Abstract
Objective: To develop a risk scoring model for screening for undiagnosed type 2 diabetes in Chinese population. Methods: A total of 5348 subjects from two districts of Jinan City, Shandong Province, China were enrolled. Group A (2985) included individuals from east of the city and Group B (2363) from west of the city. Screening questionnaires and a standard oral glucose tolerance test (OGTT) were completed by all subjects. Based on the stepwise logistic regression analysis of Group A, variables were selected to establish the risk scoring model. The validity and effectiveness of this model were evaluated in Group B. Results: Based on stepwise logistic regression analysis performed with data of Group A, variables including age, body mass index (BMI), waist-to-hip ratio (WHR), systolic pressure, diastolic pressure, heart rate, family history of diabetes, and history of high glucose were accepted into the risk scoring model. The risk for having diabetes increased along with aggregate scores. When Youden index was closest to 1, the optimal cutoff value was set up at 51. At this point, the diabetes risk scoring model could identify diabetes patients with a sensitivity of 83.3% and a specificity of 66.5%, making the positive predictive value 12.83% and negative predictive value 98.53%. We compared our model with the Finnish and Danish model and concluded that our model has superior validity in Chinese population. Conclusions: Our diabetes risk scoring model has satisfactory sensitivity and specificity for identifying undiagnosed diabetes in our population, which might be a simple and practical tool suitable for massive diabetes screening.
Keywords: Diabetes mellitus, Screening, Questionnaire, Risk factor score
1. Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has increased rapidly worldwide, particularly in developing countries such as China. According to a new study, it is estimated that 92.4 million adults aged 20 and older are diabetic in China; 60.7% of patients in this group remain undiagnosed (Yang et al., 2010). Previous studies also showed that the onset of T2DM is often insidious and may remain undiagnosed for many years until significant complications develop (Ruige et al., 1997; Park et al., 2002). Early diagnosis and treatment, therefore, are very important to prevent severe diabetic complications. It is generally accepted that the blood glucose measurements or glucose tolerance tests are the most reliable tests to identify the undiagnosed patients, but cannot be used for screening purposes in the general population due to high cost.
Several risk scoring models have been proposed to identify subjects at high risk for diabetes, which proved to be practical and effective, and most of them were developed in Caucasian populations (Lawrence et al., 2001; Smith et al., 2003; Rathmann et al., 2005; Bergmann et al., 2007), with only a few in Asians (Ko et al., 2010; Gao et al., 2010). The present study attempts to develop a risk scoring model to screen T2DM in Chinese population.
2. Materials and methods
2.1. Subjects
A total of 5 404 individuals with Han nationality, aged between 35 and 74, were approached in two districts of Jinan City, Shandong Province in eastern China. Among them, 40 were excluded due to known history of diabetes and 16 refused to participate. That left 5 348 participants in the study. They were further divided into two groups by their geographic locations: Group A (2 985 individuals from the east part of the city) and Group B (2 363 individuals from the west part of the city). Their baseline characteristics were shown in Table 1.
Table 1.
Baseline characteristics of the participants
| Group | Number |
Age# (year) | BMI# (kg/m2) | BP# (mmHg) | WHR# | Smoking (%) | Physical activity (%) | |
| Male | Female | |||||||
| A | 1125 | 1860 | 54.41±7.83 | 25.28±3.00 | 138±19/83±12 | 0.85±0.06 | 23.84 | 78.49 |
| B | 936 | 1427 | 55.62±7.41 | 24.57±3.11 | 140±20/86±13 | 0.86±0.07 | 25.67 | 77.44 |
| Total | 2061 | 3287 | 55.23±7.49 | 24.88±3.09 | 139±20/85±13 | 0.85±0.07 | 24.68 | 78.00 |
| P* | 0.15 | 0.21 | 0.33 | 0.11 | 0.45 | 0.10 | 0.34 | |
Data are expressed as xݱs
P value compared the data in Group A with that in Group B; Those characteristics did not show any significant differences among the three groups with all the P values >0.05
An informed consent was given by each participant before the survey started. Protocols were in accordance with the Helsinki declaration and were approved by the Ethnics Committee of Shandong University. A screening questionnaire with medical history and health behavior information was completed by each participant, including treatment of hypertension and/or dyslipidemia, daily consumption of vegetables, whether the work requires a lot of sitting or activities, whether the level of physical activity was 4 h per week or more, et cetera. Some simple and easy-to-obtain potential risk factors were also selected, which included measurements of weight (in light indoor clothes), height (without shoes), waist circumference (at a level midway between the lowest rib and the iliac crest), hip circumference (the maximal horizontal circumference between the waist and thigh), and seated blood pressure (measured twice with at least 5 min apart by a trained nurse from the right arm after the individual resting for 10 min in seated position and took the average). Body mass index (BMI) was calculated as body weight (kg) divided by squared height (m2). Waist-to-hip ratio (WHR) was equal to waist circumference (cm) divided by hip circumference (cm). Information about smoking and alcohol intakes was also obtained. All subjects underwent a standard 75 g oral glucose tolerance test (75 g OGTT). Diagnosis of diabetes was made according to the World Health Organization (WHO) 1999 diagnostic criteria. Individuals with fasting plasma glucose (FPG) level ≥7.0 mmol/L or 2-h post-load plasma glucose (P2hPG) level ≥11.1 mmol/L were identified as positive screening and referred to hospital for additional tests. If repeat FPG or P2hPG was still at or above the previously mentioned criteria, the individual was diagnosed with diabetes. Participants with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) were considered non-diabetic.
2.2. Establishment of a diabetes risk scoring model
A diabetes risk scoring model was developed based on data of Group A. With diabetes as the dependent variable, all the potential risk factors included in the questionnaire and clinical examinations were put in stepwise logistic regression and β-coefficients were calculated to detect risk factors. P value <0.05 was considered statistically significant. Each independent variable was assigned with a score value as 10 times of its coefficient (β). Diabetes risk score was the sum of those scores. It is assumed that the probability of diabetes increases as the diabetes risk score accumulates.
2.3. Evaluation of the diabetes risk scoring model
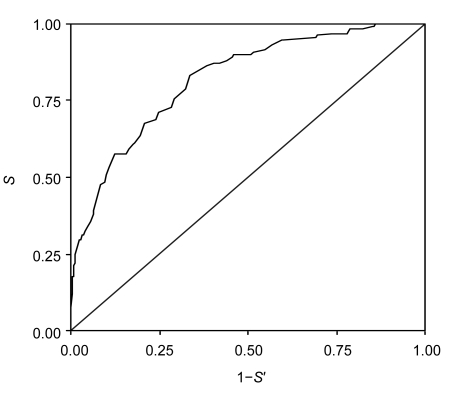
The diabetes risk scoring model developed in Group A was validated in the participants of Group B. The sensitivity (S), specificity (S′), and Youden index were calculated. Youden index was equal to S+S′−1, ranging from −1 to1. It indicated the validity of the diagnostic technique. The closer it is to 1, the better the diagnostic technique is. The optimal cutoff point was determined by the maximum values of the Youden index. The predictive performance of the risk score was evaluated with the area under the curve (AUC) in a receiver operating characteristics (ROC) curve. The sensitivity was plotted on the Y-axis, and the false-positive rate (1−S′) was plotted on the X-axis (Fig. 1). A steeper upward portion of the ROC curve and a lager AUC indicate a more discriminative test. The higher the AUC, the nearer the optimal cut-off point to the peak of the curve. The effectiveness of diagnosis was better when the AUC was closer to 1.
Fig. 1.
Receiver operating characteristics (ROC) curves showing the performance of the diabetes risk score in predicting diabetes
Diagonal segments are produced by ties. The area under the curve (AUC) was 0.82 (95% CI: 0.78–0.86). When the cut-off point of diabetes risk score was ≥51, the sensitivity (S) was 83.3%, specificity (S′) was 66.5%, positive predictive value was 12.83%, and negative predictive value was 98.53%
2.4. Statistical analysis
Statistical analysis was performed with SPSS software (Version 11.5). Stepwise logistic regression analysis was carried out using diabetes as dependent variable, with age, sex, smoking, physical activity, BMI, WHR, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse, family history of diabetes mellitus, and known hyperglycemia as independent variables. χ 2-test was used for rate comparisons. Analysis of variance was used for comparisons of normal distribution data. ROC curves were plotted, and larger AUC reflected a higher accuracy of the test, with the optimal cut-off point at the peak of the curve.
3. Results
3.1. Establishment of the diabetes risk scoring model
The data from Group A were analyzed by stepwise logistic regression analysis with diabetes as the dependent variable and the known risk factors of diabetes, including hypertension, dyslipidemia, activities, BMI, WHR, etc., as potential independent variables. By stepwise logistic regression analysis, variables with P value <0.05 were selected and defined as risk factors for the risk scoring model. The selected variables included age, BMI, WHR, SBP, DBP, heart rate, family history of diabetes, and history of hyperglycemia (Table 2). Each variable was assigned with a score calculated as 10 times of its β-coefficient in the stepwise logistic regression models. The maximum score value was 124.
Table 2.
Risk factors of diabetes and their scores in Group A
| Independent variable | β | OR | 95% CI | P | Score |
| Age | |||||
| 45–54 years | 1.48 | 4.41 | 2.39–8.14 | 0.00 | 15 |
| 55–64 years | 1.92 | 6.83 | 3.43–13.61 | 0.00 | 19 |
| ≥65 years | 2.07 | 7.95 | 2.92–21.69 | 0.00 | 21 |
| BMI | |||||
| 24.00–27.99 kg/m2 | 1.31 | 3.70 | 1.83–7.49 | 0.00 | 13 |
| ≥28.00 kg/m2 | 2.41 | 11.08 | 4.94–24.89 | 0.00 | 24 |
| WHR1 | 1.02 | 2.78 | 1.59–4.85 | 0.00 | 10 |
| SBP2 | 0.63 | 1.87 | 1.07–3.26 | 0.03 | 6 |
| DBP3 | 0.55 | 1.74 | 1.05–2.88 | 0.03 | 6 |
| Heart rate4 | 1.17 | 3.24 | 1.98–5.29 | 0.00 | 12 |
| Family history of DM5 | 1.69 | 5.41 | 3.48–8.40 | 0.00 | 17 |
| History of hyperglycemia6 | 2.80 | 16.52 | 9.00–30.30 | 0.00 | 28 |
WHR: male ≥0.90, female ≥0.85
SBP: ≥140 mmHg
DBP ≥90 mmHg
Heart rate ≥90 beats/min
Family history of diabetes mellitus (DM): either parents or siblings have diabetes
History of hyperglycemia: ever been told have hyperglycemia or latent diabetes, including gestational diabetes
3.2. Evaluation of the diabetes risk scoring model by the data from Group B
The accumulative scores and diabetes prevalence rates of the individuals in Group B were calculated with the model developed in Group A (Table 3). The odds ratio (OR) indicated the incremental times of diabetes prevalence rate. Then Youden index was calculated for every score range and it was achieved when the accumulative score reached 51. With this score value, the diabetes risk scoring model had the best validity. It could identify diabetes patients with a sensitivity of 83.3% and a specificity of 66.5%, the positive predictive value was 12.83%, and the negative predictive value was 98.53% (Fig. 1).
Table 3.
Diabetes prevalence rate of the individuals in Group B
| Score | Number of people | Number of diabetics* | Prevalence rate (%) | OR |
| 0–25 | 396 | 2 | 0.51 | 1.00 |
| 26–51 | 1138 | 26 | 2.28 | 4.61 |
| 52–77 | 725 | 65 | 8.97 | 19.40 |
| 78–124 | 104 | 39 | 37.50 | 118.20 |
The individuals who were not yet diagnosed as diabetes mellitus before
χ 2=259.44, P<0.05
Sensitivity, specificity, positive and negative predictive values, as well as AUC in the ROC curve were compared. There were no significant differences between the two groups with all P>0.05 (Table 4).
Table 4.
Comparisons of the sensitivity, specificity, Youden index, positive and negative predictive values, and AUC between the two groups
| Group | Sensitivity (%) | Specificity (%) | Youden index | Predictive value (%) |
AUC | |
| Positive | Negative | |||||
| A | 82.1 | 65.6 | 0.477 | 12.62 | 98.21 | 0.80 |
| B | 83.3 | 66.5 | 0.498 | 12.83 | 98.53 | 0.82 |
There were no significant differences between the two groups with all P>0.05
3.3. Comparisons of the three different diabetes risk scoring models
Our risk scoring model showed differences from other models used in western countries in many aspects (Table 5). In Finnish screening model, the most important factor for developing T2DM was history of hyperglycemia followed by obesity and age. It is consistent with our study. While in Danish model, the most important factor for development of T2DM was age, followed by obesity, then hypertension. When the two screening models (Finnish and Danish diabetes risk scores) were applied to the total participants of our study, we found that the AUC of Finnish model was 0.76 (95% confidence interval (CI): 0.71–0.81), and that of Danish model was 0.71 (95% CI: 0.65–0.76, P=0.00), both of which had lower validity than originally described (Table 5). The AUC of our risk scoring model was 0.82 (95% CI: 0.78–0.86) (Fig. 1).
Table 5.
Comparisons of three different models of diabetes risk score questionnaires
| Source | Variables | Performance |
| Finnish model | Age (45–54 and 55–64 years old), BMI (25–30 and ≥30 kg/m2), waist circumference (men 94 to <102, women 80 to <88; men ≥102, women ≥88), history of antihypertensive drug treatment, high blood glucose, physical activity, daily consumption of fruits, berries, or vegetables. | The cut-off point ≥9 with sensitivity of 77%, specificity of 66%, and AUC of 0.80 in original population. The sensitivity was 45%, specificity 86%, and AUC 0.76 when the model was applied to our total participants. |
| Danish model | Age (45, 50, and 55–60 years old), sex (male and female), BMI (25–29 and 30 kg/m2), known hypertension, physical activity at leisure time, family history of diabetes. | The cut-off point ≥31 with sensitivity of 76%, specificity of 72%, and AUC of 0.80 in original population. The sensitivity was 51%, specificity 76%, and AUC 0.71 when the model was applied to our total participants. |
| Our model | Age (45–54, 55–64, and ≥65 years old), BMI (24–28 and ≥28 kg/m2), WHR (male ≥0.9 and female ≥0.85), SBP (≥140 mmHg), DBP (≥90 mmHg), heart rate (≥90 beats/min), family history of diabetes mellitus, history of hyperglycemia. | The cut-off point ≥51 with sensitivity of 83%, specificity of 66%, and AUC of 0.82 in original population. |
4. Discussion
Previous studies have shown that 30%–50% T2DM patients have at least one microvascular or macrovascular complication at the time of diagnosis (Harris, 1993). Individuals with previously undiagnosed diabetes also have a higher risk for cardiovascular disease when compared with normal glucose-tolerant individuals (Fuller et al., 1980; Williams et al., 1995; Midthjell et al., 1999; Claudi et al., 2000). Therefore, it is of great importance to develop a practical screening method for early detection of the disease.
Several risk scoring models for screening diabetes have been developed (Lindström and Tuomilehto, 2003; Glümer et al., 2004; Thomas et al., 2006; Schulze et al., 2007). However, scoring models developed in western countries might yield low validity when applied to Chinese population. The low validity could be caused by differences in lifestyles and dietary habits, and possibly genetic marks in different ethnic groups (Dong et al., 2009). The above assumption was proved in our present study. Moreover, we also demonstrated different relative power of each risk factor in our model when compared with previous models (Table 5). The reasons are that we use different definitions in some risk factors; for example, the definition of overweight is BMI≥24 kg/m2, and obesity BMI≥28 kg/m2 in China, while it is ≥25 kg/m2 for overweight and ≥30 kg/m2 for obesity in western countries. When using their standards to evaluate our study population, the scores were lower and deviations occurred. Besides, Chinese diabetes patients have certain unique characteristics, such as lower BMI (Hong et al., 2007), more prominent defect in first-phase insulin secretion (Jia et al., 2007), and different dietary habit such as eat more carbohydrates and less fat. Those differences may account for the low validity when applying western models to Chinese population.
In our study, one of the risk factors was blood pressure, including SBP, DBP, and pulse, but not the “history of antihypertensive drug treatment” as reported previously (Glümer et al., 2004). This change was purposefully made due to the following: in China, many people do not know that they have hypertension until they have overt symptoms; on the other hand, some people know they have hypertension but refuse any treatment, in respect that health insurance or medicare is not as popular as it is in developed countries.
Eight variables were found to be independent risk factors for diabetes in our model. They are age, BMI, WHR, SBP, DBP, heart rate, family history of diabetes, and history of hyperglycemia. The most powerful risk factor was the history of hyperglycemia including gestational diabetes. The second important risk factor was obesity. The optimal risk score was determined when the accumulative score reached 51. With this score value, our model identified 83.3% of previously undiagnosed T2DM patients, with a specificity of 66.5%; furthermore, the positive predictive value was 12.83% and the negative predictive values was as high as 98.53%.
Family history of diabetes, which reflects the genetic predisposition for the disease, is known to be an important marker for increased risk of diabetes (Ma et al., 2008). Its role was less important than history of hyperglycemia, obesity, and age in our study, underscoring the role of environment and lifestyle in development of T2DM. If those risk factors were properly controlled, individuals with high genetic susceptibility can be prevented from developing diabetes.
With this model, automatic calculation of risk scores in the health website becomes possible for people to undergo diabetes self-assessment (Baan et al., 1999). Therefore, our study provides an easy and practical tool to screen undiagnosed diabetes in Chinese population and can be divided into two steps. In the first step, people receive the risk score automatically after filling the questionnaires. If the scores are smaller than 51, the individuals are at less risk of suffering diabetes. If the scores are over 51, the individuals are suggested to undergo a standard OGTT. In this way, many unnecessary blood tests can be saved.
In summary, our diabetes risk scoring model is a simple, inexpensive and relative reliable method to detect diabetes patients in Chinese population. A larger sample size might be needed to make some modification to this model in order to reduce its false-negative predictive value. Other limitations include the lacking of an external validation of the scoring model, and the model will need further evaluation before it can be applied to populations of other Chinese nationalities.
Acknowledgments
We thank Dr. Yun-tai LIU (President of the Staff Hospital of Jinan Ji’er Machine Tool Group Cooperation Limited Company, Shandong, China), Dr. Shu-yu DU (Head of the Medicine Department, Gaoxin District Hospital of Qilu Hospital, Shandong, China) and their coworkers for organizing and conducting the data collection.
Footnotes
Project (No. 963000052) supported by the Science and Technology Department of Shandong Province, China
References
- 1.Baan CA, Ruige JB, Stolk RP, Witteman JC, Dekker JM, Heine RJ, Feskens EJ. Performance of a predictive model to identify undiagnosed diabetes in a health care setting. Diabetes Care. 1999;22(2):213–219. doi: 10.2337/diacare.22.2.213. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann A, Li J, Wang L, Schulze J, Bornstein SR, Schwarz PE. A simplified Finnish diabetes risk score to predict type 2 diabetes risk and disease evolution in a German population. Horm Metab Res. 2007;39(9):677–682. doi: 10.1055/s-2007-985353. [DOI] [PubMed] [Google Scholar]
- 3.Claudi T, Midthjell K, Holmen J, Fougner K, Kruger O, Wiseth R. Cardiovascular disease and risk factors in persons with type 2 diabetes diagnosed in a large population screening: the Nord-Trøndelag Diabetes Study, Norway. J Intern Med. 2000;248(6):492–500. doi: 10.1111/j.1365-2796.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 4.Dong JJ, Lou NJ, Xin Y, Xing HY, Mou YR, Wang Q, LIAO L. Evaluation of various questionnaires for screening diabetes mellitus in Chinese population. Chin J Endocrinol Metab. 2009;25(1):64–65. (in Chinese) [Google Scholar]
- 5.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Coronary-heart-disease risk and impaired glucose tolerance: the Whitehall study. Lancet. 1980;315(8183):1373–1376. doi: 10.1016/S0140-6736(80)92651-3. [DOI] [PubMed] [Google Scholar]
- 6.Gao WG, Dong YH, Pang ZC, Nan HR, Wang SJ, Ren J, Zhang L, Tuomilehto J, Qiao Q. A simple Chinese risk score for undiagnosed diabetes. Diabet Med. 2010;27(3):274–281. doi: 10.1111/j.1464-5491.2010.02943.x. [DOI] [PubMed] [Google Scholar]
- 7.Glümer C, Carstensen B, Sandbǽk A, Lauritzen T, Jǿrgensen T, Johnsen KB. A Danish diabetes risk score for targeted screening. Diabetes Care. 2004;27(3):727–733. doi: 10.2337/diacare.27.3.727. [DOI] [PubMed] [Google Scholar]
- 8.Harris MI. Undiagnosed NIDDM: clinical and public health issues. Diabetes Care. 1993;16(4):642–652. doi: 10.2337/diacare.16.4.642. [DOI] [PubMed] [Google Scholar]
- 9.Hong J, Gu WQ, Zhang YF, Yang YS, Shen CF, Xu M, Li XY, Wang WQ, Ning G. The interplay of insulin resistance and β-cell dysfunction involves the development of type 2 diabetes in Chinese obeses. Endocrine. 2007;31(2):93–99. doi: 10.1007/s12020-007-0002-2. [DOI] [PubMed] [Google Scholar]
- 10.Jia WP, Pang C, Chen L, Bao YQ, Lu JX, Lu HJ, Tang JL, Wu YM, Zuo YH, Jiang SY, et al. Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai Diabetes Studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia. 2007;50(2):286–292. doi: 10.1007/s00125-006-0503-1. [DOI] [PubMed] [Google Scholar]
- 11.Ko G, So W, Tong P, Ma R, Kong A, Ozaki R, Chow C, Cockram C, Chan J. A simple risk score to identify southern Chinese at high risk for diabetes. Diabet Med. 2010;27(6):644–649. doi: 10.1111/j.1464-5491.2010.02993.x. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence JM, Bennett P, Young A, Robinson AM. Screening for diabetes in general practice: cross sectional population study. BMJ. 2001;323(7312):548–551. doi: 10.1136/bmj.323.7312.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26(3):725–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 14.Ma XJ, Jia WP, Hu C, Zhou J, Lu HJ, Zhang R, Wang CR, Wu SH, Xiang KS. Genetic characteristics of familial type 2 diabetes pedigrees: a preliminary analysis of 4468 persons from 715 pedigrees. Natl Med J China. 2008;88(36):2541–2543. (in Chinese) [PubMed] [Google Scholar]
- 15.Midthjell K, Kruger O, Holmen J, Tverdal A, Claudi T, Bjorndal A, Magnus P. Rapid changes in the prevalence obesity and known diabetes in an adult Norwegian population: the Nord-Trøndelag Health Surveys: 1984–1986 and 1995–1997. Diabetes Care. 1999;22(11):1813–1820. doi: 10.2337/diacare.22.11.1813. [DOI] [PubMed] [Google Scholar]
- 16.Park PJ, Griffin SJ, Sargeant L, Wareham NJ. The performance of a risk score in predicting undiagnosed hyperglycemia. Diabetes Care. 2002;25(6):984–988. doi: 10.2337/diacare.25.6.984. [DOI] [PubMed] [Google Scholar]
- 17.Rathmann W, Martin S, Haastert B, Icks A, Holle R, Löwel H, Giani G. Performance of screening questionnaires and risk scores for undiagnosed diabetes. Arch Intern Med. 2005;165(4):436–441. doi: 10.1001/archinte.165.4.436. [DOI] [PubMed] [Google Scholar]
- 18.Ruige JB, Bouter LM, Neeling JN, Henine RJ, Kositense PJ. Performance of an NIDDM screening questionnaire based on symptoms and risk factors. Diabetes Care. 1997;20(4):491–497. doi: 10.2337/diacare.20.4.491. [DOI] [PubMed] [Google Scholar]
- 19.Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Möhlig M, Pfeiffer AF, Spranger J, Thamer C, Häring HU, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care. 2007;30(3):510–515. doi: 10.2337/dc06-2089. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM, Holohan J, McAuliffe A, Firth RG. Irish diabetes detection programme in general practice. Diabet Med. 2003;20(9):717–722. doi: 10.1046/j.1464-5491.2003.00998.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas C, Hyppönen E, Power C. Type 2 diabetes mellitus in midlife estimated from the Cambridge risk score and body mass index. Arch Intern Med. 2006;166(6):682–688. doi: 10.1001/archinte.166.6.682. [DOI] [PubMed] [Google Scholar]
- 22.Williams DR, Wareham NJ, Brown DC, Byrne CD, Clark PM, Cox BD, Cox LJ, Day NE, Hales CN, Palmer CR, et al. Undiagnosed glucose intolerance in the community: the Isle of Ely Diabetes Project. Diabet Med. 1995;12(1):30–35. doi: 10.1111/j.1464-5491.1995.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang WY, Lu JM, Weng JP, Jia WP, Ji LN, Xiao JJ, Shan ZY, Liu J, Tian HM, Ji QH, et al. Prevalence of diabetes among man and women in China. N Engl J Med. 2010;362(12):1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]