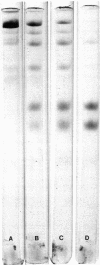
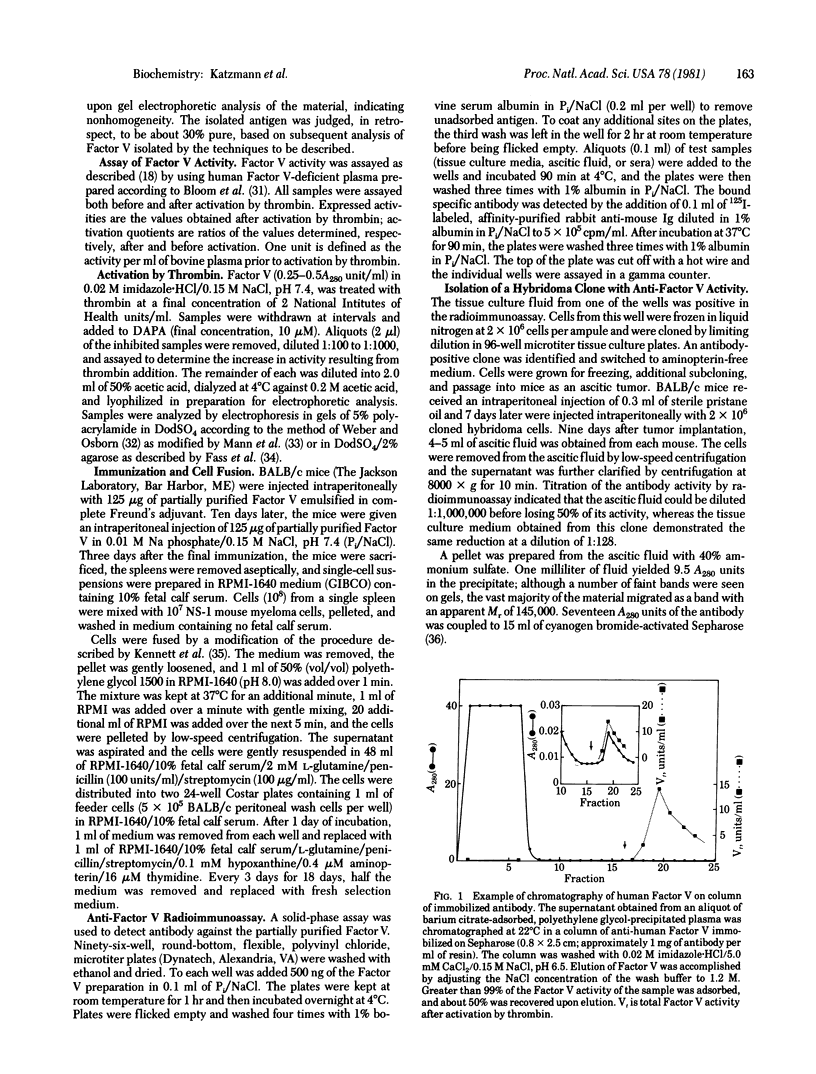
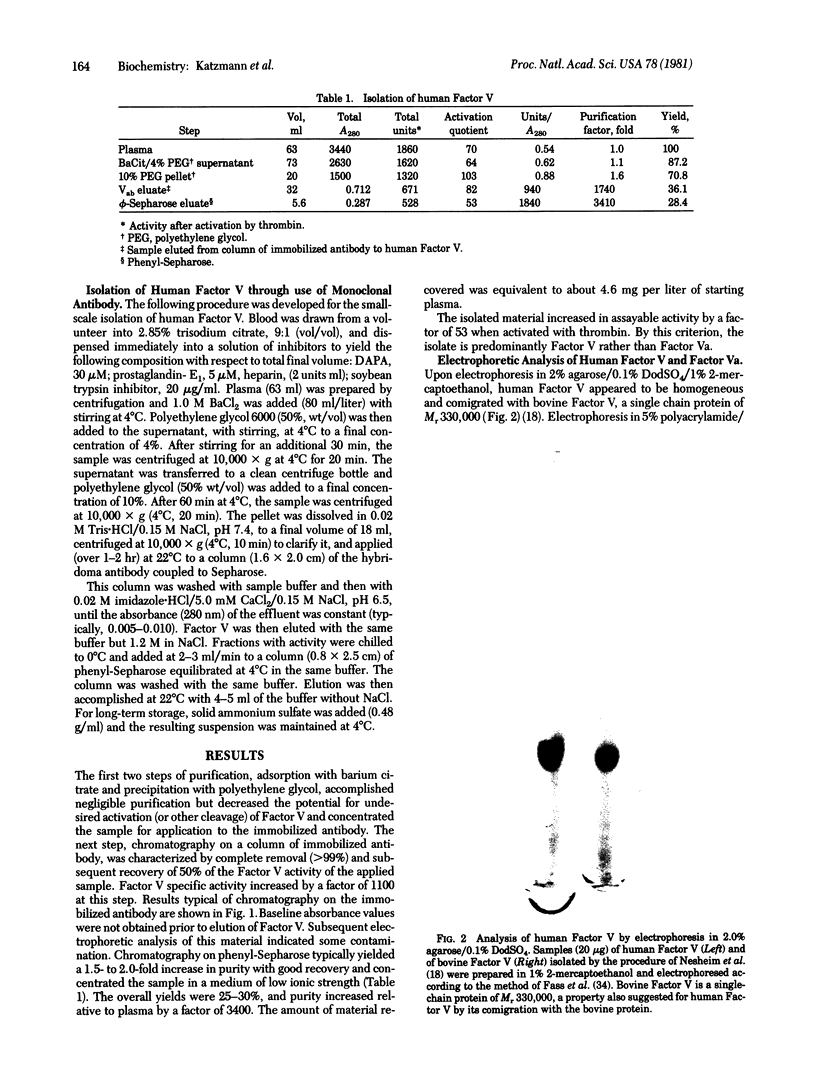
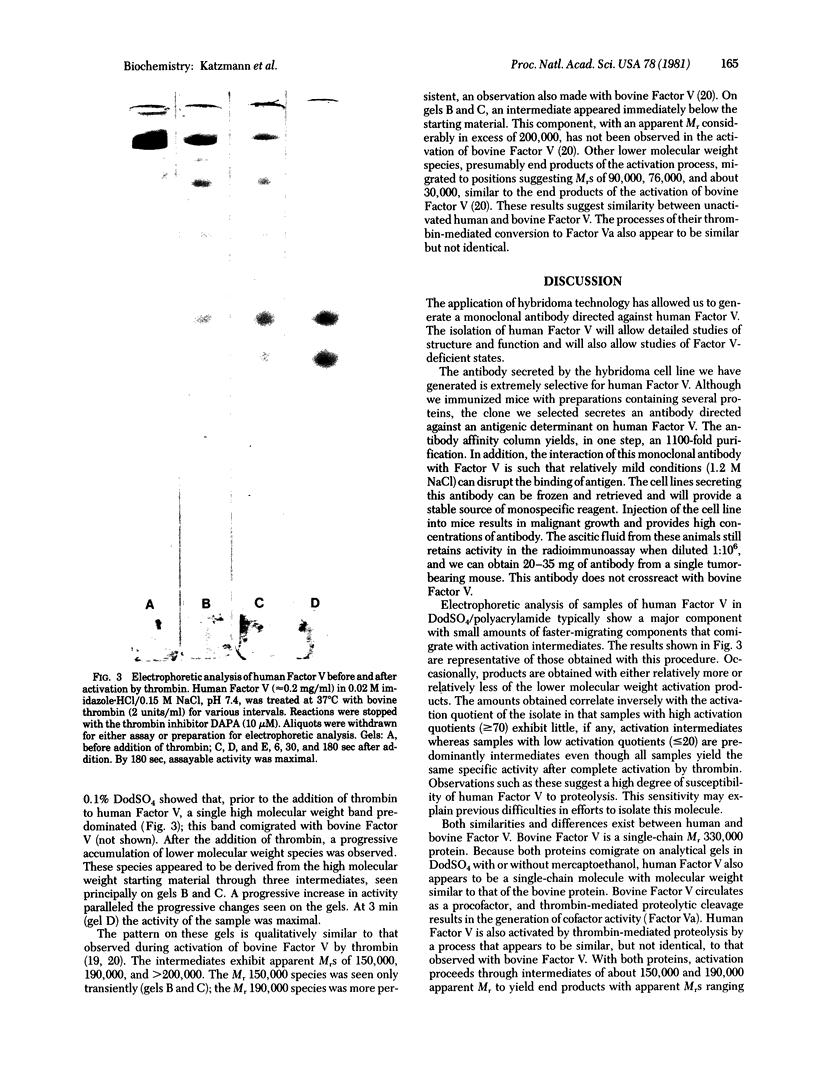
Abstract
Spleen cells obtained from mice immunized with partially purified human coagulation Factor V were fused with NS-1 mouse myeloma cells, and hybrids were selected. Culture media were screened for anti-Factor V activity, and an antibody-positive clone was obtained and passaged as an ascites tumor in mice. The ascitic fluid from the hybridoma-bearing mouse could be diluted 1:10(6) before losing reactivity in an anti-Factor V radioimmunoassay. When immobilized on agarose, the monoclonal antibody quantitatively removed Factor V activity from human plasma. Factor V activity could be eluted with 1.2 M NaCl at pH 6.5. Homogeneous Factor V was isolated by chromatography of barium citrate-adsorbed, polyethylene glycol 6000 precipitated plasma on the antibody column followed by chromatography on phenyl-Sepharose. The isolated Factor V exhibited a single band upon gel electrophoresis in sodium dodecyl sulfate with an apparent Mr comparable to that of bovine Factor V (330,000). Upon exposure to thrombin, the activity of Factor V increased 53-fold when measured in Factor V-deficient plasma. This increased activity was associated with discrete proteolytic cleavages of the parent molecule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj S. P., Butkowski R. J., Mann K. G. Prothrombin fragments. Ca2+ binding and activation kinetics. J Biol Chem. 1975 Mar 25;250(6):2150–2156. [PubMed] [Google Scholar]
- Barton P. G., Hanahan D. J. The preparation and properties of a stable factor V from bovine plasma. Biochim Biophys Acta. 1967 Apr 11;133(3):506–518. doi: 10.1016/0005-2795(67)90555-7. [DOI] [PubMed] [Google Scholar]
- Bloom J. W., Nesheim M. E., Mann K. G. A rapid technique for the preparation of factor V deficient plasma. Thromb Res. 1979;15(5-6):595–599. doi: 10.1016/0049-3848(79)90169-5. [DOI] [PubMed] [Google Scholar]
- Bloom J. W., Nesheim M. E., Mann K. G. Phospholipid-binding properties of bovine factor V and factor Va. Biochemistry. 1979 Oct 2;18(20):4419–4425. doi: 10.1021/bi00587a023. [DOI] [PubMed] [Google Scholar]
- Colman R. W. The effect of proteolytic enzymes on bovine factor V. I. Kinetics of activation and inactivation by bovine thrombin. Biochemistry. 1969 Apr;8(4):1438–1445. doi: 10.1021/bi00832a019. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Weinberg R. M. Factor V. Methods Enzymol. 1976;45:107–122. doi: 10.1016/s0076-6879(76)45015-2. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G., Jackson C. M. A plausible mechanism for prothrombin activation by factor Xa, factor Va, phospholipid, and calcium ions. J Biol Chem. 1974 Dec 25;249(24):8045–8047. [PubMed] [Google Scholar]
- Esmon C. T. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979 Feb 10;254(3):964–973. [PubMed] [Google Scholar]
- Esnouf M. P., Jobin F. The isolation of factor V from bovine plasma. Biochem J. 1967 Mar;102(3):660–665. doi: 10.1042/bj1020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass D. N., Knutson G. J., Bowie E. J. Porcine Willebrand factor: a population of multimers. J Lab Clin Med. 1978 Feb;91(2):307–320. [PubMed] [Google Scholar]
- Hibbard L. S., Mann K. G. The calcium-binding properties of bovine factor V. J Biol Chem. 1980 Jan 25;255(2):638–645. [PubMed] [Google Scholar]
- Jobin F., Esnouf M. P. Studies on the formation of the prothrombin-converting complex. Biochem J. 1967 Mar;102(3):666–674. doi: 10.1042/bj1020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandall C. L., Rosenberg R., Colman R. W. Molecular changes associated with proteolysis of bovine factor V by thrombin. Eur J Biochem. 1975 Oct 1;58(1):203–211. doi: 10.1111/j.1432-1033.1975.tb02365.x. [DOI] [PubMed] [Google Scholar]
- Kane W. H., Lindhout M. J., Jackson C. M., Majerus P. W. Factor Va-dependent binding of factor Xa to human platelets. J Biol Chem. 1980 Feb 10;255(3):1170–1174. [PubMed] [Google Scholar]
- Mann K. G., Heldebrant C. M., Fass D. N. Multiple active forms of thrombin. I. Partial resolution, differential activities, and sequential formation. J Biol Chem. 1971 Oct 10;246(19):5994–6001. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Mann K. G. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979 Feb 25;254(4):1326–1334. [PubMed] [Google Scholar]
- Nesheim M. E., Myrmel K. H., Hibbard L., Mann K. G. Isolation and characterization of single chain bovine factor V. J Biol Chem. 1979 Jan 25;254(2):508–517. [PubMed] [Google Scholar]
- Nesheim M. E., Prendergast F. G., Mann K. G. Interactions of a fluorescent active-site-directed inhibitor of thrombin: dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Biochemistry. 1979 Mar 20;18(6):996–1003. doi: 10.1021/bi00573a010. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Taswell J. B., Mann K. G. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979 Nov 10;254(21):10952–10962. [PubMed] [Google Scholar]
- PAPAHADJOPOULOS D., HOUGIE C., HANAHAN D. J. PURIFICATION AND PROPERTIES OF BOVINE FACTOR V: A CHANGE OF MOLECULAR SIZE DURING BLOOD COAGULATION. Biochemistry. 1964 Feb;3:264–270. doi: 10.1021/bi00890a021. [DOI] [PubMed] [Google Scholar]
- Rosing J., Tans G., Govers-Riemslag J. W., Zwaal R. F., Hemker H. C. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980 Jan 10;255(1):274–283. [PubMed] [Google Scholar]
- Saraswathi S., Rawala R., Colman R. W. Subunit structure of bovine factor V. Influence of proteolysis during blood collection. J Biol Chem. 1978 Feb 25;253(4):1024–1029. [PubMed] [Google Scholar]
- Smith C. M., Hanahan D. J. The activation of factor V by factor Xa or alpha-chymotrypsin and comparison with thrombin and RVV-V action. An improved factor V isolation procedure. Biochemistry. 1976 May 4;15(9):1830–1838. doi: 10.1021/bi00654a007. [DOI] [PubMed] [Google Scholar]
- Tracy P. B., Peterson J. M., Nesheim M. E., McDuffie F. C., Mann K. G. Interaction of coagulation factor V and factor Va with platelets. J Biol Chem. 1979 Oct 25;254(20):10354–10361. [PubMed] [Google Scholar]
- Travis J., Bowen J., Tewksbury D., Johnson D., Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976 Aug 1;157(2):301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]