Abstract
Introduction
Recent studies in the human adenocarcinoma cell line A549 have identified cell growth dependent equilibrative nucleoside transporter-1 (hENT1) as a modifier of 3’-fluoro-3’-deoxythymidine (FLT) uptake and retention. In the present study, we used the ability to isolate human lymphoblastoid clones deficient in thymidine kinase 1 (TK1) to study how metabolism and nucleoside transport influence FLT uptake and retention.
Methods
Transport and metabolism of FLT were measured in the human lymphoblastoid cell line TK6 and in 8 clones isolated from TK6. Four clones were TK1-proficient while four were TK1-deficient. Both influx and efflux of FLT were measured under conditions where concentrative and equilibrative transport could be distinguished.
Results
Sodium-dependent concentrative FLT transport dominated over equilibrative transport mechanisms and while inhibition of hENT1 reduced FLT uptake, there were no correlations between clonal variations in hENT1 levels and FLT uptake. There was an absolute requirement of TK1 for concentration of FLT in TK6 cells. FLT uptake reached a peak after 60min incubation with FLT after which intracellular levels of FLT and FLT metabolites declined. Efflux was rapid and was associated with reductions in FLT and each of its metabolites. Both FLT and FLT-monophosphate were found in the efflux buffer.
Conclusions
Initial rates of FLT uptake were a function of both concentrative and equilibrative transporters. TK1 activity was an absolute requirement for the accumulation of FLT. Retention was dependent on nucleoside/nucleotide efflux and retrograde metabolism of FLT nucleotides.
Keywords: FLT Transport, FLT Metabolism, Nucleoside Transporters, Nucleotide Transporters
1. Introduction
The thymidine analog FLT (3’-fluoro-3’-deoxythymidine) has been extensively studied for its potential to detect changes in tumor proliferation [1–5]. Its use as a surrogate for tumor proliferation is based on the assumption that thymidine kinase-1 (TK1), a cell cycle dependent cytosolic enzyme, is the primary factor driving nucleoside uptake and retention in tumors [6]. Recent studies have shown that variations in nucleoside transporters can influence intracellular FLT levels and therefore need to be considered when interpreting FLT scans [7–10].
Previous work with the human adenocarcinoma cell line A549 demonstrated that FLT was transported into cells primarily by the equilibrative transporter hENT1 and that inhibition of hENT1 reduced overall FLT levels [8, 10]. We also reported that hENT1 levels increased as cells moved from plateau-phase culture to exponential growth [10]. In the present work, we used the ability to easily isolate individual clones deficient in TK1 activity from the human B-lymphoblast cell line TK6 to examine how FLT transport and metabolism influence uptake and retention of FLT. Unlike A549 cells, we observed no relationship between hENT1 levels and either FLT uptake or growth related changes in TK6 cells. However, we did identify other important FLT transport processes active in TK6 but not in A549, and we identified passive and active mechanisms that led to a rapid loss of FLT and FLT metabolites from these cells under exponential growth conditions.
2. Materials and Methods
2.1 Reagents
[methyl-3H]-3’-Deoxy-3’-fluorothymidine ([3H]-FLT; 351.5GBq/mmol) and [3H]-S-(p-nitrobenzyl)-6-thioinosine ([3H]-NBMPR; 740GBq/mmol) were purchased from Moravek Biochemicals (Brea, CA). High-purity nitrobenzylmercaptopurine ribonucleoside (NBMPR), and other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Cell culture media and supplements were purchased from Invitrogen (Carlsbad, CA). Antibodies were purchased from Abcam (Cambridge, MA).
2.2 Cell Culture Conditions
The cell line TK6 was derived from a single human lymphoblastoid line, WIL2, which was isolated in 1968 [11]. Individual TK1-proficient and TK1-deficient TK6 clones were isolated as described in [12]. To select for TK1-deficient clones, cells were isolated in medium containing 2 μg/mL trifluorothymidine. Each trifluorothymidine-resistant clone was expanded in drug-free medium. Each clone was also analyzed for sensitivity to aminopterin, which is toxic to TK1-deficient cells [12]. Multiple independent clones were analyzed to reduce the possibility of any clone-specific effect influencing results. Cells were maintained in suspension at 37°C in an atmosphere of 5% CO2/95% air in RPMI 1640 medium containing 10% fetal bovine serum.
2.3 Cell Doubling Time, BrdU Incorporation, and Ki67 Index
Cell doubling time was determined by counting cell numbers at daily intervals in flasks seeded at a concentration of 2 × 105 cells/mL. Cell line doubling times were calculating using Graphpad Prism Software (version 5.01). Cell cycle phase distributions were determined by BrdU-labeling and flow cytometry as previously described [10]. Ki67 indices were determined on parallel samples by staining with FITC-conjugated anti-Ki67 antibody and propidium iodide. Samples were analyzed on a Becton-Dickinson LSR2 cytometer using FACSDiva software (version 6.0). 20,000 events were collected for each sample, and data were analyzed with FlowJo software (version 9.0, Tree Star Inc., Ashland, OR, USA).
2.4 TK1 Activity Determination
107 cells were removed from culture media, washed once in ice-cold PBS, then incubated for 30min in ice-cold lysis buffer as described by Sherley and Kelly [13]. Cells were vortexed, then centrifuged for 15min at 13,000 × g. Supernatants were kept on ice and assayed immediately or snap-frozen in liquid nitrogen and stored at −80°C for up to 2 weeks prior to analysis. Protein content in lysates was determined with a bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL). Reactions were initiated by adding 15μL of reaction mix to 25μL of lysate and incubating at 37°C. Reaction mix was as described by Rasey et. al. [6] and Hruby et. al [14], and contained 10MBq/mL [methyl-3H]-thymidine (specific activity – 740GBq/mM). TK1-specific activity was distinguished from total TK activity by using reaction mix with or without 10mM deoxycytidine triphosphate as described by van der Wilt, et. al [15]. Reactions were terminated at 0, 5, 10, and 15 minutes by removing 8μL aliquots of reaction mixture to tubes containing 40μL of ice-cold 6% trichloroacetic acid (TCA). Samples were then vortexed for 20s, centrifuged at 13,000 × g for 10min, and supernatants were removed to new tubes. Acidified supernatants were neutralized with saturated potassium bicarbonate, and relative levels of 3H-thymidine and 3H-thymidine-monophosphate were determined by HPLC analysis as described below, and as described by Huang et. al. [16].
2.5 FLT Transport Studies
FLT uptake and loss were carried out in either sodium-containing or sodium-free HEPES-buffered Ringer’s solution as previously described [10]. For tracer influx, cells were removed from culture media, washed once with buffer, and assayed in a Coulter counter (Z2, Beckman Coulter). Aliquots of counted cells were incubated in buffer containing 74kBq/mL (2μCi/mL) of 3H-labeled FLT, with gentle rocking at 37°C. After incubating for times ranging from 30–120min, cells were pelleted by centrifuging for 1min at 13,000×g. Pelleted cells were washed twice in ice-cold PBS, incubated in 5% Triton X-100 for 2 hours, and then radioactivity measured in a Tri-Carb 1900 liquid scintillation counter using Ultima Gold scintillation cocktail (Perkin Elmer) with appropriate corrections for background and quenching.
For measuring initial rates of FLT transport, the protocol was modified as follows: washed and counted cells were incubated in 200μL of buffer containing 74 KBq/mL 3H-FLT for 60s at 37°C, then influx was terminated by the addition of 1000μL of ice-cold PBS containing 100μM NBMPR and 10mM thymidine. Cells were pelleted, washed, and radioactivity was measured as described above. To study ENT-mediated transport, reactions were carried out in buffer containing 100μM NBMPR.
For efflux studies, cells were incubated for 1h in 3H-FLT, washed twice in ice-cold PBS, and placed in buffer with or without Na+ and/or 100μM NBMPR. Samples were incubated at 37°C for up to 120 min. Aliquots of cells were placed immediately into 5% Triton X-100 at the beginning of efflux to determine the starting amount of activity in the cells. Samples of efflux buffer were removed from other aliquots for scintillation counting. Activity remaining in the cells after efflux was also measured by solubilizing cell pellets in 5% Triton X-100 for 2 hours and taking samples for scintillation counting.
2.6 HPLC Analysis of Tracer Metabolites
Cells were treated as described above and then suspended in ice-cold 6% trichloroacetic acid (TCA) to measure FLT metabolites. Acidified cells were vortexed for 20s, incubated on ice for 10min, vortexed again, and centrifuged at 14,000 × g for 10 min. The resulting supernatants were stored at −80°C for up to 2 weeks and neutralized with saturated potassium bicarbonate prior to HPLC analysis. Samples were analyzed using a Hewlett-Packard HP1050 HPLC system equipped with an in-line degasser (Alltech) and a Radiomatic 625TR flow scintillation analyzer (Perkin Elmer). A Waters Symmetry C18 3.5μm (150 × 4.6mm) column equipped with a NovaPak C18 guard column was used, and maintained at 27°C for all assays. Mobile phase was delivered at 1.0 mL/min using the following gradient program: A–B (100:0) 0–4 min→ (60:40) at 15 min → (40:60) at 35–45 min. Buffer A consisted of 10mM tetrabutylammonium hydroxide, 10mM KH2PO4, and 0.25% methanol, pH 6.9. Buffer B consisted of 6mM tetrabutylammonium hydroxide, 50mM KH2PO4, and 50% methanol, pH 7.0. Sample injection volume was 50μL, and UV absorbance was at 254nm. A standard mixture containing TdR, TMP, TDP, TTP, FLT, and FMAU was run prior to each assay to validate metabolite retention times. Details on the methods are described in [10].
3. Results
3. 1 Variations in hENT1 levels were unrelated to growth characteristics
The parent TK6 cell line, four independently isolated clones of TK6, and four independently isolated trifluorothymidine-resistant clones were analyzed for a variety of cell growth characteristics as well as TK1 activity and hENT1 content (Table 1). There were only minor differences in cell doubling times, although the percentages of S phase cells were significantly lower in the trifluorothymidine-resistant clones (P < 0.0001). As expected, the trifluorothymidine-resistant clones had little or no measurable TK1 activity. There was also little or no BrdU incorporation in these clones. Ki67 levels varied from 58% to 85%. There were significant variations in hENT1 levels in the eight TK6 clones (P < 0.0001). The hENT1 values ranged from 3.3 to 11.5 × 107 NBMPR binding sites/μg protein. There was no obvious relationship between these variations and any of the growth characteristics of the clones. There was also no significant correlation between hENT1 abundance and TK1 activity (P=0.24; r2=0.54).
Table 1.
Characteristics of Cell Lines1
| Cell Line | Cell Doubling Time (h) | %S Phase Cells | % Ki67-Labeled Cells | % BrdU-Labeled Cells | TK1 Activity (fmol dTMP/30’/ug protein) | hENT1 Sites (107 sites/ug protein) |
|---|---|---|---|---|---|---|
| TK6 (parent) | 20.4 ± 0.7 | 45.9 ± 2.3 | 90.7 ± 2.1 | 46.9 ± 2.9 | 193.9 ± 9.7 | 7.0 ± 1.5 |
| Control Clones | ||||||
| TK6-Clone T02 | 19.1 ± 0.3 | 55.1 ± 3.6 | 70.4 ± 5.1 | 46.4 ± 2.4 | 298.1 ± 46.1 | 5.7 ± 0.1 |
| TK6-Clone T03 | 19.0 ± 0.3 | 57.1 ± 2.8 | 75.0 ± 7.0 | 46.0 ± 2.3 | 345.6 ± 17.1 | 6.8 ± 0.6 |
| TK6-Clone T04 | 20.3 ± 0.5 | 57.7 ± 2.9 | 73.1 ± 6.3 | 39.2 ± 0.5 | 379.9 ± 34.8 | 5.3 ± 0.1 |
| TK6-Clone T05 | 19.2 ± 0.4 | 59.7 ± 2.3 | 73.1 ± 2.8 | 43.4 ± 1.2 | 289.3 ± 23.6 | 3.9 ± 0.2 |
| Mean ± sem | 19.4 ± 0.3 | 57.4 ± 1.0 | 72.9 ± 1.0 | 43.8 ± 1.7 | 328.2 ± 21.2 | 5.43 ± 0.6 |
| Trifluorothymidine-Resistant Clones | ||||||
| TK6-Clone T2 | 21.3 ± 0.7 | 42.8 ± 4.0 | 84.8 ± 2.5 | 0.5 ± 4.0 | 5.0 ± 1.8 | 3.8 ± 0.1 |
| TK6-Clone T4 | 18.3 ± 0.0 | 47.9 ± 1.8 | 87.3 ± 0.0 | 0.8 ± 1.8 | NMA | 3.3 ± 0.2 |
| TK6-Clone T13 | 21.8 ± 0.2 | 43.6 ± 1.7 | 62.8 ± 10.1 | 2.4 ± 0.8 | NMA | 8.9 ± 0.3 |
| TK6-Clone T14 | 20.6 ± 0.1 | 42.5 ± 1.7 | 58.4 ± 6.5 | 2.4 ± 0.9 | NMA | 11.5 ± 0.3 |
| Mean ± sem | 20.5 ± 0.8 | 44.2 ± 1.3 | 73.3 ± 7.4 | 1.5 ± 0.5 | 1.3 ± 1.3 | 6.9 ± 2.0 |
TK6 is the parent cell line, TK6-T2 and TK6-T4 are independent TRIFLUOROTHYMIDINE-resistant clones, and the remaining cells are independently isolated TK6 clones.
fmol dTMP/30’/μg protein
107 sites/ug protein
No measureable activity
3.2 Absolute requirement of TK1 for concentration of FLT in cells
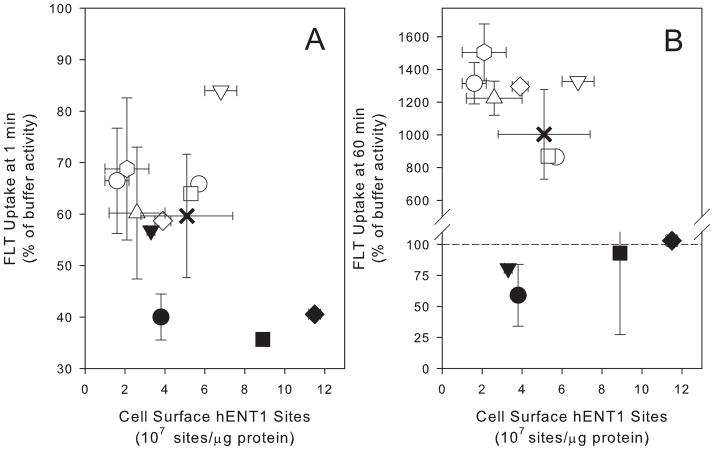
FLT uptake following 1min incubation in buffer was used to estimate initial rate of FLT uptake in the 8 TK6 clones. Trifluorothymidine-resistant (TK1-deficient) clones had lower levels of uptake at 1min as compared to the TK1-proficient clones (Fig 1A). This was likely due to the rapid metabolism in TK1-proficient cells (Table 2). There was no statistically significant (P=0.98, r2=0.10) correlation between the number of hENT1 sites and 1min FLT uptake levels for the 8 clones characterized. When the four TK1-proficient clones were evaluated separately, the relationship between the number of hENT1 sites and 1min FLT uptake levels, while not significant (P=0.09), showed a stronger correlation (r2 = 0.75) suggesting a possible relationship that might be revealed by analyzing a larger data set. Therefore an additional 3 clones were isolated and evaluated. There was no correlation between hENT1 and FLT uptake in this larger group (P=0.84, r2=0.11).
Figure 1.
Comparison between cell surface hENT1 sites intracellular concentration of FLT after (A) 1min and (B) 60min incubation with buffer containing [H-3] FLT. Uptake is expressed as a percentage of the activity in an equivalent volume of buffer. TK6 parent (x), TK1-proficient clones of TK6 (○),TK1-deficient TK6 clones (●).
Table 2.
Metabolites of FLT
| Percent of Total Activity in Cells | Total Activity in Cells (DPM/106 Cells) | ||||
|---|---|---|---|---|---|
| Influx min | FLT | FLT-MP | FLT-DP | FLT-TP | |
| 1 | 51.4 | 48.6 | 0 | 0 | 5663.6 ± 321.4 |
| 30 | 2.1 | 56.0 | 5.0 | 36.9 | 42358.4 ± 12023.3 |
| 60 | 5.3 | 44.4 | 6.5 | 43.7 | 48628.0 ± 11411.8 |
| 90 | 7.0 | 32.2 | 7.7 | 53.2 | 25003.0 ± 13803.4 |
| 120 | 8.1 | 25.4 | 8.5 | 58.0 | 34593.5 ± 7660.4 |
| Efflux min | FLT | FLT-MP | FLT-DP | FLT-TP | |
| 0 | 5.3 | 44.4 | 6.5 | 43.7 | 45689.9 ± 13501.1 |
| 30 | 2.1 | 31.0 | 10.2 | 56.6 | 22098.3 ± 3870.3 |
| 60 | 2.8 | 26.6 | 7.9 | 62.7 | 16547.4 ± 5189.7 |
| 90 | 7.1 | 31.3 | 11.2 | 50.4 | 10560.4 ± 3749.7 |
| 120 | 4.6 | 31.4 | 8.5 | 55.5 | 6816.9 ± 1521.7 |
| Percent of Total Activity in Buffer | Total Activity in Buffer | ||||
| Efflux min | FLT | FLT-MP | FLT-DP | FLT-TP | |
| 120 | 49.0 | 51.0 | 0 | 0 | 89955.6 (89.6)1 |
| 1202 | 71.0 | 29.0 | 0 | 0 | 76463.2 (75.8)1 |
Percentage of total activity recovered from cells and efflux buffer.
Efflux measured in FLT-free buffer containing Cyclosporine A.
One hour incubation led to 8- to 15-fold concentration of FLT in TK1-proficient clones as compared to buffer levels (Fig 1B). Trifluorothymidine-resistant clones did not concentrate FLT (Fig 1B). There was no significant correlation between hENT1 levels and 60min uptake values for TK1-proficient and deficient clones evaluated together (P=0.46, r2=0.31) or the TK1-proficient clones evaluated separately (P=0.92, r2=0.20).
3.3 Na-dependent transport dominated in TK6
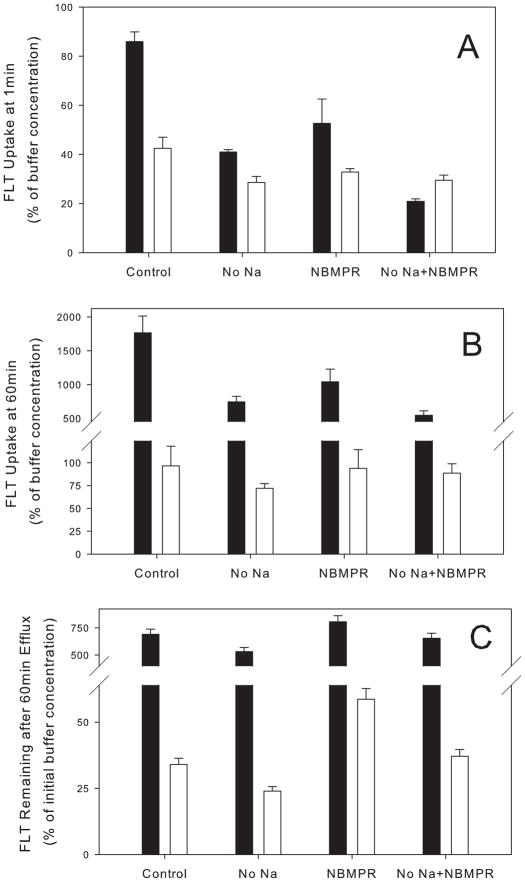
In separate experiments, 1min FLT uptake was measured in buffer containing no sodium in order to measure the contribution of concentrative transporters, and in buffer containing 100μM NBMPR to measure the contribution of equilibrative transporters to overall transport. Only TK6 and TK6-2, a TK1-deficient clone, were analyzed. Elimination of sodium inhibited 52% of FLT uptake in TK6 while 100μM NBMPR reduced FLT transport by 39%. For the TK1-deficient clone, TK6-2, elimination of sodium from the buffer inhibited FLT uptake by 33% while NBMPR inhibited uptake by 23% (Fig 2A). The difference between the two clones likely reflected the metabolism that occurs rapidly in TK6 cells. The results were consistent with a greater contribution of concentrative transporters in defining initial rates of FLT uptake in TK6 cells. Incubation in sodium-free buffer supplemented by 100μM NBMPR reduced FLT uptake by 76% in TK6 cells and by 30% in TK6-2 consistent with FLT diffusion or residual transporter activity.
Figure 2.
Inhibition of [H-3]FLT uptake in TK6 cells (filled bar) and in TK6-2, a TK1-deficient TK6 clone (open bar) by sodium-free buffer ± 100 μM NBMPR. Results are expressed as a percentage of the radioactivity normalized to the initial FLT levels in an equivalent volume of buffer. (A) 1min uptake values. (B) 60min uptake values. (C) FLT remaining in cells after a 60min efflux into FLT-free buffer.
The transport inhibition studies were expanded to address the role of nucleoside transporters in modulating peak FLT intracellular concentrations by analysis of uptake after 1h incubation with FLT (Fig 2B). Here the magnitude of inhibition in TK6 cells associated with eliminating sodium or incubating with NBMPR was similar to the 1min time points (58% and 41%, respectively). Incubation in sodium-free buffer supplemented by 100μM NBMPR reduced FLT uptake by 69%. In the TK6-2 clones, elimination of sodium reduced FLT uptake by 25% in while incubation in NBMPR had no measurable effect on 1h FLT levels. Incubation in sodium-free buffer supplemented by 100μM NBMPR reduced FLT uptake in TK6-2 clones by less than 10%. Presumably, the smaller effect of transport inhibition in TK6-2 clones were a reflection of the inability of FLT to concentrate in these TK1-deficient clones; Diffusion over 1h reduced the effect of transporter inhibition.
3.4 Transporter mediated efflux of FLT
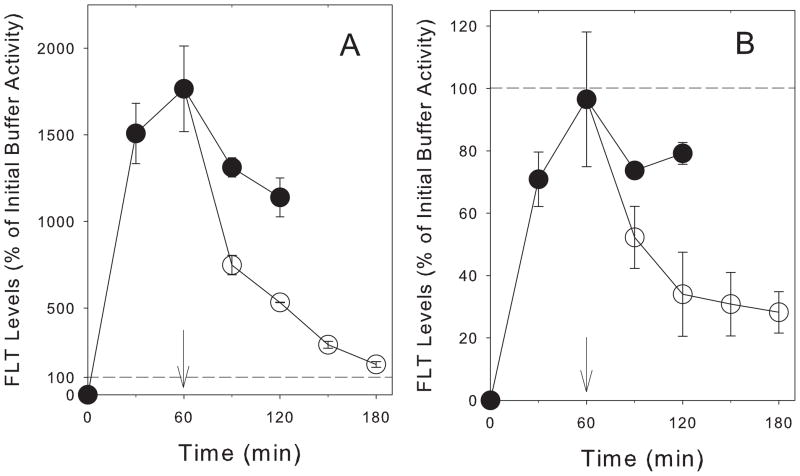
For TK6, there was an initial phase of increased intracellular tracer uptake for 1h (Fig 3A). Longer incubation in buffer containing FLT was associated with a reduction in intracellular radioactivity. After 2h incubation, the intracellular radioactivity was nearly 30% lower than the 1h concentration. The decline was not associated with any loss of membrane integrity as measured by Trypan blue exclusion studies and there was no reduction in activity for the TK1-deficient TK6-2 clones (Fig 3B). In contrast to the FLT observations, levels of radiolabeled thymidine and metabolites continued to increase at exponential rates (Fig 3A).
Figure 3.
Time-dependent changes in intracellular levels of radioactivity in (A) TK6 cells and (B) TK1-deficient TK6-2 cells incubated for up to 120min in [H-3]FLT (●), cells preloaded by incubation in [H-3]FLT for 60min, then washed and cultured in [H-3]FLT-free buffer for up to an additional 120 min (○), and cells incubated with [H-3]TdR (x). Solid line: Average initial [H-3]FLT activity in equivalent volume of buffer ± 1sd around average (dashed lines).
Efflux was measured in cells incubated in FLT for 1h and then maintained in nucleoside-free buffer for up to 2h. Under these conditions, the intracellular radioactivity in TK6 cells declined exponentially to reach levels close to the initial buffer by 2h after incubation in FLT-free buffer (Figure 3A). With the TK6-2 clone (Fig 3B) intracellular levels of FLT declined at a much slower rate.
Part of the efflux was mediated by NBMPR-sensitive transport. Incubating TK6 cells in FLT-free buffer containing NBMPR lead to increased FLT concentrations remaining in cells (Fig 2C). Incubation of TK6 cells in buffer containing no sodium increased FLT efflux slightly. Larger relative effects were observed in the TK6-2 cells. NBMPR doubled the FLT remaining in cells after a 1h efflux time.
3.5 Nucleoside metabolism
There was rapid metabolism of FLT to FLT-MP in TK6 cells (Table 2); half of the intracellular radioactivity after 1min incubation was FLT-MP. From 30min-120min, the relative proportion of intracellular FLT levels increased slowly from 2% to 8%. The proportion of intracellular activity associated with FLT-DP mirrored the increase in FLT while FLT-TP proportions increased in parallel to FLT-DP. The ratio of FLT-TP to FLT-DP was constant at 6.94 ± 0.15 over the 30–120min incubation. The ratio of FLT-TP to FLT was constant at 10.14 ± 2.48 over the same time. In contrast, FLT-MP proportions declined during this time from 56% to 25%.
Changes in the levels of different metabolites were measured in TK6 cells preloaded with FLT for 1h prior to incubation in FLT-free buffer (Table 2). Over the 120min incubation in FLT-free buffer, the total activity in cells declined by 85%. During this time, there was essentially no change in the relative proportions of intracellular FLT and its metabolites. The mean (± sem) ratio of FLT-TP to FLT-DP at 6.13 ± 0.72 was not significantly different from the influx values (p=0.35). The corresponding ratios for FLT-TP to FLT-MP and FLT were 1.89 ± 0.16 and 17.13 ± 4.57, respectively.
The buffer was analyzed for different FLT species that came out of the cells during the 120min efflux. About 50% of the total recovered activity was FLT and the remainder was FLT-MP. Incubation of cells with cyclosporine A, an inhibitor of P-glycoprotein [17], reduced the relative proportion of FLT-MP in buffer to 29% of the total activity recovered from the buffer with the remaining activity being FLT.
4. Discussion
While TK1 activity is considered to be the rate limiting factor driving FLT uptake and retention in tumors, recent publications suggest a role for nucleoside transporters as well [7, 10, 18]. Nucleoside transporter levels vary across different tissues and between different tumors [19–22]. They also can differ in cycling and non-cycling cells [10, 23, and 24]. In a previous publication [10], we reported that hENT1 levels increased as cells from the human tumor cell line A549 moved from a non-proliferative state into active cell growth. In the present study we examined whether there might be a general link between hENT1 levels and different cell growth parameters in actively growing cells. In our study, we took advantage of clonal variation in hENT1 levels to test this relationship. We observed no correlation between hENT1 levels and any cell growth characteristics suggesting no strong link between hENT1 levels and cell growth characteristics in actively-growing cells. Differences noted by us and others [10, 23, 24] might reflect movement of cells from a G0-state into active growth rather than changes in overall growth rate.
We also examined the relationship between hENT1 levels and FLT uptake in TK6 clones. Paproski et al. [8] had reported that reducing hENT gene expression in A549 cells reduced FLT uptake. We observed no correlation between hENT1 levels and either 1min or 60min uptake levels (Fig 1). The difference between our results and those of Paproski et al. [8] were probably due to the different repertoire of transporters used by A549 and TK6 cells. The predominant mechanism for FLT transport in A549 cells was reported to be by hENT1 [10] while that for TK6 was hCNT (Fig 2). As Paproski et al. [8] suggested, variations in hENT1 levels should have less effect on FLT uptake in cells where concentrative transport predominates. Our studies confirmed the absolute requirement of TK1 in concentrating FLT in cells. TK1-deficient clones did not concentrate FLT above the buffer level (Figs 1, 2).
The kinetics of uptake was qualitatively different in the TK6 cells from previously reported results in A549 human tumor cells [10, 25]. In A549 cells under exponential growth conditions, FLT activity levels increased rapidly up to about 30min and then increased more slowly for the second hour. In contrast, FLT activity in TK6 cells peaked after 60min in FLT-containing buffer (Fig 3A) and then declined slightly over the next 60min. The decline was due to a loss in activity associated with FLT-MP without compensatory changes in FLT or other FLT metabolites (Table 2). The effect was not observed with thymidine where intracellular activity associated with thymidine continued to increase over the 2h incubation.
To understand the basis for the loss in FLT-MP, we examined efflux of activity from cells incubated for 1h in buffer containing FLT, followed by incubation in FLT-free buffer for up to 2h. Removing FLT from the buffer led to a rapid loss of activity from cells. There was about a 70% loss of radioactivity from cells 60min after the transfer of cells to FLT-free buffer (Figure 3). By 2h, about 85% of the activity had been lost from cells. For comparison, Grierson et al. [25] reported a 50% drop in intracellular radioactivity after a 60min incubation of A549 cells in FLT-free medium.
In A549 cells, the loss of activity when cells were transferred to FLT-free medium was associated with reductions in FLT and FLT-MP and increases in FLT-TP [25]. About 80% of the activity recovered in the buffer was FLT with the remaining 20% being FLT-MP. In that publication [25], Grierson et al suggested that the primary factor underlying loss of activity was retrograde metabolism of FLT-MP back to the nucleoside, followed by its diffusion out of the cell. As for the observation of FLT-MP in the efflux material, Grierson et al. presented evidence to suggest that it might reflect some nucleotide transport process.
For TK6, the proportions of intracellular FLT and FLT metabolites remained constant over the 2h washout (Table 2), consistent with a loss of all four species over time. The primary species observed in the efflux buffer were FLT (49%) and FLT-MP (51%). The results are consistent with what Grierson et al. observed in A549 cells; there is both a retrograde metabolism of FLT-MP back to the nucleoside, followed by its diffusion out of the cell and a nucleotide transporter to pump FLT-MP out of cells. This diffusion of FLT out of TK6 cells was mediated in part by hENT since NBMPR inhibited FLT loss (Figure 2C). Guo et al. [26] reported that the multi-drug resistance protein MRP8, an ATP-binding cassette (ABC) transporter associated with p-glycoprotein, mediated the efflux of 5’-fluoro-2’-deoxyuridine monophosphate. Incubation of TK6 cells with cyclosporine A, an inhibitor of the ATPase activity of p-glycoprotein [17] reduced the relative levels of FLT-MP in buffer suggesting that MRP8 or a similar protein might mediate efflux of FLT-MP out of cells. Interestingly, cyclosporine A had little effect on overall activity lost from cells which would be consistent with retrograde metabolism of FLT-MP into FLT compensating for reduced FLT-MP efflux.
While the intracellular activity declined in TK6 cells incubated in FLT-free buffer, the relative proportions of FLT and its metabolites did not change. This observation supports retrograde metabolism of FLT-TP and FLT-DP in TK6 cells. There are a number of enzymatic activities that have been reported to catabolize deoxyuridine and thymidine triphosphates [27–29] that could account for the observed loss of FLT-TP. As the ratios of FLT-TP to FLT-DP were constant and the same both in the presence and absence of FLT in buffer, regulation of nucleotide pool concentrations might underlie the retrograde metabolism of these two nucleotide species.
The measurements of FLT uptake, metabolism, and efflux from TK6 cells suggest that it might not be easy to generalize FLT results from one tumor to another. While TK1 is an absolute requirement for FLT uptake, there are other activities such as nucleoside and nucleotide transporters as well as retrograde metabolism that should influence the kinetics of FLT uptake and loss. They might explain some of the FLT uptake variation reported for different tumor cell lines [30, 31]. Understanding the role of these additional non-TK1-related variables on FLT levels in vivo is needed to build confidence in our interpretation of clinical scans.
5. Conclusions
Unlike A549 cells, initial rates of FLT uptake in TK6 cells were a function of both concentrative and equilibrative transporters. Concentration of FLT was primarily dependent on TK1 activity. However, retention of FLT and FLT metabolites was a function of nucleoside and nucleotide efflux as well as retrograde metabolism of FLT nucleotides.
Acknowledgments
This work was supported by grant CA118130 from the National Institutes of Health. The authors acknowledge the help and advice of Drs. Ken Krohn and Jash Unadkat, both of the University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krohn KA, Mankoff DA, Eary JF. Imaging cellular proliferation as a measure of response to therapy. J Clin Pharmacol. 2001;(Suppl):96S–103S. [PubMed] [Google Scholar]
- 2.Bading JR, Shahinian AH, Bathija P, Conti PS. Pharmacokinetics of the thymidine analog 2'-fluoro-5-[(14)C]-methyl-1-beta-D-arabinofuranosyluracil ([(14)C]FMAU) in rat prostate tumor cells. Nucl Med Biol. 2000;27:361–8. doi: 10.1016/s0969-8051(00)00100-1. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Samuelsson L, Bergstrom M, Sato K, Fasth KJ, Langstrom B. Rat studies comparing 11C-FMAU, 18F-FLT, and 76Br-BFU as proliferation markers. J Nucl Med. 2002;43:1688–98. [PubMed] [Google Scholar]
- 4.Shields AF. PET Imaging with (18)F-FLT and Thymidine Analogs: Promise and Pitfalls. J Nucl Med. 2003;44:1432–4. [PubMed] [Google Scholar]
- 5.Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, et al. Imaging Proliferation in Lung Tumors with PET: (18)F-FLT Versus (18)F-FDG. J Nucl Med. 2003;44:1426–31. [PubMed] [Google Scholar]
- 6.Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–7. [PubMed] [Google Scholar]
- 7.Paproski RJ, Ng AM, Yao SY, Graham K, Young JD, Cass CE. The role of human nucleoside transporters in uptake of 3'-deoxy-3'-fluorothymidine. Mol Pharmacol. 2008;74:1372–80. doi: 10.1124/mol.108.048900. [DOI] [PubMed] [Google Scholar]
- 8.Paproski RJ, Wuest M, Jans H-S, Graham K, Gati WP, McQuarrie S, et al. Biodistribution and Uptake of 3'-Deoxy-3'-Fluorothymidine in ENT1-Knockout Mice and in an ENT1-Knockdown Tumor Model. J Nucl Med. 51:1447–55. doi: 10.2967/jnumed.110.076356. [DOI] [PubMed] [Google Scholar]
- 9.Paproski RJ, Young JD, Cass CE. Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3'-deoxy-3'- fluorothymidine. Biochem Pharmacol. 79:587–95. doi: 10.1016/j.bcp.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Plotnik DA, Emerick LE, Krohn KA, Unadkat JD, Schwartz JL. Different modes of transport for 3H-thymidine, 3H-FLT, and 3H-FMAU in proliferating and nonproliferating human tumor cells. J Nucl Med. 51:1464–71. doi: 10.2967/jnumed.110.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy JA, Virolainen M, Defendi V. Human lymphoblastoid lines from lymph node and spleen. Cancer. 1968;22:517–24. doi: 10.1002/1097-0142(196809)22:3<517::aid-cncr2820220305>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Liber HL, Thilly WG. Mutation assay at the thymidine kinase locus in diploid human lymphoblasts. Mutat Res. 1982;94:467–85. doi: 10.1016/0027-5107(82)90308-6. [DOI] [PubMed] [Google Scholar]
- 13.Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988;263:8350–8. [PubMed] [Google Scholar]
- 14.Hruby DE, Ball LA. Cell-free synthesis of enzymatically active vaccinia virus thymidine kinase. Virology. 1981;113:594–601. doi: 10.1016/0042-6822(81)90187-2. [DOI] [PubMed] [Google Scholar]
- 15.van der Wilt CL, Backus HH, Smid K, Comijn L, Veerman G, Wouters D, et al. Modulation of both endogenous folates and thymidine enhance the therapeutic efficacy of thymidylate synthase inhibitors. Cancer Res. 2001;61:3675–81. [PubMed] [Google Scholar]
- 16.Huang D, Zhang Y, Chen X. Analysis of intracellular nucleoside triphosphate levels in normal and tumor cell lines by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:101–9. doi: 10.1016/s1570-0232(02)00780-8. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Kokubu N, Charnick SB, Naito M, Tsuruo T, Cohen D. Interaction of cyclosporin derivatives with the ATPase activity of human P-glycoprotein. Br J Pharmacol. 1997;122:241–8. doi: 10.1038/sj.bjp.0701377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perumal M, Pillai RG, Barthel H, Leyton J, Latigo JR, Forster M, et al. Redistribution of nucleoside transporters to the cell membrane provides a novel approach for imaging thymidylate synthase inhibition by positron emission tomography. Cancer Res. 2006;66:8558–64. doi: 10.1158/0008-5472.CAN-06-0898. [DOI] [PubMed] [Google Scholar]
- 19.Belt JA, Marina NM, Phelps DA, Crawford CR. Nucleoside transport in normal and neoplastic cells. Adv Enzyme Regul. 1993;33:235–52. doi: 10.1016/0065-2571(93)90021-5. [DOI] [PubMed] [Google Scholar]
- 20.Cass CE, Young JD, Baldwin SA, Cabrita MA, Graham KA, Griffiths M, et al. Nucleoside transporters of mammalian cells. Pharm Biotechnol. 1999;12:313–52. doi: 10.1007/0-306-46812-3_12. [DOI] [PubMed] [Google Scholar]
- 21.Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol. 2001;18:53–63. [PubMed] [Google Scholar]
- 22.Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5:63–84. doi: 10.2174/1389200043489162. [DOI] [PubMed] [Google Scholar]
- 23.del Santo B, Valdes R, Mata J, Felipe A, Casado FJ, Pastor-Anglada M. Differential expression and regulation of nucleoside transport systems in rat liver parenchymal and hepatoma cells. Hepatology. 1998;28:1504–11. doi: 10.1002/hep.510280609. [DOI] [PubMed] [Google Scholar]
- 24.Soler C, Garcia-Manteiga J, Valdes R, Xaus J, Comalada M, Casado FJ, et al. Macrophages require different nucleoside transport systems for proliferation and activation. Faseb J. 2001;15:1979–88. doi: 10.1096/fj.01-0022com. [DOI] [PubMed] [Google Scholar]
- 25.Grierson JR, Schwartz JL, Muzi M, Jordan R, Krohn KA. Metabolism of 3'-deoxy-3'-[F-18]fluorothymidine in proliferating A549 cells: validations for positron emission tomography. Nucl Med Biol. 2004;31:829–37. doi: 10.1016/j.nucmedbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Kotova E, Chen ZS, Lee K, Hopper-Borge E, Belinsky MG, et al. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2',3'-dideoxycytidine and 9'-(2'-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278:29509–14. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 27.Rampazzo C, Gallinaro L, Milanesi E, Frigimelica E, Reichard P, Bianchi V. A deoxyribonucleotidase in mitochondria: involvement in regulation of dNTP pools and possible link to genetic disease. Proc Natl Acad Sci U S A. 2000;97:8239–44. doi: 10.1073/pnas.97.15.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams MV, Holliday J, Glaser R. Induction of a deoxyuridine triphosphate nucleotidohydrolase activity in Epstein-Barr virus-infected cells. Virology. 1985;142:326–33. doi: 10.1016/0042-6822(85)90341-1. [DOI] [PubMed] [Google Scholar]
- 29.Schultes BC, Fischbach E, Dahlmann N. Purification and characterization of two different thymidine-5'-triphosphosphate-hydrolysing enzymes in human serum. Biol Chem Hoppe Seyler. 1992;373:237–47. doi: 10.1515/bchm3.1992.373.1.237. [DOI] [PubMed] [Google Scholar]
- 30.Toyohara J, Waki A, Takamatsu S, Yonekura Y, Magata Y, Fujibayashi Y. Basis of FLT as a cell proliferation marker: comparative uptake studies with [3H]thymidine and [3H]arabinothymidine, and cell-analysis in 22 asynchronously growing tumor cell lines. Nucl Med Biol. 2002;29:281–7. doi: 10.1016/s0969-8051(02)00286-x. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz JL, Tamura Y, Jordan R, Grierson JR, Krohn KA. Monitoring tumor cell proliferation by targeting DNA synthetic processes with thymidine and thymidine analogs. J Nucl Med. 2003;44:2027–32. [PubMed] [Google Scholar]