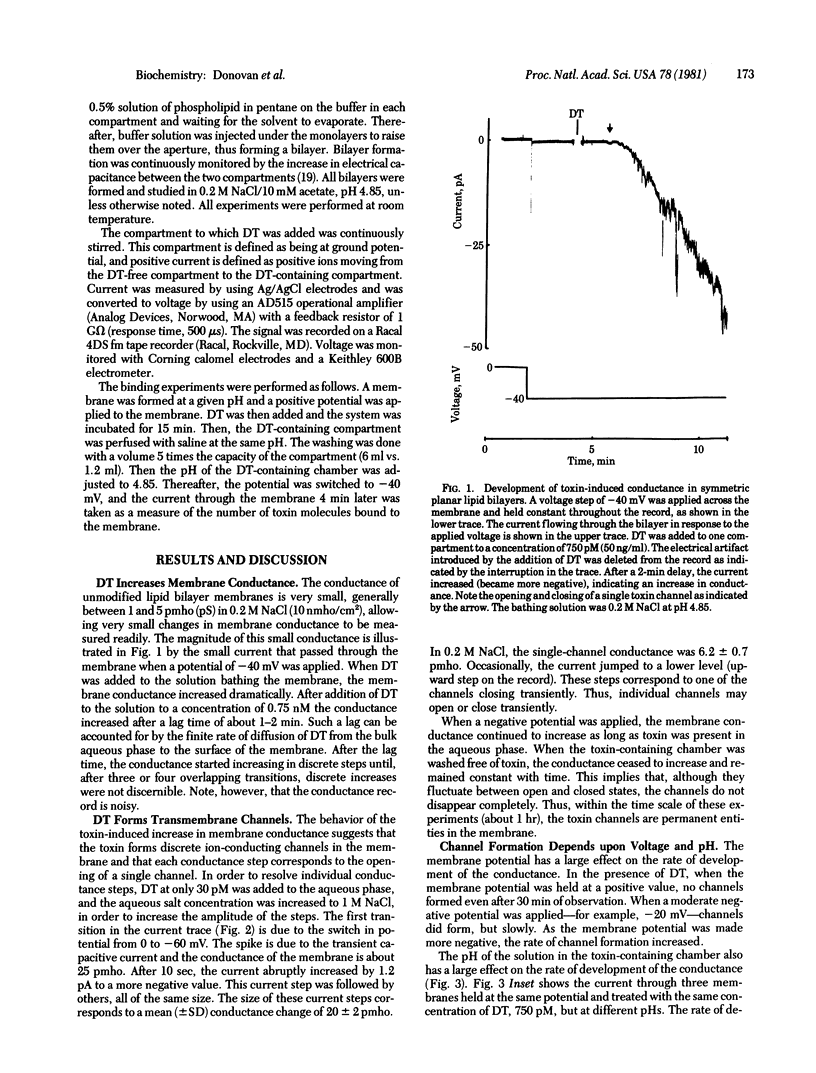
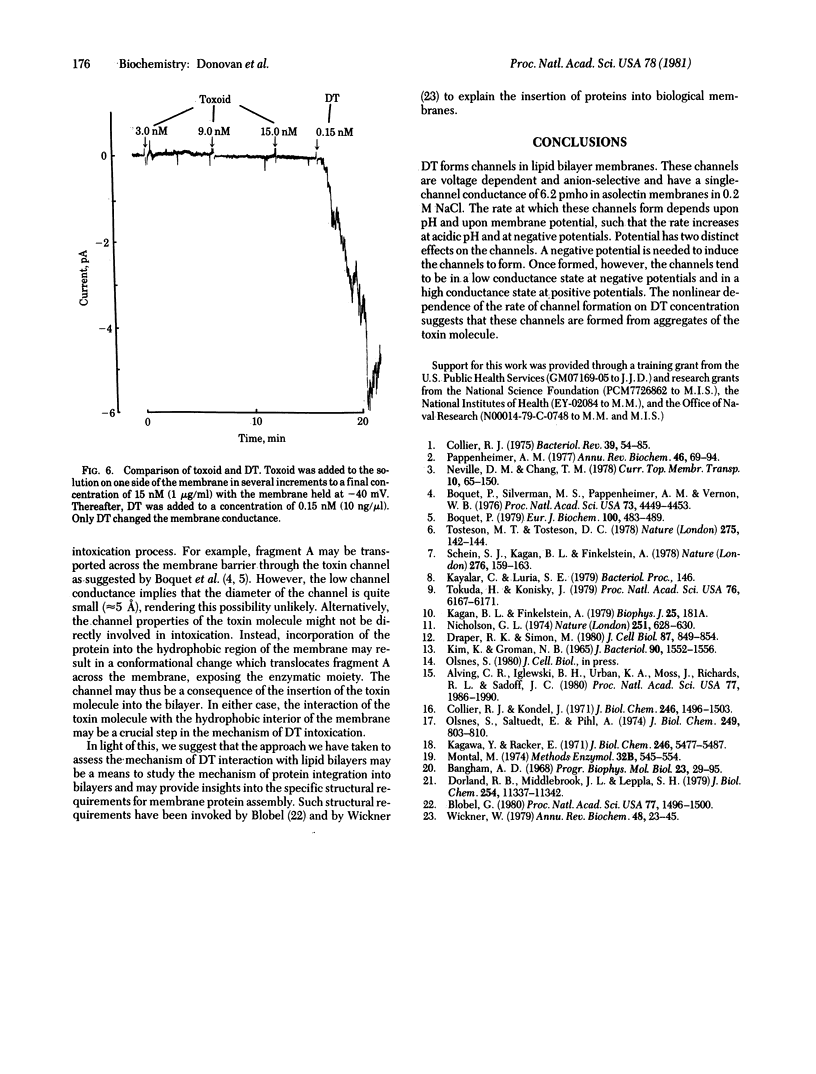
Abstract
When exposed to a lipid bilayer, diphtheria toxin binds to it and forms transmembrane, voltage-dependent, anion-selective channels. The mean (+/- SD) conductance of these channels in asolectin membranes is 6.2 +/- 0.7 pmho (pS) in 0.2 M NaCl and 20 +/- 2 pmho in 1.0 M NaCl. The rate of channel formation depends on the pH in the toxin-containing compartment; it is very low at pH greater than 5.0 and increases abruptly as the pH decreases from 4.9 to 4.0. Binding of toxin to the membrane is also pH dependent, being unmeasurable at pH 7 and increasing monotonically with decreasing pH. The rate of channel formation depends upon membrane potential as well; channels form only at negative potentials. These channels are permanent in the time scale of the experiments (about 1 hr). The membrane conductance caused by the channels is also voltage dependent, being constant at positive potentials and decreasing at negative potentials. Hence, the current-voltage curve is linear at positive potentials and sublinear at negative potentials. The conditions necessary for insertion of toxin into the bilayer and formation of channels are similar to those that prevail inside the lysosome. Thus, these results lend credence to the idea that toxin enters the cytoplasm from the lysosomal compartment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Iglewski B. H., Urban K. A., Moss J., Richards R. L., Sadoff J. C. Binding of diphtheria toxin to phospholipids in liposomes. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1986–1990. doi: 10.1073/pnas.77.4.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A. D. Membrane models with phospholipids. Prog Biophys Mol Biol. 1968;18:29–95. doi: 10.1016/0079-6107(68)90019-9. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P. Interaction of diphtheria toxin fragments A, B and protein crm 45 with liposomes. Eur J Biochem. 1979 Oct 15;100(2):483–489. doi: 10.1111/j.1432-1033.1979.tb04192.x. [DOI] [PubMed] [Google Scholar]
- Boquet P., Silverman M. S., Pappenheimer A. M., Jr, Vernon W. B. Binding of triton X-100 to diphtheria toxin, crossreacting material 45, and their fragments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4449–4453. doi: 10.1073/pnas.73.12.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Receptor-mediated internalization and degradation of diphtheria toxin by monkey kidney cells. J Biol Chem. 1979 Nov 25;254(22):11337–11342. [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Groman N. B. In vitro inhibition of diphtheria toxin action by ammonium salts and amines. J Bacteriol. 1965 Dec;90(6):1552–1556. doi: 10.1128/jb.90.6.1552-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Ultrastructural analysis of toxin binding and entry into mammalian cells. Nature. 1974 Oct 18;251(5476):628–630. doi: 10.1038/251628a0. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Effect of colicins Ia and E1 on ion permeability of liposomes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6167–6171. doi: 10.1073/pnas.76.12.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Tosteson D. C. Bilayers containing gangliosides develop channels when exposed to cholera toxin. Nature. 1978 Sep 14;275(5676):142–144. doi: 10.1038/275142a0. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]



