Abstract
Objective: Mitogen-activated protein kinases (MAPKs) are correlated with a more malignant phenotype in many cancers. This study was designed to evaluate the predictive value of the expression of MAPK phosphatase-1 (MKP-1) and phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2), as the key regulatory mechanism of the MAPKs, in lung squamous cell carcinoma (SCC). Methods: We assessed the expressions of MKP-1 and p-ERK1/2 in twenty subjects at different differentiation degree of SCC and five normal lungs by immunohistochemistry and real-time reverse transcriptase polymerase chain reaction (RT-PCR) analysis. Results: Immunohistochemistry and real-time RT-PCR assay showed that the expression of MKP-1 was gradually decreased as tissue type went from normal lung tissues to increasingly undifferentiated carcinoma, and it was negatively correlated with tumor differentiation (P<0.01). However, the expression of p-ERK1/2 or ERK1/2 was gradually increased as tissue type went from normal lung tissues to increasingly undifferentiated carcinoma, and it was positively correlated with tumor differentiation (P<0.01). Conclusions: Our data indicates the relevance of MKP-1 and p-ERK1/2 in SCC as a potential positive and negative prognostic factor. The imbalanced expression of MKP-1 and p-ERK1/2 may play a role in the development of SCC and these two molecules may be new targets for the therapy and prognosis of SCC.
Keywords: Mitogen-activated protein kinase phosphatase-1 (MKP-1), Extracellular signal-regulated kinase (ERK), Lung squamous cell carcinoma (SCC), Prognostic factor
1. Introduction
Lung cancer continues to be the main cause of cancer-related deaths worldwide, with more than one million deaths every year (Jemal et al., 2009). The majority of lung cancers (85%) are non-small cell lung cancers (NSCLCs), which include squamous cell carcinoma (SCC) and adenocarcinoma (Herbst et al., 2008). The pathogenesis of SCC involves the accumulation of genetic and epigenetic alterations in a long multi-step process, partly owing to the continuing exposure to carcinogens such as tobacco smoke. Few changes that occur during the pathogenesis of SCC have been identified. In recent years, studies have shown that intracellular signal transduction pathways play an important role in the occurrence or development of tumors, through which numerous extracellular stimulations mediate cell proliferation, apoptosis, and invasion.
Mitogen-activated protein kinases (MAPKs) are widespread intracellular protein kinases that act in the regulation of cellular functions, including gene transcription, protein translation, cytoskeletal remodeling, endocytosis, cell metabolism, cell proliferation, and survival (Bogoyevitch, 2006; Bogoyevitch and Arthur, 2008). These processes are regulated by protein-protein interactions spatially and temporarily, generating specific cell behavior (Murphy and Blenis, 2006). MAPKs in mammals can be grouped into three structural families, the c-jun NH2-terminal kinase (JNK), p38 kinase, and extracellular signal-regulated kinase (ERK) (Johnson and Lapadat, 2002; Roux and Blenis, 2004). The MAPK superfamily consists of several serine/threonine protein kinases, which can phosphorylate considerable downstream effectors when activated, including cytoskeletal proteins, transcription factors, and other phosphoproteins, to play an important role in varieties of cellular functions ranging from migration, proliferation, to synthesis of fibrosis (Chang and Karin, 2001). The MAPK pathway has been shown to be extremely relevant in human carcinogenesis (Dunn et al., 2003). One of the MAPK pathways that is the best studied and understood is the ERK pathway, which lies on the downstream of the cellular proto-oncogene Ras and is involved in a wide range of cellular activities including cell differentiation, proliferation, and survival (Shaul and Seger, 2007). The ERK signaling has been shown to play an essential role in tumorigenesis and tumor metastasis (Ward et al., 2001; Hu et al., 2010). Activated or elevated ERK expression has been detected in a great number of human tumors, including the lung (Blackhall et al., 2003; Mukohara et al., 2003), kidney (Huang et al., 2008), breast (Adeyinka et al., 2002; Eralp et al., 2008; Chen et al., 2009), liver (Gailhouste et al., 2010), and prostate (Moro et al., 2007) tumors, and so on. Sustained activation of ERK has been a requirement for angiogenesis (Mavria et al., 2006). MAPK phosphatases (MKPs) are a family of protein phosphatases, which inactivate MAPKs by dephosphorylation of threonine and/or tyrosine residues. MAPK signaling is integrated at the level of regulation by MKPs through a negative feedback mechanism (Farooq and Zhou, 2004; Dickinson and Keyse, 2006). MKP-1 is a most important one (Wada and Penninger, 2004; Bermudez et al., 2010). It is encoded by an early response gene, which is temporarily induced by mitogens and stress signals such as cytokines, serum, and heat shock (Wong et al., 2005). MKP-1 is a nuclear phosphatase that is required for cell growth and proliferation and is expressed in various malignancies (Denkert et al., 2002; Tsujita et al., 2005). In general, MKP-1 deactivates phosphorylated ERK 1/2 (p-ERK1/2) by phosphorylation, playing the negative feedback regulation, and it can directly affect the amount and the duration of ERK.
In this study, the objective was to examine the relationship between MKP-1, p-ERK1/2, and tumor differentiation of SCC to find potential positive or negative prognostic factors and new molecular targets for lung cancer therapy.
2. Materials and methods
2.1. Patients
Surgical specimens of human lung SCC and normal lung tissues were collected from 20 subjects from the Second Affiliated Hospital, School of Medicine, Zhejiang University, China. The subjects were all native Chinese patients, including 15 males and 5 females. Written-signed informed consent was obtained from each subject. The inclusion criteria included: 34–71 years old, active or previous smokers, the stage of IB or IIB, no prior chemotherapy or radiotherapy treatment before tissue samples obtained, surgery as the initial treatment, gemcitabine- and cisplatin-based chemotherapy for four times after surgery. The study was approved by the Ethical Committee of the Second Affiliated Hospital of Zhejiang University. We also obtained five normal samples from tissues distant from the tumor or adjacent tissues to the tumor of the subjects studied. The specimens were fixed in 10% formalin and embedded in paraffin. Hematoxylin and eosin (H&E) staining was performed for tumor morphology assessment. Two pathologists in our group evaluated the SCC.
2.2. Histologic staining and immunohistochemistry
Specimens were preserved in 4% paraformaldehyde for 24 h. Histologic staining and immunohistochemistry were performed on 8-μm sections of the paraffin-embedded tissues. Sections were then deparaffinized and rehydrated. For histological analysis, some sections were stained with 3 g/L cresyl violet (VWR International, Buffalo Grove, IL, USA). For immunohistochemistry, other sections were treated with 10.2 mmol/L sodium citrate buffer (pH 6.1) at 95 °C for 20 min. Then these sections were rinsed in 0.01 mol/L phosphate buffer solution (PBS) containing 3 g/L Triton X-100 (PBS-T, pH 7.4), immersed in 2% normal goat serum in PBS at 37 °C for 2 h, and incubated with polyclone MKP-1 antibody (Santa Cruz Biotechnology, USA) or polyclone p-ERK1/2 antibody (Santa Cruz Biotechnology, USA) in PBS containing 0.01 g/ml bovine serum albumin overnight at 4 °C. After washing three times with PBS, there sections were incubated in biotinylated goat-anti-rabbit IgG (Boster) in PBS for 2 h at room temperature, then washed in PBS-T three times, and incubated in avidin-biotin-peroxidase complex solution (ABC, Boster) at room temperature for 2 h, followed by rinsing with PBS-T three times again. Immunolabeling was visualized with 0.5 g/L diaminobenzidine (DAB) and 0.3% H2O2 in PBS. After staining, the sections were counterstained by hematoxylin. The sections were then dehydrated by ethanol and xylene before coverslips with Permount. As a negative control, rat IgG (Biomeda Corporation, USA) was used instead of primary antibody.
2.3. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) analyses of MKP-1 and ERK1/2
Total RNA was extracted from the specimens using the TRIzol reagent kit (Invitrogen, USA) following the manufacturer’s procedures. RNA concentration was measured spectrophotometrically for reverse transcription, and the cDNA synthesis reaction system (20 μl) on a FTC2000 (Funglyn, Canada) contained 2 μg of total RNA. The reaction mixture included 4 μl 5× RT-buffer, 5 mmol/L deoxyribonucleotide (dNTPs), 2.5 μmol/L Oligo (dT), and 20 U RNase inhibitor. Then the samples were incubated at 70 °C for 5 min to anneal the hexamers. After 200 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, USA) was added, the samples were incubated at 42 °C for 60 min. The reaction was stopped through heating at 72 °C for 10 min. For real-time RT-PCR, the reaction mixture (40 μl) consisted of 4 μl cDNA, 35.2 μl SYBR® Premix Ex Taq™ (TaKaRa, China), 0.5 μl of 5 U Taq DNA polymerase, and 0.3 μl of 20 pmol/μl MKP-1 or ERK1/2 primer (Invitrogen, USA). The cDNA was denatured by heating at 94 °C for 3 min, followed by 40 cycles of three-step PCR (10 s at 94 °C, 30 s at 62 °C, and 30 s at 72 °C), before fluorescence measurement at 72 °C. Meanwhile, the primers were used for the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), in real-time RT-PCR to amplify GAPDH (forward: 5′-CTGCTCCTCCTGTTCGACAGT-3′, reverse: 5′-CCGTTGACTCCGACCTTCAC-3′) as an internal control of MKP-1 (forward: 5′-CGATGCCTATGACCTGGTCAAGA-3′, reverse: 5′-CTGCGCTCAAAGTCCAGCAACT-3′) and ERK1/2 (forward: 5′-CCATTGGGTTGTGGAGGCAATG-3′, reverse: 5′-CACTCTGGGGATCAGTAAGGAC-3′).
2.4. Image analysis and statistics
The sections were examined using UTHSCSA Image Tools 3.0 (School of Medicine, University of Texas at San Antonio, TX, USA) at 400× magnification. The optical densities and numbers of the MKP-1 and p-ERK1/2 positive cells were determined. The probability taken to indicate significant differences was 95%. The means±standard deviation (SD) for all data were calculated. SPSS® Version 12.0 statistical software (SPSS Inc., St. Louis, MO, USA) was used for statistical analysis. The Mann-Whitney U-test was taken to evaluate the significance of any difference between the groups.
3. Results
3.1. Histologic staining and immunohistochemistry
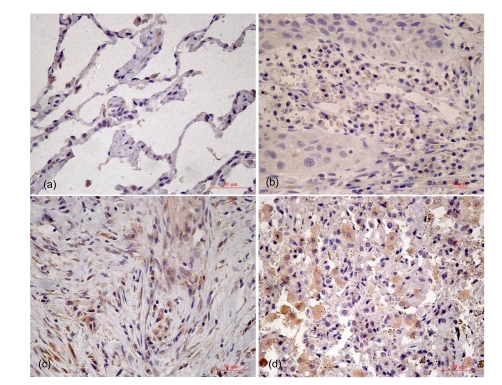
MKP-1 and p-ERK1/2 immunostainings were found both in the nucleus and cytoplasm. However, in the normal bronchial epithelium, some ciliated and endothelial cells showed MKP-1 for both nuclear and cytoplasmic staining cases studied. The staining score of MKP-1 was gradually decreased as tissue type went from normal lung tissues to increasingly undifferentiated (Fig. 1; Table 1). One-way analysis of variance (ANOVA) showed that MKP-1 expression was associated with tumor differentiation (P<0.01). Furthermore, by pairwise comparison, MKP-1 expression decreased when the degree of differentiation was reduced (P<0.01), but there was no significant difference between normal lung tissues and well differentiated tissues in this protein’s expression. We also assessed the expression of p-ERK1/2 in type II cell hyperplasia in alveolar fields close to the tumor and found that the staining score of p-ERK1/2 gradually increased from normal lung tissues to increasingly undifferentiated (Fig. 2; Table 2), and p-ERK1/2 expression was associated with tumor differentiation (P<0.01). Pairwise comparison showed that the expression increased when the degree of differentiation was less (P<0.01).
Fig. 1.
Immunohistochemistry staining for MKP-1 in tumor differentiation
(a) Normal lung tissue; (b) Well differentiation; (c) Moderate differentiation; (d) Poor differentiation
Table 1.
Immunohistochemistry staining scores for MKP-1 in tumor differentiation
| Group | n | Median staining score | P* |
| Normal lung tissue | 5 | 1.90 | |
| Well differentiation | 3 | 1.68 | <0.01 |
| Moderate differentiation | 10 | 1.03 | <0.01 |
| Poor differentiation | 7 | 0.72 | <0.01 |
Compared to the normal lung tissue group
Fig. 2.
Immunohistochemistry staining for p-ERK1/2 in tumor differentiation
(a) Normal lung tissue; (b) Well differentiation; (c) Moderate differentiation; (d) Poor differentiation
Table 2.
Immunohistochemistry staining scores for p-ERK1/2 in tumor differentiation
| Group | n | Median staining score | P* |
| Normal lung tissue | 5 | 0.55 | |
| Well differentiation | 3 | 0.94 | <0.01 |
| Moderate differentiation | 10 | 1.32 | <0.01 |
| Poor differentiation | 7 | 1.80 | <0.01 |
Compared to the normal lung tissue group
3.2. Real-time RT-PCR analysis of MKP-1 and ERK1/2
The real-time RT-PCR results showed that the MKP-1 mRNA level gradually decreased from normal lung tissues to carcinoma and was negatively correlated with tumor differentiation, as indicated by the increased ΔC T values (comparative threshold cycle (C T) method). However, the expression of ERK1/2 was gradually increased from normal lung tissues to carcinoma, and it was positively correlated with tumor differentiation (Table 3).
Table 3.
Relative quantification of MKP-1 and ERK1/2 mRNA levels using the comparative C T method followed by real-time RT-PCR
| Group | Average CT |
Fold difference 2−ΔΔCT |
|||
| MKP-1 | ERK1/2 | GAPDH | MKP-1 | ERK1/2 | |
| Poor differentiation | 24.87 | 21.19 | 17.89 | 0.51 | 3.14 |
| Moderate differentiation | 25.93 | 23.00 | 19.25 | 0.62 | 2.30 |
| Well differentiation | 26.03 | 23.84 | 19.56 | 0.72 | 1.59 |
| Normal lung tissue | 24.13 | 23.08 | 18.13 | 1.00 | 1.00 |
ΔCT=CT (MKP-1 or ERK1/2)−CT (GAPDH), ΔΔCT=ΔCT−ΔCT (control)
3.3. Impact of molecular markers on survival
The survival time was calculated from the day of surgical resection. Spearman’s correlation coefficient of MKP-1 expression and prognosis was 0.589 (P<0.01), showing a positive correlation, which indicates that MKP-1 expression was a positive prognostic factor for patients with lung SCC. The correlation between p-ERK expression and prognosis was negative, and the Spearman’s correlation coefficient was 0.693 (P<0.01), indicating p-ERK1/2 expression was an adverse prognostic factor.
4. Discussion
The MAPK pathway is one of the most crucial intracellular signaling cascades conserved from yeast to mammals (Widmann et al., 1999). Cells are always responsive to physiological stimuli and environmental cues, such as hormones, growth factors, cytokines, and stress including radiation, ischemic injury, and osmotic shock, through activation of MAPKs (Chang and Karin, 2001). ERK, at the end of the MAPK pathway, is activated by growth factor, ionic rays, and hydrogen dioxide, and changes into p-ERK1/2. p-ERK enters the cell nucleus, and stimulates the transcription and expression of related genes by acting at transcription factors such as c-Myc, activator protein-1 (AP-1), and nuclear factor-kappa B (NF-κB), thus playing an important role in cell growth, development, split, death, and malignant transformation. MAPK dephosphorylation and deactivation are carried out by some phosphatases, of which MKP-1 is the most important (Dickinson and Keyse, 2006; Owens and Keyse, 2007). MKP-1 deactivates p-ERK1/2 by phosphorylation, for negative feedback regulation, and it can directly affect the amount and duration of ERK. Our studies have shown that the imbalanced expression of MKP-1 and ERK may be one of the important reasons for the occurrence and development of tumors. Greenberg et al. (2002) reported the activation of p38 in a small number of NSCLC patients, but they did not consider the activation of ERK in their study. Serini et al. (2008) silenced the expression of MKP-1 through the RNA interference, which blocks the apoptosis of malignant cells induced by ducosahexenoic acid, so that the mechanism may be relevant to the increased expression of MKP-1 and the decreased expression of ERK. It is thus clear that the dynamic balance between MKP-1 and p-ERK1/2 has a great influence on cell survival and apoptosis. However, concerning SCC, the most common form of lung cancer, the specific mechanism has not been reported.
In this work, we describe the differential expression of MKP-1 in different stages of SCC. We found high expression of the MKP-1 protein in normal lung tissue and low expression in SCC. And with the development of cancer stages, the level of MKP-1 expression decreased. Inversely, p-ERK1/2 showed a low expression in normal lung tissue and a high expression in lung cancer tissue, and had an ascending tendency with the development of cancer stages. Meanwhile, both of MKP-1 and p-ERK1/2 were correlated with prognosis. In summary, MKP-1 and p-ERK1/2 showed an inverse and positive correlation in tumor differentiation of SCC. Thus, we suggest that, with the development of SCC, the expression of the MKP-1 protein decreases, leading the deactivation of p-ERK1/2 to be reduced. The increased expression of the p-ERK1/2 protein inhibits apoptosis and promotes proliferation of cancer cells, leading to the development of cancer.
This study showed a low expression of the MKP-1 protein and high expression of the p-ERK1/2 protein in SCC. The present study provides an experimental foundation for therapeutic strategy that RNA interference technology, or other relevant methods could be used to increase the level of MKP-1 and decrease the level of p-ERK1/2, subsequently, leading to apoptosis of cancer cells.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30900654), the Science and Technology Department of Zhejiang Province (No. 2009R10031), and the Health Bureau of Zhejiang Province (No. 2009QN010), China
References
- 1.Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res. 2002;8(6):1747–1753. [PubMed] [Google Scholar]
- 2.Bermudez O, Pages G, Gimond C. The dual-specificity MAP kinase phosphatases: critical roles in development and cancer. Am J Physiol Cell Physiol. 2010;299(2):C189–C202. doi: 10.1152/ajpcell.00347.2009. [DOI] [PubMed] [Google Scholar]
- 3.Blackhall FH, Pintilie M, Michael M, Leighl N, Feld R, Tsao MS, Shepherd FA. Expression and prognostic significance of kit, protein kinase B, and mitogen-activated protein kinase in patients with small cell lung cancer. Clin Cancer Res. 2003;9(6):2241–2247. [PubMed] [Google Scholar]
- 4.Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28(9):923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 5.Bogoyevitch MA, Arthur PG. Inhibitors of c-Jun N-terminal kinases: JuNK no more? Biochim Biophys Acta. 2008;1784(1):76–93. doi: 10.1016/j.bbapap.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang LF, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan ZK, Liu K, Huang S. Extracellular signal-regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression. Cancer Res. 2009;69(24):9228–9235. doi: 10.1158/0008-5472.CAN-09-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denkert C, Schmitt WD, Berger S, Reles A, Pest S, Siegert A, Lichtenegger W, Dietel M, Hauptmann S. Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int J Cancer. 2002;102(5):507–513. doi: 10.1002/ijc.10746. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119(22):4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2003;83(1):1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 11.Eralp Y, Derin D, Ozluk Y, Yavuz E, Guney N, Saip P, Muslumanoglu M, Igci A, Kücücük S, Dincer M, et al. MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol. 2008;19(4):669–674. doi: 10.1093/annonc/mdm522. [DOI] [PubMed] [Google Scholar]
- 12.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16(7):769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Gailhouste L, Ezan F, Bessard A, Frémin C, Rageul J, Langouët S, Baffet G. RNAi-mediated MEK1 knock-down prevents ERK1/2 activation and abolishes human hepatocarcinoma growth in vitro and in vivo. Int J Cancer. 2010;126(6):1367–1377. doi: 10.1002/ijc.24950. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg AK, Basu S, Hu J, Yie TA, Tchou-Wong KM, Rom WN, Lee TC. Selective p38 activation in human non-small cell lung cancer. Am J Respir Cell Mol Biol. 2002;26(5):558–564. doi: 10.1165/ajrcmb.26.5.4689. [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu JA, Li Y, Fang J. Effect of ERK inhibitor on pulmonary metastasis of inoculated human adenoid cystic carcinoma cells in nude mice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(1):117–123. doi: 10.1016/j.tripleo.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 17.Huang D, Ding Y, Luo WM, Bender S, Qian CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH, et al. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer Res. 2008;68(1):81–88. doi: 10.1158/0008-5472.CAN-07-5311. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 19.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 20.Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006;9(1):33–44. doi: 10.1016/j.ccr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Moro L, Arbini AA, Marra E, Greco M. Constitutive activation of MAPK/ERK inhibits prostate cancer cell proliferation through upregulation of BRCA2. Int J Oncol. 2007;30(1):217–224. doi: 10.3892/ijo.30.1.217. [DOI] [PubMed] [Google Scholar]
- 22.Mukohara T, Kudoh S, Yamauchi S, Kimura T, Yoshimura N, Kanazawa H, Hirataa K, Wanibuchib H, Fukushimab S, Inouec K, et al. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC) Lung Cancer. 2003;41(2):123–130. doi: 10.1016/S0169-5002(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 23.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31(5):268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 25.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serini S, Trombino S, Oliva F, Piccioni E, Monego G, Resci F, Boninsegna A, Picci N, Ranelletti FO, Calviello G. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis. 2008;13(9):1172–1183. doi: 10.1007/s10495-008-0246-1. [DOI] [PubMed] [Google Scholar]
- 27.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773(8):1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Tsujita E, Taketomi A, Gion T, Kuroda Y, Endo K, Watanabe A, Nakashima H, Aishima S, Kohnoe S, Maehara Y. Suppressed MKP-1 is an independent predictor of outcome in patients with hepatocellular carcinoma. Oncology. 2005;69(4):342–347. doi: 10.1159/000089766. [DOI] [PubMed] [Google Scholar]
- 29.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23(16):2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 30.Ward Y, Wang W, Woodhouse E, Linnoila I, Liotta L, Kelly K. Signal pathways which promote invasion and metastasis: critical and distinct contributions of extracellular signal-regulated kinase and Ral-specific guanine exchange factor pathways. Mol Cell Biol. 2001;21(17):5958–5969. doi: 10.1128/MCB.21.17.5958-5969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 32.Wong HR, Dunsmore KE, Page K, Shanley TP. Heat shock-mediated regulation of MKP-1. Am J Physiol Cell Physiol. 2005;289(5):C1152–C1158. doi: 10.1152/ajpcell.00138.2005. [DOI] [PubMed] [Google Scholar]