Abstract
It is well known that ethacrynic acid (EA) can potentiate the ototoxicity of aminoglycoside antibiotics (AmAn) such as kanamycin (KM), if they were applied at the same time. Currently, to create the model of EA-KM-induced cochlear lesion in rats, adult rats received a single injection of EA (75 mg/kg, intravenous injection), or followed immediately by KM (500 mg/kg, intramuscular injection). The hearing function was assessed by auditory brainstem response (ABR) measurement in response to click and/or tone bursts at 4, 8, 12, 16, 20, 24, and 32 kHz. The static microcirculation status in the stria vascularis after a single EA injection was evaluated with eosin staining. The pathological changes in cochlear and vestibular hair cells were also quantified after co-administration of EA and KM. After a single EA injection, blood flow in vessels supplying the stria vascularis rapidly diminished. However, the blood supply to the cochlear lateral wall partially recovered 5 h after EA treatment. Threshold changes in ABR were basically parallel to the microcirculation changes in stria vascularis after single EA treatment. Importantly, disposable co-administration of EA and KM resulted in a permanent hearing loss and severe damage to the cochlear hair cells, but spared the vestibular hair cells. Since the cochlear lateral wall is the important part of the blood-cochlea barrier, EA-induced anoxic damage to the epithelium of stria vascularis may enhance the entry of KM to the cochlea. Thus, experimental animal model of selective cochlear damage with normal vestibular systems can be reliably created through co-administration of EA and KM.
Keywords: Ototoxicity, Ethacrynic acid, Kanamycin, Rat, Blood-cochlea barrier
1. Introduction
Aminoglycoside antibiotics (AmAn) have been widely used in clinics for treatment of bacterial infections (Bailey and Peddie, 1976; Shuman and Smith, 1978; Graham et al., 2004). However, the use of AmAn in clinics was restricted with discretion owing to its serious ototoxicity and nephrotoxicity (Ding et al., 1990a; Humes, 1999; Wu et al., 2001; de Jager and van Altena, 2002; Rougier et al., 2003; 2004). The antibacterial mechanism of AmAn arises from their ability to selectively bind to prokaryotic ribosome at the adenosine decoding region of 16S ribosomal RNA (rRNA), then resulting in codon misreading and suppression of translation, and finally causing inhibition of protein synthesis in bacteria. In contrast, the cytoplasmic rRNA of mammalian cells is weakly bound by AmAn due to an adenosine-to-guanosine substitution in 16S rRNA in eukaryotic cells. However, the mitochondria in mammalian cells had adenosine at the 1 408 position on the 16S rRNA, which was similar to bacteria that can be specifically affected by AmAn (Gutell et al., 1994; Vellai et al., 1998; Guan et al., 2000; Ding and Salvi, 2005). Therefore, mitochondria were proved to be the crucial target of AmAn in ototoxicity (Ding et al., 1991; 1995a; 1997; 2010; Ding and Salvi, 2005). Because of the obstruction of blood-cochlea barrier, AmAn-induced hair cell damage developed slowly when subjects were systemically treated only with AmAn over several days until AmAn accumulation reached the damage concentration in the inner ear (Aran, 1982; Hangfu et al., 1992; Zhu et al., 1993; Ding et al., 1995c; 1995d; Aran et al., 1999).
Much evidence from recent studies revealed that the blood-cochlea barrier can be temporally eliminated by a treatment of loop diuretic, such as ethacrynic acid (EA) and furosemide (Akiyoshi et al., 1975; Abbruzzese et al., 1990; Ding et al., 2002; 2004; 2007; 2010; Versnel et al., 2007; Li et al., 2011). EA is a kind of potent diuretic, and excess dose of EA can lead to profound diuresis with fluid and electrolyte loss. Two major side effects of EA are nephrotoxicity and temporal ototoxicity. Recent studies revealed that the ototoxic effect of EA is actually involved in selectively blocking the lateral spiral artery in the cochlea and suppressing the blood flow supply to the cochlear lateral wall. The epithelial ischemia and anoxia in stria vascularis resulted in a significant depression of endolymphatic potential which was equivalent to cutting off the power supply of the cochlea (Ding et al., 1990b; 2002; 2003; 2004; 2008; 2010; Ding and Salvi, 2005). However, when the blood supply to the cochlear lateral wall was restored and the epithelial damage was repaired, EA-induced hearing loss could be recovered completely (Ding et al., 1990b; 1996; 2002; 2004). During the ischemic damage to the epithelium and capillaries on the cochlear lateral wall, the vascular permeability and membranous permeability in stria vascularis were also affected so that the ototoxic drug can penetrate through the broken blood-cochlea barrier to enter the cochlea. When the ototoxic drug passed through the blood-cochlea barrier, it easily permeated into the endolymph in the scala media and into the perilymph in the crack of connective tissue cells (fibrocytes) in the spiral ligament which communicates the perilymph in the scala tympani. It is believed that the perilymph in the scala tympani is connected with the cortilymph. Therefore, the ototoxic drug can reach the cochlear hair cells either through the cuticular plate facing the endolymph or through the under parts of the hair cells in the cortilymph. In our previous studies, we had authenticated that a single co-administration of EA and gentamicin (GM) significantly enhanced the GM concentration in the perilymph, which was equivalent to the drug concentration in the perilymph from fourteen or twenty reiterative GM systemic injections (Ding et al., 1995d; 2003; 2004; Ding and Salvi, 2005). As a result, those findings indicated that the elimination of blood-cochlea barrier by EA can effectively enhance the afflux of ototoxic drug into the cochlea.
Rats are increasingly used in experimental studies because of the understanding in its genomic sequence. However, the ototoxic experimental models in rats with co-administration of EA and other ototoxic drugs have not been fully established yet, although similar models were reliably studied in guinea pigs, chinchillas, and cats (Xu et al., 1993; Ding et al., 1995d; 2002; 2004; 2007; 2008; 2010; Yamasoba et al., 2003; Ding and Salvi, 2005). Despite that Yamasoba et al. (2003) had mentioned the EA-kanamycin (KM) treatment in rats, it was focused only on the cell proliferation and it did not provide any detailed information of cochlear lesions in this model. To develop an animal model with rapid KM-induced hearing loss and cochlear pathology in rats, EA was injected via the caudal vein, followed by intramuscular injection of KM. The hearing electrophysiology and cochlear pathology were evaluated as evidence of this ideal experimental model.
2. Materials and methods
2.1. Subjects
Subjects were 18 healthy adult rats (Sasco Sprague-Dawley rats) weighing between 245 and 326 g, and were divided into three groups. Six rats were used for assessment of ischemia in stria vascularis 1, 3, and 5 h post-EA (75 mg/kg), respectively, and four cochleae from two animals per time point were observed. Another six rats were studied to monitor the threshold shifts in auditory brainstem response (ABR) for 24 h following a single EA (75 mg/kg) intravenous injection. The remaining six rats were simultaneously treated with EA (75 mg/kg, intravenous injection) and KM (500 mg/kg, intramuscular injection), and the functional changes and the pathological damages in the cochleae were examined 10 d after EA-KM treatment. All procedures regarding the use and care of animals in this study were approved by the Institutional Animal Care and Use Committee of the University at Buffalo, USA.
2.2. EA and KM treatment
EA was purchased from Aton Pharma, Inc. EA solution for intravenous injection was freshly prepared with saline before use. Animals were anesthetized by inhalation of 4% (v/v) isoflurane in a chamber, then maintained under anaesthesia with 1.5% (v/v) isoflurane, and kept warm. The rat tail was placed in warm water for vasodilatation, and then the skin was gently swabbed with alcohol for sterilization. EA solution was injected with a dose of 75 mg/kg through the visible tail vein at the lateral side of tail using a hypodermic needle.
KM (product No. K4000) was purchased from Sigma. KM solution for intramuscular injection was freshly prepared. For the experimental group in co-administration of EA and KM, KM solution was intramuscularly injected at the inside of the thigh with a dose of 500 mg/kg for one treatment following the EA injection.
2.3. Auditory brainstem response (ABR) testing
ABR was tested before and after the treatment in all experimental animals (Chen et al., 2010). Briefly, rats were initially anesthetized in a soundproof chamber by inhalation of 4% (v/v) isoflurane, maintained with 1.5% (v/v) isoflurane, and kept warm on a heating pad. Prior to the ABR testing, the external auditory canal of animals was examined under electrical otoscope to exclude tympanic membrane perforation, middle ear infection, and cerumen. Under effective anaesthesia, subdermal needle electrodes (Grass-Telefactor, West Warwick, RI) were placed at the scalp vertex (inverting), posterior bulla (non-inverting), and lower back (ground), respectively, for recording ABR. The test stimuli were 4, 8, 12, 16, 20, 24, and 32 kHz tone bursts (5-ms duration with 1-ms rise/fall time) and click, generated using Tucker Davis Technologies (TDT) hardware and software (SigGen) and presented at the rate of 21 times per second. Stimuli were attenuated with a computer-controlled attenuator (TDT PA-5) and routed through a high frequency speaker (FT28D, Fostex Co.). Under control of TDT hardware and software, the stimulus level was attenuated in 5 dB steps from 100 to 0 dB sound pressure level (SPL). ABR thresholds were defined as the lowest repeatable intensity that elicited a response. The responses were amplified 50 000 times, filtered (10–3 000 Hz), and averaged over 600 stimuli. Monaural thresholds were obtained by occluding the contralateral ear with silicone ear molds to isolate the test ear.
2.4. Histology
At the end of the experiments, animals were deeply anesthetized with CO2 and decapitated. The temporal bones were quickly removed, and the apex of the cochlea, the round window, and the oval window were punctured. Fixative was perfused through the cochlear apex with 10% (v/v) formalin in phosphate-uffered saline (PBS) (pH 7.4, 4 °C), and then the sample was immersed in the fixative for 24 h. The cochlear basilar membrane, the spiral ligament, and the vestibular end-organs were routinely microdissected as described previously (Wilhelm et al., 1978; Moazed and Noller, 1987; Recht et al., 1999). The tissue of spiral ligament was stained with 0.5% (w/v) eosin solution for 5 min. The tissues of basilar membrane and vestibular end-organs were stained with hematoxylin. Cochlear specimens were prepared as flat surface preparations, then mounted in glycerin on glass slides and examined under a light microscope (Zeiss Axioskop) and photographed (Olympus PM-10ADS). To localize and quantify the cochlear hair cell damage induced by co-administration of EA and KM, the numbers of missing inner hair cells (IHCs) and outer hair cells (OHCs) were counted over 0.24-mm intervals along the entire length of the cochlea. Using data of laboratory norms from normal Sasco Sprague-Dawley rats, cochleograms were constructed to show the percentage of missing hair cells as a function of percentage distance from the apex of the cochlea. The mean cochleogram from the group of six 10-d EA-KM-treated animals was compared with the cochlear data from normal Sasco Sprague-Dawley rats. Cochlear place was transformed to frequency using the frequency-place map formula (Greenwood, 1990). To quantify the hair cell density in vestibular end-organs, the density of vestibular hair cells in macular of saccule and utricle, as well as in the three crista of ampullae was counted and calculated as described by Ding et al. (1992).
3. Results
3.1. Temporary hearing loss after a single injection of EA
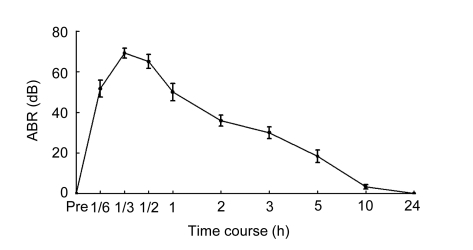
After injection of EA, click-evoked ABR thresholds were rapidly and dramatically elevated as shown in Fig. 1. ABR threshold was completely recovered 24 h after EA treatment.
Fig. 1.
Temporary changes in mean ABR thresholds (SPL) induced by click stimulation following single EA treatment (75 mg/kg)
Injection of EA could result in a dramatic and rapid elevation of ABR thresholds as early as 10 min post-EA. The threshold shifts of ABR can last more than 5 h, but gradually recovered about 10 h after EA treatment
3.2. Selective ischemia in stria vascularis after a single injection of EA
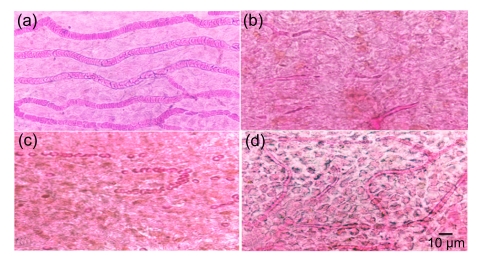
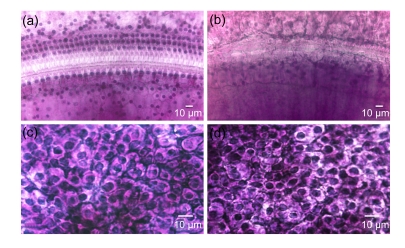
Eosin-stained red blood cells in vessels in normal stria vascularis were visible with dark staining (Fig. 2a). However, the blood flow in all vessels supplying the cochlear lateral wall rapidly and dramatically reduced after EA injection (Figs. 2b and 2c). Three hours after EA injection, many small vacant spaces were seen in epithelium of stria vascularis (Fig. 2c). Five hours after EA injection, the blood supply to the stria vascularis was gradually recovered. However, large vacuoles were still presented in intercellular spaces (Fig. 2d). Those intracellular and extracellular vacuoles in stria vascularis were definitely caused by the ischemia and hypoxia which affected the cellular permeability and broke the blood-cochlea barrier. In contrast, the red blood cells in the vessels of cochlear basilar membrane, vestibular end-organs in macula of saccule, macula of utricle, and crista of ampullae were normal at all time points after EA treatment (data not shown). This conformed to our previous findings in guinea pigs and in chinchillas (Zhao et al., 1988b; Ding et al., 1990b; 1995d; 2002; 2004; McFadden et al., 2002; Ding and Salvi, 2005).
Fig. 2.
Surface preparation of the cochlear spiral ligament
(a) The blood vessels in the stria vascularis were fully filled with red blood cells in normal rat; (b) 1 h after EA injection, the blood supply to the cochlear lateral wall was greatly reduced; (c) 3 h after EA injection, the blood vessels in the stria vascularis retained empty; (d) The red blood cells were visible again in stria vascularis 5 h after EA injection, whereas the epithelium of stria vascularis was still swollen with obvious cavities in intercellular space due to the temporary ischemia
3.3. Severe deafness induced by co-administration of EA and KM (EA-KM)
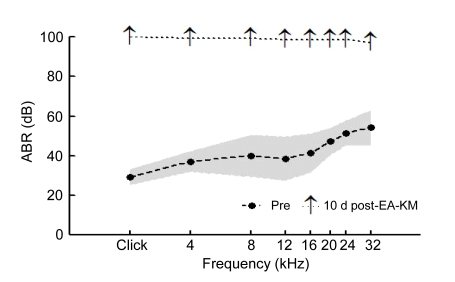
Ten days after EA-KM treatment, obvious threshold elevations in ABR were detected. Note that ABR thresholds could not be measured at all frequencies (Fig. 3).
Fig. 3.
Significant threshold elevations of ABR (SPL) at click, 4, 8, 12, 16, 20, 24, and 32 kHz as functions 10 d after EA-KM treatment
Shaded region showed 95% confidence interval (CI) around the mean of the pretesting data
3.4. Pathological changes in the inner ear induced by co-administration of EA and KM
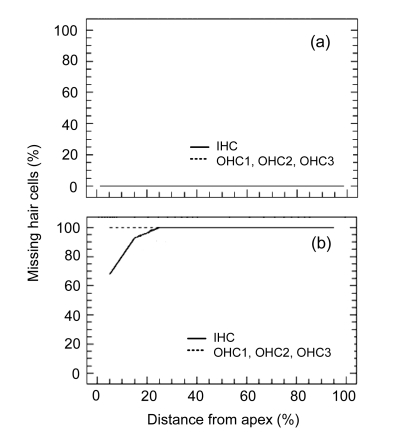
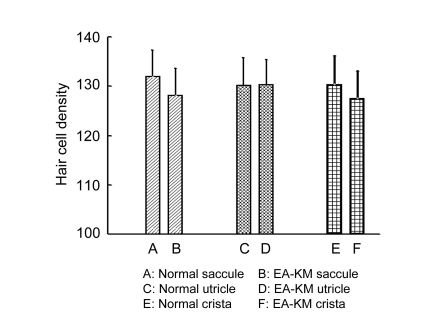
Ten days post-EA-KM, a complete OHC missing was found along the entire length of the cochlea, and IHCs destruction was also close to 100% in the middle turn and basal turn of the cochlea. However, about 30% IHCs were still surviving in the extremely apical turn of the cochlea (Fig. 4). This was consistent with the base to apex gradient pattern of hair cells missing by AmAn treatment. The quantitative statistical analysis of the total percentage of missing cochlear hair cells showed a significant difference between the normal animals [(0.2±0.05)%] and EA-KM-treated animals [(99.3±0.07)%]. There was a statistically significant difference between the normal control and EA-KM groups (P<0.0001, one-way analysis of variance ANOVA). Despite the fact that EA-KM treatment caused massive damage to hair cells in the cochlea (Fig. 5b), the vestibular end-organs appeared morphologically normal in surface preparations in the macula of saccule, the macula of utricle, and the cristae of ampullae (Figs. 5c and 5d). Consistent with the morphological findings, the density of vestibular hair cells was not significantly changed in the group of EA-KM treated animals (P>0.05) (Fig. 6).
Fig. 4.
Mean cochleograms showing the percentage of missing inner hair cells (IHC) and outer hair cells (OHC1, OHC2, OHC3) in the normal group (a) and the group 10 d after EA (75 mg/kg) and KM (500 mg/kg) treatment (b)
Rat N=6. Note that most cochlear hair cells were destroyed by KM 10 d post-EA-KM
Fig. 5.
Surface preparations of the organ of Corti and the macula of utricle
(a) Cochlear hair cells were intact in normal rats; (b) 100% OHCs and IHCs were missing 10 d post-EA-KM; (c) Vestibular hair cells were intensively located in the macula of utricle in normal rats; (d) Density of vestibular hair cells in the macula of utricle was normal 10 d post-EA-KM
Fig. 6.
Hair cell density in vestibular end-organs
There was no significant difference in hair cell density between the normal animals and the EA-KM-treated animals in macula of saccule, macula of utricle, and crista of ampullae (crista measures were averaged across the lateral, superior, and posterior cristae). Hair cell density is expressed as hair cell numbers in 0.01 mm2 vestibular epithetlium
4. Discussion
The access for many drugs entering the inner ear is limited by the presence of blood-cochlea barrier, which is functionally and anatomically similar to the blood-brain barrier (Torihara et al., 1994; Swan et al., 2008). Some AmAn, such as streptomycin, can easily traverse the blood-brain barrier and the blood-cochlea barrier to affect the central nervous system and the cochlea with the typical toxic effects like the curare to block the acetylcholine (Pittinger and Adamson, 1972; Zucca et al., 1992; Ding et al., 1993; 1995b; Ding and Zhang, 1995). However, it is difficult for aminoglycosides, such as GM and KM, to cross the blood-brain barrier and the blood-cochlea barrier. Such kind of AmAn has very limited entry to the inner ear; therefore, single treatment of GM or KM usually does not cause any damage to the inner ear. Nevertheless, because of the difficult clearance of GM or KM from the inner ear due to their long period of half-life in the cochlea, repetitious or intermittent treatment of AmAn can gradually induce drug accumulation in the inner ear and finally reach damage concentration. To create a shortcut avoiding the blocks of blood-cochlea barrier, a local approach was developed and widely used for drug delivery to the inner ear via penetrable round window membrane or scala tympani. This novel delivery approach was considered to be very helpful for protection and treatment in various inner ear ailments such as sudden sensorineural hearing loss and autoimmune inner ear disease, and for preserving spiral ganglion neurons and regenerating sensory hair cells (Ajodhia and Dix, 1976; Akiyoshi, 1978; Au et al., 1986; Akisada, 1987). This approach was also widely used for chemical ablation of cochlear and vestibular labyrinth (Akiyoshi et al., 1974; 1975; Becvarovski et al., 2002; Li et al., 2004; Kraus et al., 2009; Zhou et al., 2009). Even though the round window could be a good approach for ototoxic drug entry into the inner ear, this approach did not mimic the systemic treatment in clinics.
EA is a potent loop diuretic which can lead to temporary hearing loss by abolishing the blood flow in vessels supplying the lateral wall of the cochlea, and result in the ischemia and anoxia in epithelium of stria vascularis (Zhao et al., 1988a; 1988b; Ding et al., 1990b; 2002; 2004). Since the cochlear lateral wall is the important section of the blood-cochlea barrier, EA-induced anoxic degeneration in epithelium of stria vascularis can contemporarily open up the blood-cochlea barrier on the cochlear lateral wall. More importantly, when another ototoxic drug was treated at the moment when the blood-cochlea barrier was eliminated, EA can enhance the entry of the ototoxic drug into the inner ear to reach their damage concentration. Furthermore, it is a crucial condition that the concentration of ototoxic drug must be higher in blood serum than in the perilymph when the blood-cochlea barrier is open. Therefore, the simultaneous injection of both EA and other ototoxic drug or a little delay injection of other ototoxic drug is the keypoint in causing the permanent hearing loss and cochlear degeneration (Ding et al., 1995d; McFadden et al., 2002; Ding et al., 2003; 2004; 2007; 2008; 2010).
According to the pathological occurrence in the cochlea following EA and AmAn treatments, the changes in hearing function can be divided into two phases. The hearing loss in the first phase should be mainly in relation to EA-induced ischemia and anoxia in the stria vascularis that suppressed the endolymphatic potential as a result of “power failure” in the cochlea. The microcirculatory disturbance in the cochlear lateral wall and the ABR threshold shift following single EA injection suggested that the blood supply to the cochlear lateral wall could be interrupted by EA for at least 5 h, resulting in a temporary hearing dysfunction over 5 h with no damage to the hair cells (Zhao et al., 1988b; Ding et al., 1996; 2002; 2004; 2007; Ding and Salvi, 2005). Therefore, the first phase of hearing dysfunction may last over 5 h. The hearing loss in the second phase may start 5 h after simultaneous treatment of EA and AmAn, which should be mainly involved in AmAn-induced complete destruction to the cochlear hair cells and irreversible hearing loss (Ding et al., 1995d; 2007; 2010; Ding and Salvi, 2005). Many studies have confirmed that simultaneous EA and AmAn injection causes permanent hearing loss and hair cell destruction (Xu et al., 1993; Ding et al., 1995d; 2002; 2004; 2007; 2008; 2010; Yamasoba et al., 2003; Ding and Salvi, 2005). In our recent study of investigating the apoptotic mechanisms by co-administration of EA and GM, we discovered that the hair cells start to release apoptotic signals (cytochrome C) 5 h after EA-GM treatment, and the process of programmed self-destruction in cochlear hair cells can be completed in 12 h post-EA-GM (Ding et al., 2010). This suggested that even though EA induced reduction of endolymphatic potential can return to normal after 5 h, the permanent hearing loss was going to occur 5 h after EA-AmAn injection, and the sensory hair cells started to be destroyed at this moment. Therefore, it seemed almost reasonable to expect that the first phase in hearing loss in EA-AmAn model was only related to EA-induced decline of endolymphatic potential for about 5 h. In contrast, the second phase of hearing loss might only depend on AmAn-induced hair cell destruction 5 h after EA-AmAn treatment. Even though the blood supply to the cochlear lateral wall and the endolymphatic potential were recovering 5 h after EA injection, the permanent deafness is sure to happen from the beginning of co-administration of EA and AmAn.
In the current study, EA treatment with a dose of 75 mg/kg by tail vein injection could effectively abolish the blood flow to the cochlear stria vascularis in rats for at least 5 h. This approach and dosage of EA injection were confirmed to be a valid treatment to temporally break the blood-cochlea barrier. If KM was injected at this time, KM can easily enter the cochlea just at the right moment through the broken blood-cochlea barrier. Single concurrent treatment of EA and KM therefore caused permanent hearing loss and extensive hair cell destruction in the cochlea, which was much more severe than the chronic KM treatment with a high dose for many days. Unexpectedly, the vestibular hair cells were intact from EA-KM invasion and a similar phenomenon was discovered in other animal models with concurrent treatment of EA and GM (Ding et al., 1995d; 2004; McFadden et al., 2002). Considering the single treatment of EA and AmAn which caused a brief increase of AmAn concentration in the perilymph, it happened that the cochlear hair cells were rightly immersed in cortilymph which was believed to be connected with perilymph in scala tympani. However, the vestibular hair cells were tightly packaged by surrounding supporting cells while they did not directly expose the perilymph. It may be one of the reasons to explain why the brief attack of AmAn only damages the cochlear hair cells whilst it spares the vestibular hair cells. Of course, this hypothesis still needs to be further studied in the future.
5. Conclusions
The present data indicate that co-administration of EA intravenous injection via tail vein and KM intramuscular injection can cause a rapid and permanent hearing loss as well as irreversible hair cell lesions in the cochlea of rats. This experimental model will be useful for further study in cochlear microcirculatory disturbance, temporary blood-cochlea barrier elimination, and ototoxic effects and mechanisms of KM in rats.
Footnotes
Project (No. R01 DC006630) supported by the National Institutes of Health (NIH) of USA
References
- 1.Abbruzzese JL, Amato R, Schmidt S, Raber MN, Frost P. Phase I clinical trial of cisplatin given i.v. with 5-fluorouracil and high-dose folinic acid. Cancer Chemother Pharmacol. 1990;26(3):159–162. doi: 10.1007/BF02897192. [DOI] [PubMed] [Google Scholar]
- 2.Ajodhia JM, Dix MR. Drug-induced deafness and its treatment. Practitioner. 1976;216(1295):561–570. [PubMed] [Google Scholar]
- 3.Akisada T. Evaluation of cochlear damage in kanamycin administered hamster and protective effect of cepharanthine against KM induced ototoxicity. An experimental study. Nippon Jibiinkoka Gakkai Kaiho. 1987;90(8):1229–1244. doi: 10.3950/jibiinkoka.90.1229. [DOI] [PubMed] [Google Scholar]
- 4.Akiyoshi M. Evaluation of ototoxicity of tobramycin in guinea pigs. J Antimicrob Chemother. 1978;4(Suppl. A):69–72. doi: 10.1093/jac/4.suppl_a.69. [DOI] [PubMed] [Google Scholar]
- 5.Akiyoshi M, Sato K, Nakada H, Tajima T. Audiometric and histopathologic evaluation of ototoxicity of 3′,4′-dideoxykanamycin B, a new aminoglycoside antibiotic (author’s transl.) Jpn J Antibiot. 1974;27(1):15–26. (in Japanese) [PubMed] [Google Scholar]
- 6.Akiyoshi M, Sato K, Nakada H, Tajima T, Suzuki K. Evaluation of ototoxicity of amikacin (BB-K8) by animal test (author’s transl.) Jpn J Antibiot. 1975;28(3):288–304. (in Japanese) [PubMed] [Google Scholar]
- 7.Aran JM. Evaluation of the ototoxicity of aminoglycosides. Comparative study of dibekacin, gentamicin and tobramycin. Nouv Presse Med. 1982;11(46):3426–3431. [PubMed] [Google Scholar]
- 8.Aran JM, Erre JP, Lima da Costa D, Debbarh I, Dulon D. Acute and chronic effects of aminoglycosides on cochlear hair cells. Ann N Y Acad Sci. 1999;884:60–68. doi: 10.1111/j.1749-6632.1999.tb08636.x. [DOI] [PubMed] [Google Scholar]
- 9.Au S, Weiner N, Schacht J. Membrane perturbation by aminoglycosides as a simple screen of their toxicity. Antimicrob Agents Chemother. 1986;30(3):395–397. doi: 10.1128/aac.30.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey RR, Peddie B. Tobramycin in the treatment of severe and complicated urinary tract infections. N Z Med J. 1976;84(567):1–3. [PubMed] [Google Scholar]
- 11.Becvarovski Z, Bojrab DI, Michaelides EM, Kartush JK, Zappia JJ, LaRouere MJ. Round window gentamicin absorption: an in vivo human model. Laryngoscope. 2002;112(9):1610–1613. doi: 10.1097/00005537-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Chen GD, Kermany MH, D′Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265(1-2):63–69. doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jager P, van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis. 2002;6(7):622–627. [PubMed] [Google Scholar]
- 14.Ding D, Zhang Z. Acoustical transmission blockage caused by urethane and streptomycin. J Audiol Speech Disord. 1995;3(1):36–38. (in Chinese) [Google Scholar]
- 15.Ding D, Salvi R. Review of cellular changes in the cochlea due to aminoglycoside antibiotics. Volta Rev. 2005;105(3):407–438. [Google Scholar]
- 16.Ding D, Luo D, Huangfu M. The kanamycin toxic relation between the ear and kidney. J Clin Otorhinolaryngol. 1990;4(3):142–144. (in Chinese) [Google Scholar]
- 17.Ding D, Zhao J, Luo D, Huangfu M. The microcirculation static quantitative observation of the stria vascularis. Acta Otolaryngol. 1990;4(2):1–2. (in Chinese) [Google Scholar]
- 18.Ding D, Luo D, Guo Y, Huangfu M. Probe into the ototoxic mechanism of aminoglycoside antibiotic. Chin J Otorhinolaryngol. 1991;26(3):154–155. (in Chinese) [Google Scholar]
- 19.Ding D, Chen X, Jin X. Observation of vestibular end organ with a small field vision count technique. Chin J Otorhinolaryngol. 1992;27(4):202–203. (in Chinese) [Google Scholar]
- 20.Ding D, Jin X, Huangfu M. Acoustical transmission blockage caused by streptomycin. J Audiol Speech Disord. 1993;1(1):29–31. (in Chinese) [Google Scholar]
- 21.Ding D, Jin X, Zhao J. Accumulative sites of kanamycin in cochlea basal membrane cells. Chin J Otorhinolaryngol. 1995;30(6):323–325. (in Chinese) [PubMed] [Google Scholar]
- 22.Ding D, Jin X, Zhao J. Different binding sites of kanamycin and streptomycin in the organs of Corti. J Clin Otorhinolaryngol. 1995;9(6):346–347. (in Chinese) [Google Scholar]
- 23.Ding D, Jin X, Zhang Z, Zhu Q. Different susceptibility in gentamycin ototoxicity between red and black eye guinea pigs. Acta Otorhinolaryngol. 1995;9(2):70–74. (in Chinese) [Google Scholar]
- 24.Ding D, Zhang Z, Zhu Q. Experimental study of concurrent ototoxicity between ethacrynic acid and gentamycin. J Audiol Speech Disord. 1995;3(2):76–79. (in Chinese) [Google Scholar]
- 25.Ding D, Jin X, Zhao J. The changes of cochlear bioelectric potential on guinea pigs deafened with ethacrynic acid. J Clin Otorhinolaryngol. 1996;10(6):330–332. (in Chinese) [Google Scholar]
- 26.Ding D, Jin X, Zhao J. Accumulative sites of kanamycin in the organ of Corti by microautoradiography. Chin J Otorhinolaryngol. 1997;32(6):348–349. (in Chinese) [PubMed] [Google Scholar]
- 27.Ding D, McFadden SL, Woo JM, Salvi R. Ethacrynic acid rapidly and selectively abolishes blood flow in vessels supplying the lateral wall of the cochlea. Hear Res. 2002;173(1-2):1–9. doi: 10.1016/S0378-5955(02)00585-3. [DOI] [PubMed] [Google Scholar]
- 28.Ding D, McFadden SL, Browne RW, Salvi R. Late dosing with ethacrynic acid can reduce gentamicin concentration in perilymph and protect cochlear hair cells. Hear Res. 2003;185(1-2):90–96. doi: 10.1016/S0378-5955(03)00258-2. [DOI] [PubMed] [Google Scholar]
- 29.Ding D, Jiang H, McFadden SL, Salvi R. Ethacrynic acid is the key for opening of the blood-labyrinth barrier. Chin J Otol. 2004;1(2):42–47. (in Chinese) [Google Scholar]
- 30.Ding D, Jiang H, Wang P, Salvi R. Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res. 2007;226(1-2):129–139. doi: 10.1016/j.heares.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Ding D, Qi W, Zhang M, Wang P, Jiang H, Salvi R. Cisplatin and its ototoxicity. Chin J Otol. 2008;6(2):125–133. (in Chinese) [Google Scholar]
- 32.Ding D, Jiang H, Salvi R. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res. 2010;259(1-2):16–23. doi: 10.1016/j.heares.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham AC, Mercier RC, Achusim LE, Pai MP. Extended-interval aminoglycoside dosing for treatment of enterococcal and staphylococcal osteomyelitis. Ann Pharmacother. 2004;38(6):936–941. doi: 10.1345/aph.1D514. [DOI] [PubMed] [Google Scholar]
- 34.Greenwood DD. A cochlear frequency-position function for several species-29 years later. J Acoust Soc Am. 1990;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 35.Guan MX, Fischel-Ghodsian N, Attardi G. A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum Mol Genet. 2000;9(12):1787–1793. doi: 10.1093/hmg/9.12.1787. [DOI] [PubMed] [Google Scholar]
- 36.Gutell RR, Larsen N, Woese CR. Lessons from an evolving rRNA: 16S and 23S rRNA structures from a comparative perspective. Microbiol Rev. 1994;58(1):10–26. doi: 10.1128/mr.58.1.10-26.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hangfu M, Zhao J, Ding D. The prophylactic effect of thyroxin on kanamycin ototoxicity in guinea pigs. Hear Res. 1992;61(1-2):132–136. doi: 10.1016/0378-5955(92)90043-M. [DOI] [PubMed] [Google Scholar]
- 38.Humes HD. Insights into ototoxicity. Analogies to nephrotoxicity. Ann N Y Acad Sci. 1999;884(1):15–18. doi: 10.1111/j.1749-6632.1999.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 39.Kraus KS, Ding D, Zhou Y, Salvi R. Central auditory plasticity after carboplatin-induced unilateral inner ear damage in the chinchilla: up-regulation of GAP-43 in the ventral cochlear nucleus. Hear Res. 2009;255(1-2):33–43. doi: 10.1016/j.heares.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Ding D, Zheng XY, Salvi R. Vestibular destruction by slow infusion of gentamicin into semicircular canals. Acta Otolaryngol. 2004;124(Suppl. 552):35–41. doi: 10.1080/03655230410017102. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Ding D, Jiang H, Fu Y, Salvi R. Co-administration of cisplatin and furosemide causes rapid and massive loss of cochlear hair cells in mice. Neurot Res. 2011 doi: 10.1007/s12640-011-9262-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McFadden SL, Ding D, Jiang H, Woo JM, Salvi R. Chinchilla models of selective cochlear hair cell loss. Hear Res. 2002;174(1-2):230–238. doi: 10.1016/S0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 43.Moazed D, Noller HF. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 44.Pittinger C, Adamson R. Antibiotic blockade of neuromuscular function. Annu Rev Pharmacol. 1972;12:169–184. doi: 10.1146/annurev.pa.12.040172.001125. [DOI] [PubMed] [Google Scholar]
- 45.Recht MI, Douthwaite S, Dahlquist KD, Puglisi JD. Effect of mutations in the A site of 16S rRNA on aminoglycoside antibiotic-ribosome interaction. J Mol Biol. 1999;286(1):33–43. doi: 10.1006/jmbi.1998.2446. [DOI] [PubMed] [Google Scholar]
- 46.Rougier F, Claude D, Maurin D, Sedoglavic A, Ducher M, Corvaisier S, Jelliffe R, Maire P. Aminoglycoside nephrotoxicity: modeling, simulation, and control. Antimicrob Agents Chemother. 2003;47(3):1010–1016. doi: 10.1128/AAC.47.3.1010-1016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rougier F, Claude D, Maurin M, Maire P. Aminoglycoside nephrotoxicity. Curr Drug Targets Infect Disord. 2004;4(2):153–162. doi: 10.2174/1568005043340858. [DOI] [PubMed] [Google Scholar]
- 48.Shuman RD, Smith CR. Intrathecal gentamicin for refractory gram-positive meningitis. JAMA. 1978;240(5):469–471. doi: 10.1001/jama.240.5.469. [DOI] [PubMed] [Google Scholar]
- 49.Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60(15):1583–1599. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torihara K, Suganuma T, Ide S, Morimitsu T. Anionic sites in blood capillaries of the mouse cochlear duct. Hear Res. 1994;77(1-2):69–74. doi: 10.1016/0378-5955(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 51.Vellai T, Takacs K, Vida G. A new aspect to the origin and evolution of eukaryotes. J Mol Evol. 1998;46(5):499–507. doi: 10.1007/PL00006331. [DOI] [PubMed] [Google Scholar]
- 52.Versnel H, Agterberg MJ, de Groot JC, Smoorenburg GF, Klis SF. Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear Res. 2007;231(1-2):1–12. doi: 10.1016/j.heares.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Wilhelm JM, Pettitt SE, Pettitt SE. Aminoglycoside antibiotics and eukaryotic protein synthesis: structure-function relationships in the stimulation of misreading with a wheat embryo system. Biochemistry. 1978;17(7):1143–1149. doi: 10.1021/bi00600a001. [DOI] [PubMed] [Google Scholar]
- 54.Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158(1-2):165–178. doi: 10.1016/S0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 55.Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res. 1993;70(2):205–215. doi: 10.1016/0378-5955(93)90159-X. [DOI] [PubMed] [Google Scholar]
- 56.Yamasoba T, Kondo K, Miyajima C, Suzuki M. Changes in cell proliferation in rat and guinea pig cochlea after aminoglycoside-induced damage. Neurosci Lett. 2003;347(3):171–174. doi: 10.1016/S0304-3940(03)00675-X. [DOI] [PubMed] [Google Scholar]
- 57.Zhao J, Ding D, Huangfu M. The influence of enthacrynic acid on the activity of enzyme in the stria vascularis in guinea pigs. J Clin Otorhinolaryngol. 1988;2(3):65–67. (in Chinese) [Google Scholar]
- 58.Zhao J, Ding D, Wang J, Huangfu M. Influence of ethacrynic acid on microcirculation of stria vascularis of cochlea in guinea pigs. Acta Univ Med Second Shanghai. 1988;8(2):34–37. (in Chinese) [Google Scholar]
- 59.Zhou Y, Ding D, Kraus KS, Yu D, Salvi R. Functional and structural changes in the chinchilla cochlea and vestibular system following round window application of carboplatin. Audiol Med. 2009;7(4):189–199. doi: 10.3109/16513860903335795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Q, Liu G, Ding D, Jin X. Accumulation of gentamycin in perilymph of guinea pigs. Acad J Second Mil Med Univ. 1993;14(6):568–571. (in Chinese) [Google Scholar]
- 61.Zucca G, Vega R, Botta L, Perez ME, Valli P, Soto E. Streptomycin blocks the afferent synapse of the isolated semicircular canals of the frog. Hear Res. 1992;59(1):70–74. doi: 10.1016/0378-5955(92)90103-T. [DOI] [PubMed] [Google Scholar]