Abstract
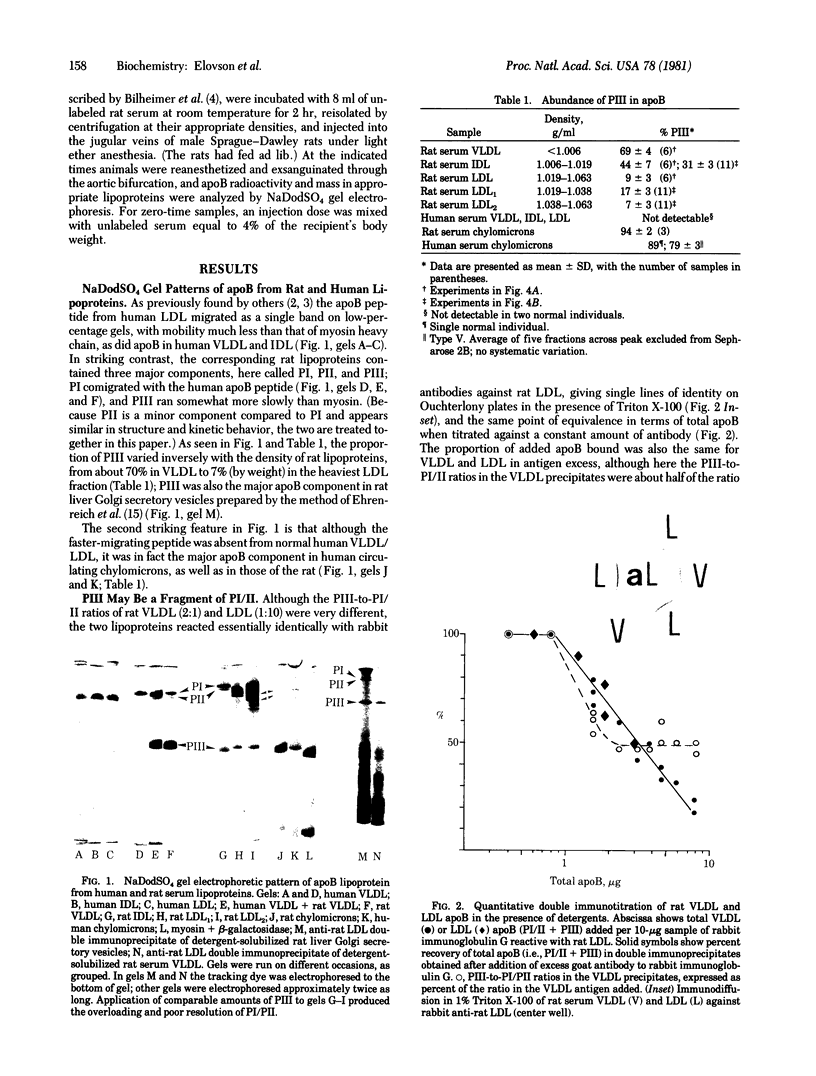
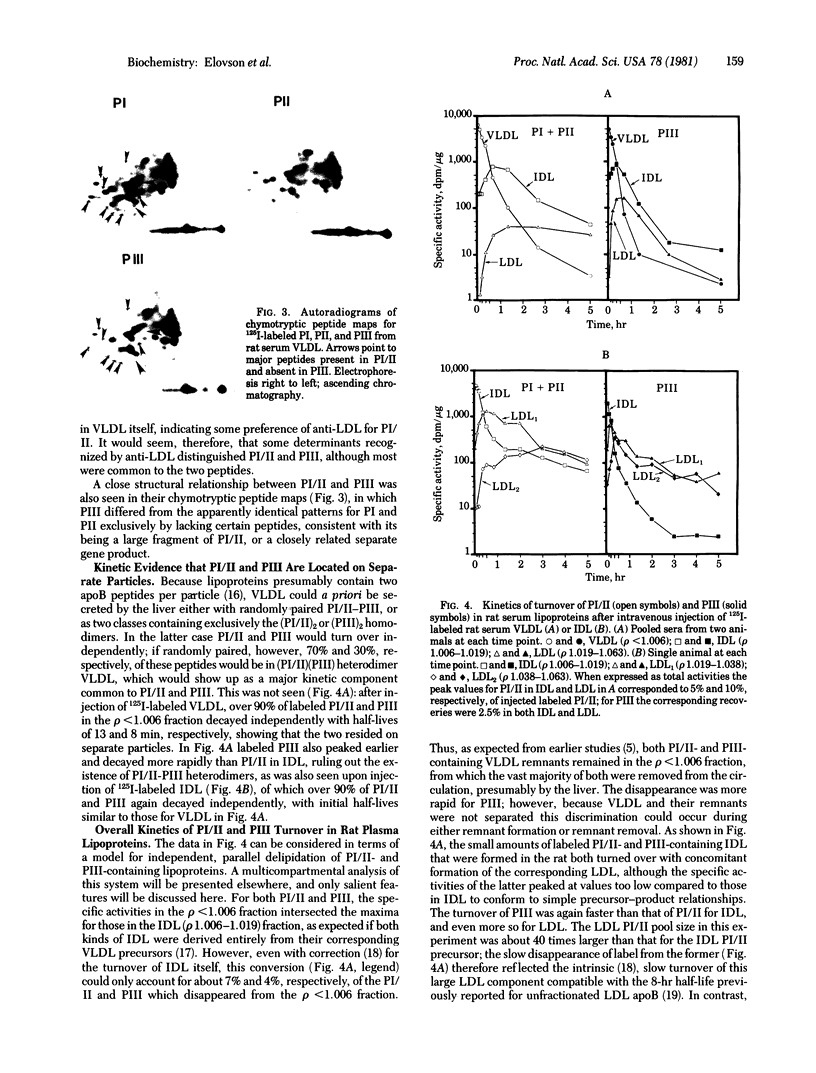
Electrophoresis of rat apolipoprotein B (apoB) on 5% polyacrylamide gels in the presence of NaDodSO4 separates three major components: PI, which comigrates with human low density lipoprotein (LDL) apoB; PII, a slightly faster-moving satellite band; and PIII, which migrates somewhat more slowly than myosin heavy chain. The proportion of PIII decreases with increasing density of the parent rat lipoprotein, from 90% an 70%, respectively, in chylomicrons and very low density lipoproteins (VLDL), to 7% in the major LDL2 (density 1.038-1.063 g/ml) fraction. A major component that comigrates with rat PIII is a marker for human chylomicron apoB, being absent from human VLDL, intermediate density lipoprotein (IDL), and LDL. Preliminary immunological and peptide mapping data show that rat apoB PI and PIII are closely related structurally, with the latter possibly being a large fragment of the former. Both peptides are synthesized in rat liver and found in Golgi secretory vesicles. Kinetic tracer experiments show that rat PI and PIII are present on separate VLDL particles, both of which are extensively removed from the circulation at the remnant stage, and that the declining PIII-to-PI/II ratios in IDL and LDL may be attributed to the more rapid turnover of PIII-containing lipoproteins at all levels, particularly within the LDL density range.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown A. E., Elovson J. Subfractionation of liver membrane preparations by specific ligand-induced density perturbation. Biochim Biophys Acta. 1980 Apr 10;597(2):247–262. doi: 10.1016/0005-2736(80)90103-0. [DOI] [PubMed] [Google Scholar]
- Carrella M., Cooper A. D. High affinity binding of chylomicron remnants to rat liver plasma membranes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):338–342. doi: 10.1073/pnas.76.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Rachmilewitz D. Metabolism of rat plasma very low density lipoprotein. II. Fate in circulation of apoprotein subunits. Biochim Biophys Acta. 1973 Dec 20;326(3):391–405. doi: 10.1016/0005-2760(73)90140-9. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Elovson J. Biogenesis of plasma membrane glycoproteins. Purification and properties of two rat liver plasma membrane glycoproteins. J Biol Chem. 1980 Jun 25;255(12):5807–5815. [PubMed] [Google Scholar]
- Fidge N. H., Poulis P. Metabolic heterogeneity in the formation of low density lipoprotein from very low density lipoprotein in the rat: evidence for the independent production of a low density lipoprotein subfraction. J Lipid Res. 1978 Mar;19(3):342–349. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist L., Carlson K. Selective extraction of human serum very low density apolipoproteins with organic solvents. Biochim Biophys Acta. 1977 Aug 23;493(2):400–409. doi: 10.1016/0005-2795(77)90196-9. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaiah K. V., Walker L. F., Borensztajn J., Schonfeld G., Getz G. S. Apolipoprotein B variant derived from rat intestine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3806–3810. doi: 10.1073/pnas.77.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaiah K. V., Wiegandt H. Demonstration of protease-like activity in human serum low density lipoprotein. FEBS Lett. 1974 Apr 1;40(2):265–268. doi: 10.1016/0014-5793(74)80241-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morrisett J. D., Jackson R. L., Gotto A. M., Jr Lipoproteins: structure and function. Annu Rev Biochem. 1975;44:183–207. doi: 10.1146/annurev.bi.44.070175.001151. [DOI] [PubMed] [Google Scholar]
- Nestruck A. C., Rubinstein D. The synthesis of apoproteins of very low density lipoproteins isolated from the Golgi apparatus of rat liver. Can J Biochem. 1976 Jul;54(7):617–628. doi: 10.1139/o76-091. [DOI] [PubMed] [Google Scholar]
- Risser T. R., Reaven G. M., Reaven E. P. Intestinal contribution to secretion of very low density lipoproteins into plasma. Am J Physiol. 1978 Mar;234(3):E277–E281. doi: 10.1152/ajpendo.1978.234.3.E277. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Jenkins L. L., Brewer H. B., Jr Human chylomicron apolipoprotein metabolism. Biochem Biophys Res Commun. 1978 Jan 30;80(2):405–412. doi: 10.1016/0006-291x(78)90691-5. [DOI] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson G., Noel S. P., Havel R. J. Catabolism of the apoprotein of low density lipoproteins by the isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):628–634. [PubMed] [Google Scholar]
- Smith R., Dawson J. R., Tanford C. The size and number of polypeptide chains in human serum low density lipoprotein. J Biol Chem. 1972 Jun 10;247(11):3376–3381. [PubMed] [Google Scholar]
- Steele J. C., Jr, Reynolds J. A. Characterization of the apolipoprotein B polypeptide of human plasma low density lipoprotein in detergent and denaturation solutions. J Biol Chem. 1979 Mar 10;254(5):1633–1638. [PubMed] [Google Scholar]
- Williams D. L. Apoproteins of avian very low density lipoprotein: demonstration of a single high molecular weight apoprotein. Biochemistry. 1979 Mar 20;18(6):1056–1063. doi: 10.1021/bi00573a019. [DOI] [PubMed] [Google Scholar]
- Wu A. L., Windmueller H. G. Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J Biol Chem. 1979 Aug 10;254(15):7316–7322. [PubMed] [Google Scholar]
- Zilversmit D. B. Atherogenesis: a postprandial phenomenon. Circulation. 1979 Sep;60(3):473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]