk for several types of cancer (Pufulete et al., 2003; Hsiung et al., 2007; Moore et al., 2008; Choi et al., 2009; Cho et al., 2010; Mirabello et al., 2010). In the present study, we demonstrated for the first time that individuals with global hypomethylation measured in LINE-1 repeats in leukocyte DNA had an increased HCC risk. Taken together, these findings support the idea that there exist common epigenetic basis for the pathogenesis of these different cancers. DNA methylation is a reversible epigenetic mechanism and DNA methylation patterns can be changed in response to exposure to exogenous and endogenous factors (Hsieh, 2000; Gluckman et al., 2008). Global hypomethylation in leukocyte DNA has been reported to be associated with exposure to risk factors for carcinogenesis (Bollati et al., 2007; Rusiecki et al., 2008; Breton et al., 2009; Liu et al., 2010; Wan et al., 2010), suggesting that leukocyte DNA hypomethylation may reflect cumulative effects from carcinogenic exposures. On the other hand, global methylation change has been indicated to be partly under genetic control (Bjornsson et al., 2008; Hillemacher et al., 2008), suggesting that DNA hypomethylation in leukocyte DNA may also reflect transgenerational risks for common human diseases including cancers (Bjornsson et al., 2004). Thus, global methylation levels in leukocyte DNA could provide a useful biomarker of susceptibility to certain cancer types.
Global DNA hypomethylation has been proposed to contribute to activation of oncogenes and genomic instability in cancer tissues, thereby causing aberrant activation of a wide spectrum of genes, formation of abnormal chromosomal structures and increased mutation rates that convey various growth advantages (Gaudet et al., 2003; Feinberg et al., 2006). LINE-1 hypomethylation is often found in association with hypermethylation of specific genes in cancer tissues (Cho et al., 2007; Ogino et al., 2008). Thus, global DNA hypomethylation may also contribute to carcinogenesis by inactivating tumor suppression genes. Moreover, LINE-1 methylation status by itself is likely to have biological effects, which may contribute directly to carcinogenesis through new retrotransposon insertions in human genomes (Iskow et al., 2010). Global DNA hypomethylation has been demonstrated to lead to the transcriptional activation of LINE-1 promoters, which can alter the transcriptome both in primary bladder tumors and in their premalignant urothelium counterparts (Wolff et al., 2010). These findings suggested that hypomethylation of LINE-1 may play a role not only in cancer but also in cancer predisposition.
Our study had the advantages of being based on a relatively large sample size, careful matching of controls to HCC cases by age, sex and HBsAg status, and pyrosequencing-based quantitative analysis which produced individual measures of methylation at three CpG dinucleotide positions, thus more accurately reflecting DNA methylation in the region. Several limitations in the present study should be noted. Firstly, this was a hospital-based case-control study, which may result in selection bias of participants. Moreover, given that DNA methylation in cancer patients was measured after cancer diagnosis and DNA hypomethylation could be detected in sera as well as HCC cells in peripheral blood of HCC cases (Tangkijvanich et al., 2007; Lee et al., 2009), blood DNA hypomethylation in cancer patients we observed may partly represent the methylation level of DNA derived from HCC cells. As degree of hypomethylation in tumor cells and number of HCC cells in circulating peripheral blood progress according to increased tumor stage, further analyses of LINE-1 methylation in relation to HCC risk were repeated using cases only with early stage of tumor (Stage I/II), which showed no major differences in risk estimates from the results based on the total number of cases (data not shown). In this context, the potentially-confounding effect of contaminating DNA from HCC cells on the association between LINE-1 hypomethylation and HCC risk observed in the present study would be minimal. However, further prospective studies using HCC-free subjects are needed to verify the risk-effect of global hypomethylation in blood DNA on HCC development. Secondly, information on diet was not available here, which may also influence methylation levels in genomic DNA (Suzuki et al., 2006; Schernhammer et al., 2010). Lastly, although LINE-1 repetitive elements have been used as a surrogate for global DNA methylation content, its methylation level is not equivalent to global DNA methylation content (Yang et al., 2004; Choi et al., 2009). Moreover, PCR primers were designed towards a consensus LINE-1 sequence and allowed for the amplification of only a small pool of LINE-1 repetitive elements dispersed throughout the genome (Yang et al., 2004).
In conclusion, global hypomethylation measured in LINE-1 repeats in blood leukocyte DNA shows an increased risk for HCC. Our data provide the evidence that global hypomethylation detected in the easily obtainable DNA source of blood leukocytes may help identify individuals at risk for HCC.
References
- 1.Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20(8):350–358. doi: 10.1016/j.tig.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, Yu W, Rongione MA, Ekstrom TJ, Harris TB, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299(24):2877–2883. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, Byun HM, Jiang J, Marinelli B, Pesatori AC, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67(3):876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 4.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burden AF, Manley NC, Clark AD, Gartler SM, Laird CD, Hansen RS. Hemimethylation and non-CpG methylation levels in a promoter region of human LINE-1 (L1) repeated elements. J Biol Chem. 2005;280(15):14413–14419. doi: 10.1074/jbc.M413836200. [DOI] [PubMed] [Google Scholar]
- 6.Cho NY, Kim BH, Choi M, Yoo EJ, Moon KC, Cho YM, Kim D, Kang GH. Hypermethylation of CpG island loci and hypomethylation of LINE-1 and Alu repeats in prostate adenocarcinoma and their relationship to clinicopathological features. J Pathol. 2007;211(3):269–277. doi: 10.1002/path.2106. [DOI] [PubMed] [Google Scholar]
- 7.Cho YH, Yazici H, Wu HC, Terry MB, Gonzalez K, Qu M, Dalay N, Santella RM. Aberrant promoter hypermethylation and genomic hypomethylation in tumor, adjacent normal tissues and blood from breast cancer patients. Anticancer Res. 2010;30(7):2489–2496. [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JY, James SR, Link PA, McCann SE, Hong CC, Davis W, Nesline MK, Ambrosone CB, Karpf AR. Association between global DNA hypomethylation in leukocytes and risk of breast cancer. Carcinogenesis. 2009;30(11):1889–1897. doi: 10.1093/carcin/bgp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nat Rev Genet. 2009;10(10):691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22(22):4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 11.El-Maarri O, Becker T, Junen J, Manzoor SS, Diaz-Lacava A, Schwaab R, Wienker T, Oldenburg J. Gender specific differences in levels of DNA methylation at selected loci from human total blood: a tendency toward higher methylation levels in males. Hum Genet. 2007;122(5):505–514. doi: 10.1007/s00439-007-0430-3. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7(1):21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 14.Formeister EJ, Tsuchiya M, Fujii H, Shpyleva S, Pogribny IP, Rusyn I. Comparative analysis of promoter methylation and gene expression endpoints between tumorous and non-tumorous tissues from HCV-positive patients with hepatocellular carcinoma. Mutat Res. 2010;692(1-2):26–33. doi: 10.1016/j.mrfmmm.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300(5618):489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14(27):4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillemacher T, Frieling H, Moskau S, Muschler MA, Semmler A, Kornhuber J, Klockgether T, Bleich S, Linnebank M. Global DNA methylation is influenced by smoking behaviour. Eur Neuropsychopharmacol. 2008;18(4):295–298. doi: 10.1016/j.euroneuro.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CL. Dynamics of DNA methylation pattern. Curr Opin Genet Dev. 2000;10(2):224–228. doi: 10.1016/S0959-437X(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 20.Hsiung DT, Marsit CJ, Houseman EA, Eddy K, Furniss CS, McClean MD, Kelsey KT. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16(1):108–114. doi: 10.1158/1055-9965.EPI-06-0636. [DOI] [PubMed] [Google Scholar]
- 21.Iskow RC, McCabe MT, Mills RE, Torene S, Pittard WS, Neuwald AF, van Meir EG, Vertino PM, Devine SE. Natural mutagenesis of human genomes by endogenous retrotransposons. Cell. 2010;141(7):1253–1261. doi: 10.1016/j.cell.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YJ, Lee HS. Single nucleotide polymorphisms associated with hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Intervirology. 2005;48(1):10–15. doi: 10.1159/000082089. [DOI] [PubMed] [Google Scholar]
- 23.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 24.Lee HS, Kim BH, Cho NY, Yoo EJ, Choi M, Shin SH, Jang JJ, Suh KS, Kim YS, Kang GH. Prognostic implications of and relationship between CpG island hypermethylation and repetitive DNA hypomethylation in hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):812–820. doi: 10.1158/1078-0432.CCR-08-0266. [DOI] [PubMed] [Google Scholar]
- 25.Lei HJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Wu CW. Prognostic value and clinical relevance of the 6th Edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg. 2006;203(4):426–435. doi: 10.1016/j.jamcollsurg.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Lim U, Flood A, Choi SW, Albanes D, Cross AJ, Schatzkin A, Sinha R, Katki HA, Cash B, Schoenfeld P, et al. Genomic methylation of leukocyte DNA in relation to colorectal adenoma among asymptomatic women. Gastroenterology. 2008;134(1):47–55. doi: 10.1053/j.gastro.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, Davis S, Zhang Y, Hussain M, Xi S, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29(25):3650–3664. doi: 10.1038/onc.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirabello L, Savage SA, Korde L, Gadalla SM, Greene MH. LINE-1 methylation is inherited in familial testicular cancer kindreds. BMC Med Genet. 2010;11(1):77. doi: 10.1186/1471-2350-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore LE, Pfeiffer RM, Poscablo C, Real FX, Kogevinas M, Silverman D, Garcia-Closas R, Chanock S, Tardon A, Serra C, et al. Genomic DNA hypomethylation as a biomarker for bladder cancer susceptibility in the Spanish bladder cancer study: a case-control study. Lancet Oncol. 2008;9(4):359–366. doi: 10.1016/S1470-2045(08)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127(5):S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Ogino S, Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, Kirkner GJ, Fuchs CS. LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Int J Cancer. 2008;122(12):2767–2773. doi: 10.1002/ijc.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda H. Hepatocellular carcinoma development in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(1):161–173. doi: 10.1016/j.bpg.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 34.Pufulete M, Al-Ghnaniem R, Leather AJ, Appleby P, Gout S, Terry C, Emery PW, Sanders TA. Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology. 2003;124(5):1240–1248. doi: 10.1016/S0016-5085(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 35.Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116(11):1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schernhammer ES, Giovannucci E, Kawasaki T, Rosner B, Fuchs CS, Ogino S. Dietary folate, alcohol and B vitamins in relation to LINE-1 hypomethylation in colon cancer. Gut. 2010;59(6):794–799. doi: 10.1136/gut.2009.183707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3(4):353–367. doi: 10.1586/egh.09.35. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki H, Toyota M, Sato H, Sonoda T, Sakauchi F, Mori M. Roles and causes of abnormal DNA methylation in gastrointestinal cancers. Asian Pac J Cancer Prev. 2006;7(2):177–185. [PubMed] [Google Scholar]
- 39.Tangkijvanich P, Hourpai N, Rattanatanyong P, Wisedopas N, Mahachai V, Mutirangura A. Serum LINE-1 hypomethylation as a potential prognostic marker for hepatocellular carcinoma. Clin Chim Acta. 2007;379(1-2):127–133. doi: 10.1016/j.cca.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 40.Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bollati V, Marinelli B, Cantone L, Rizzo G, Hou L, Schwartz J, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117(2):217–222. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas GA, Williams ED. Production of thyroid tumours in mice by demethylating agents. Carcinogenesis. 1992;13(6):1039–1042. doi: 10.1093/carcin/13.6.1039. [DOI] [PubMed] [Google Scholar]
- 42.Wan YJ, Li YY, Xia W, Chen J, Lv ZQ, Zeng HC, Zhang L, Yang WJ, Chen T, Lin Y, et al. Alterations in tumor biomarker GSTP gene methylation patterns induced by prenatal exposure to PFOS. Toxicology. 2010;274(1-3):57–64. doi: 10.1016/j.tox.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, Fiala E, Ehrlich M, Laird PW. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33(21):6823–6836. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson MJ, Shivapurkar N, Poirier LA. Hypomethylation of hepatic nuclear DNA in rats fed with a carcinogenic methyl-deficient diet. Biochem J. 1984;218(3):987–990. doi: 10.1042/bj2180987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff EM, Byun HM, Han HF, Sharma S, Nichols PW, Siegmund KD, Yang AS, Jones PA, Liang G. Hypomethylation of a LINE-1 promoter activates an alternate transcript of the MET oncogene in bladders with cancer. PLoS Genet. 2010;6(4):e1000917. doi: 10.1371/journal.pgen.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3):e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu ZZ, Sparrow D, Hou L, Tarantini L, Bollati V, Litonjua AA, Zanobetti A, Vokonas P, Wright RO, Baccarelli A, et al. Repetitive element hypomethylation in blood leukocyte DNA and cancer incidence, prevalence, and mortality in elderly individuals: the normative aging study. Cancer Causes Control. 2011;22(3):437–447. doi: 10.1007/s10552-010-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]