Abstract
BACKGROUND
Recent data suggest that highly elevated HDL-C may not always protect against cardiovascular disease. To what degree this is true in type 1 diabetes is unknown, although cardiovascular risk is increased despite elevated mean HDL-C.
OBJECTIVE
To reassess the association between HDL-C and its subfractions with coronary artery disease (CAD) in childhood-onset type 1 diabetes.
METHODS
Epidemiology of Diabetes Complications study participants free of CAD at baseline (301 men, 298 women; mean age, 27.1 and diabetes duration, 18.9 years) were studied. CAD was defined as angina, ischemic ECG changes, confirmed MI, angiographic stenosis ≥50%, revascularization, or CAD death. Cholesterol in the HDL fraction and HDL3 cholesterol subfraction was measured enzymatically after precipitation with heparin/manganese and dextran sulphate, respectively.
RESULTS
During 18 years of follow-up, 29.5% of men and 25.5% of women developed CAD. While a linear decrease in incidence was observed with increasing HDL-C concentration in men, incidence increased in women below 47 mg/dL and above 80 mg/dL. These patterns largely reflected the HDL3 cholesterol-CAD association. After multivariable adjustment, the linear, inverse, HDL-C-CAD association persisted in men (HR=0.97, 95% CI=0.94–0.99); in women, the U-shaped relationship lost significance. HDL3 cholesterol remained multivariably associated with CAD in both men (linear association, p=0.03) and women (HR=2.31 (95% CI=1.31–4.08) and HR=1.80 (95% CI=1.01–3.23) for the lowest and highest versus the middle quintiles, respectively).
CONCLUSION
The increased CAD risk in women for an HDL-C >80 mg/dL in type 1 diabetes merits further study. Gender specificity could not be determined as only two men had HDL-C >80 mg/dL.
Keywords: HDL cholesterol, coronary artery disease, type 1 diabetes, gender
Introduction
Though both the atherogenicity of apolipoprotein B containing lipoproteins, as well as, the atheroprotection conferred by higher high density lipoprotein cholesterol (HDL-C) concentrations is well established, the lipoprotein-cardiovascular disease association remains, in some regards, puzzling to date. Thus, whether or not raising HDL-C pharmacologically is of benefit in terms of reducing risk, is still unclear. Indeed, achieving higher plasma HDL-C concentrations via blockage of the cholesteryl ester transfer protein resulted in an increased (not decreased) risk of all cause mortality and cardiovascular morbidity in the ILLUMINATE trial (1) and failed to slow atherosclerosis progression in another two clinical trials (2, 3). Later reports suggested that the pharmacologic agent used to increase HDL-C levels, torcetrapib, may have had off-target toxicity, raising blood pressure and inducing increases in plasma sodium and decreases in potassium levels (4, 5). However, a subsequent meta-regression analysis of randomized controlled trials testing lipid modifying interventions also concluded that increasing circulating HDL-C does not reduce coronary heart disease morbidity or mortality (6). Moreover, independent reports from the IDEAL trial, comparing the efficacy of high to moderate dose statin regimen for the secondary prevention of cardiovascular disease, and two prospective cohort studies, the EPIC-Norfolk (7) and Regress (8), both of which did not include pharmacologic interventions, recently showed that highly elevated HDL-C concentrations do not protect against cardiovascular disease. Rather, an increased risk was suggested in the IDEAL and EPIC-Norfolk cohorts at the higher end of the HDL-C and HDL particle size distributions after adjustment for apolipoproteins A1 and B (7).
These contradictory findings concerning HDL-C likely reflect the concept that the functionality of the HDL fraction may be more important than its cholesterol content. Interestingly, the hypothesis of dysfunctional HDL-C in terms of its most critical activity, reverse cholesterol transport, has long been proposed in type 1 diabetes (9), in which HDL-C is generally increased, as is cardiovascular risk, yet an inverse association between HDL-C and cardiovascular disease incidence still remains (10–15). Furthermore, we have previously shown in a case-control study that different HDL subfractions by nuclear magnetic resonance (NMR) spectroscopy relate to coronary artery disease (CAD) incidence differently (i.e., large HDL particle concentration decreased CAD risk whereas medium HDL mass was an independent positive predictor of CAD incidence) (16). Given these observations, we aimed to reassess the prospective relationship between plasma levels of HDL-C, its subfractions (HDL2 and HDL3) and apolipoproteins A1 (Apo A-I) and B (ApoB) in more detail, with the incidence of CAD in our cohort of individuals with childhood onset type 1 diabetes given the greater power afforded by 18 years of follow-up, with a particular focus on those at the highest end of the HDL-C range.
Methods
Participants from the Pittsburgh Epidemiology of Diabetes Complications (EDC) study who were free of CAD at study initiation were selected for study (n=599). The EDC is a historical cohort study based on incident cases of childhood onset (<17 years) type 1 diabetes, diagnosed or seen within one year of diagnosis (1950–80) at Children’s Hospital of Pittsburgh (17). The cohort has been shown to be epidemiologically representative of the Allegheny County, Pennsylvania, type 1 diabetes population (18). The first clinical assessment for the EDC study was between 1986 and 1988, when the mean participant age and diabetes duration were 28 and 19 years, respectively. Subsequently, biennial examinations were conducted for 10 years, with a further examination at 18 years. The University of Pittsburgh IRB approved the study protocol.
Prior to each clinic visit, participants were sent questionnaires concerning demographic, health and diabetes self-care, and medical history information. Blood pressure was measured with a random zero sphygmomanometer, after a five minute rest (19) and hypertension was defined as ≥140/90 mmHg or use of antihypertensive medication. Stable glycosylated hemoglobin (HbA1) was measured by ion exchange chromatography (Isolab, Akron, OH) and subsequently by automated high-performance liquid chromatography (Diamat, BioRad, Hercules, CA). The two assays were highly correlated (r=0.95).
High density lipoprotein cholesterol (HDL-C) was determined enzymatically after precipitation with heparin and manganese chloride, with a modification (20) of the Lipid Research Clinics method (21). The concentration of HDL3 was measured after precipitation of HDL2 by dextran sulfate (22). Cholesterol and triglycerides were measured enzymatically (23, 24). Non-HDL cholesterol was calculated as total minus HDL cholesterol. Apolipoproteins A1 (Apo A-I) and B (Apo B) were determined via immunoelectrophoresis (25), whereas apolipoprotein A-II (Apo A-II) was determined by an enzyme-linked immunosorbent assay methodology (26).
White blood cell count was obtained using a counter S-plus IV and fibrinogen using a biuret colorimetric procedure and a clotting method. Urinary albumin was measured by immunonephelometry (27) and creatinine was assayed by an Ectachem 400 Analyzer (Eastman Kodak Co., Rochester, NY). All assays were conducted at baseline and thus, prolonged storage would not have affected measurements performed in this study.
CAD was determined by EDC study physician diagnosed angina, myocardial infarction confirmed by Q-waves on electrocardiogram (Minnesota codes 1.1 or 1.2) or hospital records, angiographic stenosis >50 percent, coronary artery bypass surgery, angioplasty, ischemic electrocardiogram changes (Minnesota codes 1.3, 4.1–4.3, 5.1–5.3, 7.1), or CAD death.
Statistical analysis
Univariate associations were determined using the student’s t-test or Wilcoxon 2-sample test for continuous variables and the χ2 or Fisher’s exact test, as appropriate, for categorical variables. To evaluate differences in baseline risk factors by HDL cholesterol category, general linear regression models were run and Bonferroni adjustment for multiple comparisons was conducted. Cox proportional hazards models with backward elimination were constructed to assess the multivariable association between HDL-C or its subfractions and the incidence of CAD adjusting for traditional risk factors and univariately significant variables. Survival time was defined as the time in years from study entry to either an incident event or censorship during the 18-year follow-up. Non-normally distributed variables were logarithmically transformed for entry into multivariable models. Statistical analyses were conducted using Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC).
Results
During 18 years of follow-up, 29.6% of 301 men and 25.5% of 298 women had an incident CAD event. Higher levels of HDL cholesterol (HDL-C), HDL2 and HDL3 cholesterol were observed among female participants compared to their male counterparts (all p-values <0.0001). Descriptive characteristics of participants at study entry by gender and subsequent CAD status are shown in Table 1. Among both men and women, those who developed a CAD event were more likely to be older, with a longer duration of type 1 diabetes, larger waist to hip ratios, and elevated blood pressure, Apo B, non-HDL cholesterol, AER, white blood cell count and fibrinogen levels. Incident cases were also more likely to have reported smoking, use of blood pressure and ACE/ARB medications, and a lower dose of insulin per kilogram body weight. BMI and serum creatinine were increased in subsequent cases only among men, whereas pulse was elevated among women who developed a CAD event compared to controls. Although both HDL-C and HDL3 cholesterol were decreased among cases, these differences were only significant among men. HDL2 cholesterol was not related to CAD in either gender.
Table 1.
Participant characteristics at study entry by gender and subsequent CAD status
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Characteristics | No CAD (n=212) | CAD (n=89) | p-value | No CAD (n=222) | CAD (n=76) | p-value |
| Age (years) | 25.4 (7.4) | 30.8 (6.5) | <0.0001 | 25.2 (7.2) | 33.2 (6.7) | <0.0001 |
| Age at onset (years) | 8.1 (4.3) | 7.8 (3.9) | 0.54 | 8.3 (3.9) | 8.9 (4.1) | 0.24 |
| Diabetes duration (years) | 17.4 (6.7) | 23.1 (7.1) | <0.0001 | 17.0 (6.8) | 24.4 (6.8) | <0.0001 |
| Body mass index (kg/m2) | 23.3 (3.1) | 24.3 (2.7) | 0.008 | 23.3 (3.3) | 23.9 (3.7) | 0.17 |
| Waist to hip ratio | 0.86 (0.05) | 0.89 (0.04) | <0.0001 | 0.77 (0.05) | 0.79 (0.07) | 0.01 |
| Percent ever smoked | 35.4 (75) | 51.7 (46) | 0.009 | 28.8 (64) | 51.3 (39) | 0.0004 |
| HbA1c (%) | 8.8 (1.5) | 8.8 (1.6) | 0.87 | 8.7 (1.6) | 8.7 (1.4) | 0.79 |
| Insulin dose/weight | 0.83 (0.26) | 0.76 (0.20) | 0.008 | 0.80 (0.25) | 0.71 (0.25) | 0.02 |
| Systolic blood pressure (mmHg) | 113.7 (12.7) | 121.9 (17.6) | <0.0001 | 107.3 (11.9) | 115.5 (16.5) | 0.0001 |
| Diastolic blood pressure (mmHg) | 73.0 (10.3) | 79.2 (10.8) | <0.0001 | 68.9 (8.7) | 73.2 (13.2) | 0.009 |
| Blood pressure medication (%) | 4.1 (8) | 23.0 (20) | <0.0001 | 4.2 (9) | 17.3 (13) | 0.0002 |
| Percent hypertensive | 9.4 (20) | 31.5 (28) | <0.0001 | 6.8 (15) | 27.6 (21) | <0.0001 |
| Pulse (beats/min) | 76.8 (10.2) | 78.2 (9.0) | 0.25 | 78.4 (9.2) | 81.7 (10.5) | 0.01 |
| HDL cholesterol (mg/dL) | 50.8 (9.8) | 46.4 (9.2) | 0.0004 | 59.2 (12.5) | 56.3 (13.8) | 0.10 |
| HDL2 (mg/dL) | 10.2 (6.8–16.1) | 9.9 (6.8–13.2) | 0.29 | 17.3 (10.6–23.0) | 15.6 (10.0–22.5) | 0.43 |
| HDL3 (mg/dL) | 38.6 (34.3–43.3) | 35.6 (31.4–40.2) | 0.001 | 40.7 (36.5–44.5) | 39.0 (34.1–46.6) | 0.17 |
| Apolipoprotein A-I (mg/dL) | 135.0 (123.0–146.0) | 135.0 (125.0–149.0) | 0.77 | 140.0 (128.0–154.0) | 140.0 (130.0–152.5) | 0.98 |
| Apolipoprotein A-II (mg/dL) | 44.0 (38.0–50.0) | 45.0 (38.0–48.0) | 0.93 | 43.5 (38.0–52.0) | 44.5 (38.0–51.5) | 0.66 |
| Apolipoprotein B (mg/dL) | 97.0 (81.0–116.0) | 108.0 (93.0–127.0) | 0.0008 | 91.0 (76.0–112.0) | 107.5 (85.0–126.5) | 0.001 |
| Non-HDL cholesterol (mg/dL) | 129.3 (36.6) | 159.6 (49.0) | <0.0001 | 128.6 (40.8) | 146.7 (36.4) | 0.0007 |
| ACE/ARB use (%) | 1.5 (3) | 5.7 (5) | 0.06 | 1.8 (4) | 6.7 (5) | 0.05* |
| Serum creatinine (mg/dL) | 0.90 (0.80–1.10) | 1.0 (0.90–1.3) | 0.001 | 0.80 (0.60–0.90) | 0.80 (0.60–1.00) | 0.29 |
| Glomerular filtration rate by Cockcroft-Gault (mL/min/1.73 m2) | 122.4 (36.1) | 107.8 (45.0) | 0.008 | 114.1 (46.8) | 100.2 (41.2) | 0.02 |
| Albumin excretion rate (μg/min) | 11.8 (7.0–52.4) | 71.3 (11.7–1006.9) | <0.0001 | 10.9 (6.6–44.4) | 30.9 (7.8–281.5) | 0.003 |
| White blood cell count × 103/mm2 | 6.1 (1.6) | 7.0 (2.0) | 0.0006 | 6.5 (1.9) | 7.3 (2.2) | 0.002 |
Note. Data presented are means (std) or percentages (n); for non-normally distributed variables, median (interquartile range) is presented. Sample size was reduced for the following variables: Body mass index (men: 211 non-cases and 88 cases; women: 221 non-cases and 76 cases); waist to hip ratio (men: 210 non-cases and 89 cases; women: 219 non-cases and 75 cases); HbA1c (men: 211 non-cases and 89 cases; women: 222 non-cases and 75 cases); insulin dose/weight (men: 197 non-cases and 87 cases; women: 215 non-cases and 75 cases); blood pressure medication (men: 197 non-cases and 87 cases; women: 215 non-cases and 75 cases); pulse (men: 210 non-cases and 89 cases); HDL2 (men: 205 non-cases and 86 cases; women: 216 non-cases and 75 cases); Apolipoprotein A-I and Apolipoprotein B (men: 207 non-cases and 89 cases; women: 220 non-cases and 76 cases); Apolipoprotein A-II (men: 207 non-cases and 89 cases; women: 118 non-cases and 76 cases); ACE/ARB use (men: 200 non-cases and 88 cases; women: 217 non-cases and 75 cases); albumin excretion rate (men: 210 non-cases and 89 cases; women: 221 non-cases and 75 cases); and white blood cell count (men: 210 non-cases and 88 cases; women: 220 non-cases and 76 cases).
Fisher’s exact test p-value
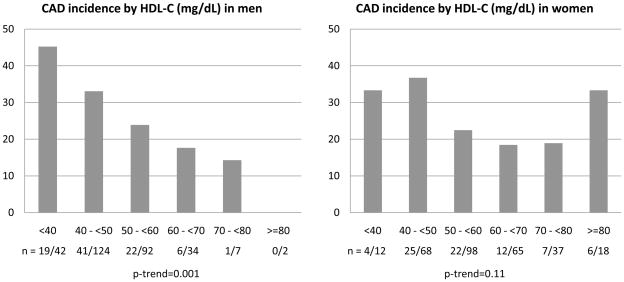
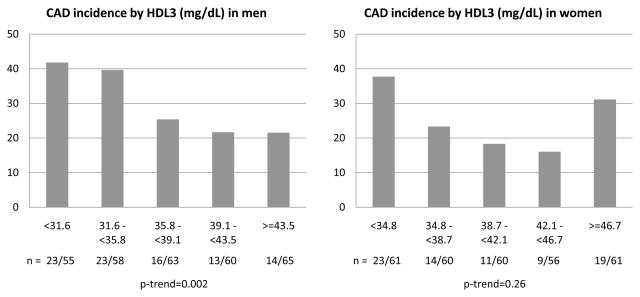
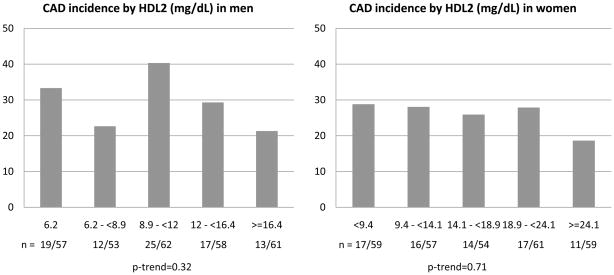
Figure 1 depicts the 18 year incidence of CAD events by unit based cut off points of HDL-C at study entry for men and women. Interestingly, although a linear decrease in incidence was observed among men with increasing concentrations of HDL-C (χ2 p=0.05, p-trend=0.001), among women, incidence increased at both the lower (below 50 mg/dL) and the upper (above 80 mg/dL) ends of the HDL-C distribution (p=0.13, p-trend=0.11) with little gradient between 50 and 80 mg/dL. Despite the considerably reduced number of events, similar results were obtained for the incidence of MI or CAD death (Supplemental Figure 1). These patterns largely reflected the HDL3 cholesterol association with CAD incidence (p=0.02, p-trend=0.002 and p=0.04, p-trend=0.26 for men and women, respectively, Figure 2). Indeed, the proportion of incident events was similar across quintiles of HDL2 cholesterol (p=0.13, p-trend=0.32 and p=0.71, p-trend=0.26 for men and women, respectively, Figure 3). In evaluating baseline risk factors by unit based cut off points for HDL cholesterol in men and women, no potential confounders were identified that could explain the increased risk observed among women with HDL-C≥80 mg/dL (Supplemental Tables 1 (men) and 2 (women) show these results). In men, as HDL cholesterol increased, significant decreasing trends were observed for BMI, waist to hip ratio, systolic and diastolic blood pressure, non-HDL cholesterol, serum creatinine, AER and white blood cell count, whereas significant increasing trends were seen for HDL2, HDL3 and Apo A-I. Similar associations were observed in women with the exception of no difference in blood pressure levels by HDL-C category and a significant decreasing trend in Apo B. The type of first CAD event by gender and HDL-C category is shown in Supplemental Table 3. It should be noted here, however, that decisions concerning cardiac procedures were driven by the individual’s health care provider, not the study investigators.
Figure 1.
Incident CAD events by baseline concentration of HDL cholesterol (mg/dL) in male and female participants of the EDC study
Figure 2.
Incident CAD events by baseline quintiles of HDL3 (mg/dL) in male and female participants of the EDC study
Figure 3.
Incident CAD events by baseline quintiles of HDLc2 (mg/dL) in male and female participants of the EDC study
The linear, inverse, association between HDL-C and CAD in men persisted after allowing for Apo A-I and B (or, in alternative models, LDL-C and triglycerides), diabetes duration, waist to hip ratio, smoking status, HbA1c, hypertension, AER and white blood cell count in Cox proportional hazard models with backward elimination (HR=0.97, 95% CI=0.95–0.99). Although Apo A-I and B were not selected for inclusion into the final model, forcing them in did not alter the effect of HDL-C (HR=0.97, 95% CI=0.94–0.99). To test the presence of a non-linear, i.e. U-shaped, association among women, a quadratic term of HDL-C was included in the models. Indeed, the quadratic association remained significant after adjustment for Apo A-I and Apo B (p=0.03), although further adjustments rendered the quadratic term insignificant. For HDL3, however, there was a linear independent association in men (HR=0.97, 95% CI=0.94–0.997, p=0.03), whereas in women, a U-shaped association again emerged and remained borderline significant after full multivariable adjustment (p=0.06). As an increased incidence of CAD was observed in the lowest two quintiles in men and the lowest and highest quintiles in women (Figure 2), multivariable Cox proportional hazard models were also constructed, using categories of HDL3 as suggested in the descriptive graphs (Table 2). Indeed, CAD risk was increased with HDL3 concentrations below 35.8 mg/dL in men and below 34.8 mg/dL in women, although among the latter an increased risk was also observed above 46.7 mg/dL.
Table 2.
Hazard ratios (95% confidence intervals) for the prediction of CAD incidence associated with HDL3 category at study entry
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Men (n=289; 88 incident events) | ||||
| HDL3 (mg/dL) | ||||
| <35.8 (n=110) | 1.93 (1.27–2.94) | 2.15 (1.40–3.30) | 2.15 (1.40–3.30) | 1.96 (1.27–3.04) |
| ≥35.8 (n=179) | Referent | Referent | Referent | Referent |
| Akaike’s information criterion | 895.993 | 847.064 | 837.484 | 830.697 |
| Women(n=285; 73 incident events) | ||||
| HDL3 (mg/dL) | ||||
| <34.8 (n=55) | 2.47 (1.42–4.28) | 2.92 (1.66–5.14) | 2.41 (1.36–4.27) | 2.31 (1.31–4.08) |
| 34.8 – <46.7 (n=172) | Referent | Referent | Referent | Referent |
| ≥46.7 (n=58) | 1.85 (1.04–3.29) | 1.84 (1.03–3.28) | 1.87 (1.05–3.34) | 1.80 (1.01–3.22) |
| Akaike’s information criterion | 756.918 | 688.295 | 671.617 | 670.341 |
Model 1: allowed for Apolipoprotein A-I, A-II and Apo B
Model 2: allowed for variables in Model 1, in addition to diabetes duration, waist to hip ratio, and smoking status (ever/never)
Model 3: allowed for variables in Model 2, in addition to HbA1c and hypertension
Discussion
Individuals with type 1 diabetes exhibit a markedly increased risk for cardiovascular disease compared to the general population, notwithstanding generally normal lipid concentrations and frequently elevated mean HDL-C levels (30). Nonetheless, the inverse association with cardiovascular disease risk observed in epidemiologic studies of the general population is confirmed in diabetes, including in our study population (10–15). However, previous reports have not generally evaluated the very high end of the HDL-C distribution or the impact of gender. The present analyses strongly suggest that although a smooth linear inverse association between HDL-C and CAD incidence exists in men, in women this simple pattern is disturbed, with apparent increases in risk below an HDL-C of 50 mg/dL as well as above 80 mg/dL and with little gradient in between (Figure 1). We therefore suggest that this gender specific HDL-C-CAD risk difference may play a significant role in the relatively greater impact of type 1 diabetes to cardiovascular disease risk in women. The small number of men with an HDL-C above 80 mg/dL (n=2) precludes defining this effect in men. While it could be argued that it might be more appropriate for equivalency to use a lower cut-off point for the high HDL-C group in men, there is no suggestion from Figures 1 and 2 of any increased risk in men at lower cut-off points for either HDL-C or HDL3, suggesting that the absolute rather than specific level is the critical determinant. As also pointed out by van der Steeg et al. (7), the increased risk associated with highly elevated HDL-C would be missed by the mere assessment of the association between HDL-C as a continuous variable, or even as quintiles (Supplemental Figure 2), with incident cardiovascular events. Our findings further indicate that the total HDL-C – CAD patterns are mainly reflective of the relationship between the HDL3 subfraction, the “precursor” of HDL2 in conventional understanding, for HDL2 showed no association with CAD. Which subfraction, HDL2 or HDL3, is the key cardioprotective subfraction is controversial, although most suggest HDL2.
HDL particles are extremely heterogeneous in size, shape, density and properties, much of the controversy, and confusion, in this area results from the multitude of different methodologies to quantify the subclasses of lipoproteins, which include electrophoresis, ultracentrifugation, precipitation and nuclear magnetic resonance spectroscopy. It is generally thought that the formation of HDL begins when discoidal, lipid poor, Apo A-I acquires cholesterol and phospholipids through its interaction with the ATP-binding cassette A1 (ABCA1), forming pre-β1 HDL particles. These particles progressively accumulate more cholesterol, which gets esterified by the enzyme lecithin-cholesterol acyltransferase (LCAT) and transferred to the core of the particle, forming larger, spherical, α-mobility HDL particles. The latter may be cleared by the hepatic scavenger receptor. Alternatively, cholesteryl esters can be transferred to VLDL/LDL for catabolism through the enzyme CETP in exchange for triglycerides, rendering triglyceride-rich particles susceptible to catabolism by various lipases yielding smaller, delipidated particles which could conceivably repeat the metabolic cycle. It thus seems likely that simply measuring the cholesterol content of the HDL fraction will at best only provide a very crude reflection of this sequence of events, as will the arbitrary division of HDL-C into HDL3 and HDL2 cholesterol (or any other subfractionation). Furthermore, the HDL2/HDL3 separation technique used in our study, though standard at the time, is now little used. Our past and current methodologies are thus poorly predictive of the efficiency of the reverse cholesterol transport and other functions of HDL.
Nevertheless, it is clear that the relationship between HDL-C and CAD in the current study paralleled the association between the smaller, denser HDL3 particles and CAD incidence whereas no relationship was observed with the larger, less dense, HDL2 subclass. Interestingly, using non-denaturing two-dimensional electrophoresis, Asztalos and Schaefer (28) also showed deficiencies in the α1 and pre-α1–3 HDL subspecies but elevations in the α3 HDL subspecies among individuals with CAD compared to normal controls, suggesting a disturbance in the progressive increase of HDL particle size in those with CAD. However, this report only included differences in the mean HDL-C concentration between CAD cases and controls, and therefore, it is not possible to evaluate differences over the whole range of the HDL-C distribution. In contrast to these findings, the EPIC-Norfolk study noted that HDL particle size was directly related to the incidence of major coronary events (7). Unfortunately, information on particle size and NMR spectroscopy subfractions is currently only available for a small number of participants selected for a nested, within the EDC, case-control study, measured at the earliest examination prior to an incident event among cases. Nevertheless, this case-control analysis showed that while large HDL particle concentration (by NMR spectroscopy) decreased CAD risk, medium HDL mass (along with total VLDL particle concentration) were also independent positive predictors of CAD incidence (16). Overall, these findings are therefore consistent with the hypothesis of a disturbed natural progression of HDL particle size change with a “hold up” at a key point, leading to an increase at that size (medium HDL by NMR, or HDL3 by electrophoresis) and a deficiency thereafter. The picture is further complicated by the potential for additional alterations in HDL size distribution during the progressive reductions of HDL size by passage of cholesterol ester to the scavenger receptor in the liver and hydrolysis of the triglyceride component.
Though reverse cholesterol transport, i.e. the HDL-C-mediated transfer of cholesterol from peripheral tissues to the liver for excretion, is the key pathway by which HDL-C reduces atherogenesis (31), measuring it clinically is still not currently feasible. We thus cannot confirm that it is decreased at very high concentrations of HDL-C (or with increased the HDL3 fraction), but such studies would be of great interest. Other potential protective mechanisms that may become defective, and thus worthy of exploration in individuals with very high HDL-C concentrations include its antioxidant, anti-inflammatory and anti-thrombotic properties, as well as endothelial properties (32, 33).
A limitation of the present analyses is the sample size after categorization of study participants by concentration of HDL-C or HDL3, which highlights the necessity of validating these results in other type 1 diabetes populations. Another shortcoming of this assessment is the lack of information on NMR spectroscopy lipoprotein subfractions on all study participants, which would allow for a broader evaluation of lipoprotein subspecies, their particle size and concentration. A third limitation is the inadequate number of male participants with very high HDL-C concentrations, prohibiting risk assessment in such a group. Finally, as the EDC study does not include non-diabetic participants, we cannot determine whether our observations reflect a specific diabetes effect or are a more general phenomenon. Given the disordered HDL-C metabolism seen in type 1 diabetes, however, we suspect that this effect is likely to be at least exacerbated by the presence of type 1 diabetes.
Conclusion
In conclusion, among individuals with long-standing type 1 diabetes, highly elevated (above 80 mg/dL) HDL-C was associated with increased CAD risk. This association largely reflected the relationship between the smaller, denser HDL3 particles and incident events and was not observed in men, for whom the sample size above 80 mg/dL was inadequate for study. Subsequent studies should address the gender specificity of this association as well as potential biological mechanisms.
Supplementary Material
Supplemental Figure 1. Incident MI/CAD death by baseline concentration of HDL cholesterol (mg/dL) in male and female participants of the EDC study
Supplemental Figure 2. CAD incidence by quintiles of baseline HDL cholesterol (mg/dL) in male and female participants of the EDC study
Acknowledgments
This research was supported by NIH grant DK34818
Abbreviations
- Apo A-I
Apolipoprotein A-I
- Apo A-II
apolipoprotein A-II
- Apo B
apolipoprotein B
- ABCA1
ATP-binding cassette A1
- CAD
coronary artery disease
- HDL-C
high density lipoprotein cholesterol
- HbA1
glycosylated hemoglobin
- LCAT
lecithin-cholesterol acyltransferase
- LDL-C
low density lipoprotein cholesterol
- NMR
nuclear magnetic resonance
- EDC
Epidemiology of Diabetes Complications
- VLDL
very low density lipoprotein
Footnotes
The authors declare no conflicts of interest related to this research paper
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 2.Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, Revkin JH, Grobbee DE, Riley WA, Shear CL, Duggan WT, Bots ML RADIANCE 1 Investigators. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–1630. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 3.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM ILLUSTRATE Investigators. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 4.Vergeer M, Bots ML, van Leuven SI, Basart DC, Sijbrands EJ, Evans GW, Grobbee DE, Visseren FL, Stalenhoef AF, Stroes ES, Kastelein JJ. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the rating atherosclerotic disease change by imaging with a new CETP inhibitor (RADIANCE) trials. Circulation. 2008;118:2515–2522. doi: 10.1161/CIRCULATIONAHA.108.772665. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118:2506–2514. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 6.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I, Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O’Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ES, Tikkanen MJ, Wareham NJ, Faergeman O, Olsson AG, Pedersen TR, Khaw KT, Kastelein JJ. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol. 2008;51:634–642. doi: 10.1016/j.jacc.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 8.van Acker BA, Botma GJ, Zwinderman AH, Kuivenhoven JA, Dallinga-Thie GM, Sijbrands EJ, Boer JM, Seidell JC, Jukema JW, Kastelein JJ, Jansen H, Verhoeven AJ REGRESS Study Group. High HDL cholesterol does not protect against coronary artery disease when associated with combined cholesteryl ester transfer protein and hepatic lipase gene variants. Atherosclerosis. 2008;200:161–167. doi: 10.1016/j.atherosclerosis.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Orchard TJ. Dyslipoproteinemia and diabetes. Endocrinology and Metabolism Clinics of North America. 1991;19:361–380. [PubMed] [Google Scholar]
- 10.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 11.Miller NE, Thelle DS, Forde OH, Mjos OD. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1977;1:965–968. doi: 10.1016/s0140-6736(77)92274-7. [DOI] [PubMed] [Google Scholar]
- 12.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 14.Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27:2898–2904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 15.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soedamah-Muthu SS, Chang YF, Otvos J, Evans RW, Orchard TJ. Pittsburgh Epidemiology of Diabetes Complications Study: Lipoprotein subclass measurements by nuclear magnetic resonance spectroscopy improve the prediction of coronary artery disease in Type 1 diabetes. A prospective report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2003;46:674–682. doi: 10.1007/s00125-003-1094-8. [DOI] [PubMed] [Google Scholar]
- 17.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications of IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 18.Wagener DK, Sacks JM, LaPorte RE, MacGregor JM. The Pittsburgh Study of insulin-dependent diabetes mellitus: risk for diabetes among relatives of IDDM. Diabetes. 1982;31:136–144. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]
- 19.Borhani NO, Kass EH, Langford HG, Payne GH, Remington RD, Stamler J. The hypertension detection and follow-up program. Prev Med. 1976;5:207–215. [Google Scholar]
- 20.Warnick GR, Albers JJ. Heparin-Mn2+ quantitaion of high density lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem. 1978;24:900–904. [PubMed] [Google Scholar]
- 21.National Institutes of Health and Dept. of Health. Lipid Research Clinics Program. Washington, DC: US Government Printing Office; 1975. [Google Scholar]
- 22.Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res. 1982;23:1206–1223. [PubMed] [Google Scholar]
- 23.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 24.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 25.Mendoza SG, Zerpa A, Carrasco H, Colemenares O, Rangel A, Gartside PS, Kashyap ML. Estradiol, testosterone, apolipoproteins, lipoprotein cholesterol and lipolytic enzymes in men with premature myocardial and angiographically assessed coronary occlusion. Artery. 1983;12:1–23. [PubMed] [Google Scholar]
- 26.Stein EA, Dipersio L, Pesce AJ, Kashyap M, Kao JT, Srivastava L, McNerney C. Enzyme-linked immunoabsorbent assay of apolipoprotein AII in plasma with use of monoclonal antibody. Clin Chem. 1986;32:967–971. [PubMed] [Google Scholar]
- 27.Ellis D, Buffone GJ. A new approach to the evaluation of proteinuric states. Clin Chem. 1977;23:666–670. [PubMed] [Google Scholar]
- 28.Asztalos BF, Schaefer EJ. High-density lipoprotein subpopulations in pathologic conditions. Am J Cardiol. 2003;91(Suppl):12E–17E. doi: 10.1016/s0002-9149(02)03383-0. [DOI] [PubMed] [Google Scholar]
- 29.Grover SA, Kaouache M, Joseph L, Barter P, Davignon J. Evaluating the Incremental Benefits of Raising High-Density Lipoprotein Cholesterol Levels During Lipid Therapy After Adjustment for the Reductions in Other Blood Lipid Levels. Arch Intern Med. 2009;169:1775–1780. doi: 10.1001/archinternmed.2009.328. [DOI] [PubMed] [Google Scholar]
- 30.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–2538. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- 31.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the atheroprotective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 32.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 33.Bisoendial RJ, Hovingh GK, Levels JHM, Lerch PG, Andresen I, Hayden MR, Kastelein JJP, Stroes ESG. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Incident MI/CAD death by baseline concentration of HDL cholesterol (mg/dL) in male and female participants of the EDC study
Supplemental Figure 2. CAD incidence by quintiles of baseline HDL cholesterol (mg/dL) in male and female participants of the EDC study