Abstract
Rationale
In addition to its rewarding actions, cocaine has profound negative effects that are unmasked as the rewarding impact of the drug fades. While much is known about the neurobiology of cocaine reward, the mechanisms underlying the negative actions of the drug remain unclear.
Objectives
The current study investigates the role of three brain regions each implicated in the modulation of negative affective states—the bed nucleus of the stria terminalis (BNST), the central (CeA), and the basolateral (BLA) nucleus of the amygdala.
Methods
The dual actions of cocaine were assessed using a runway self-administration procedure in which rats exhibit both approach to and avoidance of a goal box associated with cocaine administration (retreat behaviors). Here, rats ran a straight alley once/day for i.v. cocaine (1.0 mg/kg/injection) over 14 days during which the BNST, CeA, or BLA was inactivated via bilateral intracranial infusions of lidocaine (0 or 20 μg/0.5 μl/side) administered 15 min prior to testing. The impact of lidocaine on spontaneous locomotor activity was also assessed to rule out nonspecific actions of the treatments.
Results
Control animals running for cocaine developed the expected pattern of approach–avoidance retreat behavior. Inactivation of the BNST attenuated such behavior, BLA inactivation had no appreciable effects, and CeA inactivation produced intermediate and more variable results. Locomotor activity was unaffected by any of the treatments.
Conclusions
These data suggest that the BNST and to a lesser extent the CeA, but not the BLA, play a role in mediating the opponent-process actions of self-administered cocaine.
Keywords: Drug reinforcement, Self-administration, Operant runway, Extended amygdala, Anxiety
Introduction
Human drug users report that the pleasure produced by cocaine is typically followed by feelings of anxiety, dysphoria, and craving (Anthony et al. 1989; Resnick et al. 1977; Williamson et al. 1997; Rohsenow et al. 2007). Cocaine’s negative effects have also been reported after acute administration in animal studies (Blanchard et al. 1999; Rogerio and Takahashi 1992; Yang et al. 1992; Ettenberg et al. 1999). The presence of these dual and opposing consequences suggests that both positive and negative reinforcement processes likely motivate cocaine self-administration. Indeed, Zernig et al. (2007) have suggested that cocaine’s aversive action may serve as a self-regulating factor that limits the escalation of psychostimulant consumption compared to that observed with opioid drugs. The current study was therefore devised to investigate the neuronal substrates underlying cocaine’s dual actions using a model of drug seeking that is sensitive to both the positive and negative effects of the drug in the same animal on the same trial (Ettenberg 2004; 2009).
Animals running an alley once a day for intravenous (i.v.) cocaine develop over trials a progressive tendency to quickly approach, but then stop at, the goal box entryway and retreat back toward the start box (Ettenberg and Geist 1991, 1993). This unique pattern of “retreat” behavior has been shown to reflect an approach–avoidance conflict stemming from mixed positive (rewarding) and negative (anxiogenic) associations with the cocaine-paired goal box (Ettenberg and Geist 1991; Geist and Ettenberg 1997; Ettenberg et al. 1999; Guzman and Ettenberg 2007; see review by Ettenberg 2004). Here, we investigated the functional roles of three brain regions in modulating the negative/aversive actions of cocaine by examining the development of cocaine-induced approach–avoidance retreat behaviors in animals with lidocaine-induced inactivation of the bed nucleus of the stria terminalis (BNST), the central nucleus of the amygdala (CeA), or the basolateral nucleus of the amygdala (BLA). All three regions contain dopamine and norepinephrine synapses (Freedman and Cassell 1994; Forray et al. 1997; Asan 1998; Young and Rees 1998) where cocaine acts to inhibit the activity of the presynaptic transporter (Richelson and Pfenning 1984; Rothman et al. 2001). All three exhibit elevated levels of the stress neuropeptide corticotropin-releasing factor (CRF) in response to cocaine (Mello and Mendelson 1997; Koob 1999; Corominas et al. 2010). All are responsive to negative emotional, anxiogenic, and/or fearful stimuli (Hale et al. 2008; Walker and Davis 2008; de la Mora et al. 2010; Tanimoto et al. 2003), and each has been implicated in the aversive effects of cocaine withdrawal (Koob 2008; Smith and Aston-Jones 2008; Erb 2010). The goal of the present research was therefore to assess the putative role of the BNST, CeA, and BLA in the opponent-process actions of acute cocaine administration as reflected by the development of approach–avoidance retreat behaviors in animals running a straight alley for i.v. cocaine.
Materials and methods
Subjects
The subjects were 93 male Sprague–Dawley rats (Charles River Labs, Hollister, CA) weighing 300–350 g at the time of surgery. Rats were individually housed within a temperature-controlled (22°C) vivarium maintained on a 12-h light/dark cycle (lights on at 0800 hours) and had ad libitum access to both food and water throughout the experiment. All methods were conducted in strict adherence to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCSB Institutional Animal Care and Use Committee.
Surgery
Subjects were deeply anesthetized by inhalation of isoflurane gas (4% for induction and 1.5–2.5% for maintenance) and each fitted with both an indwelling i.v. catheter (PE tubing; 0.3 mm inner diameter, 0.64 mm outer diameter; Dow Corning Corp., Midland, MI) and bilateral intracranial (i.c.) guide cannula (22 gauge, 9 mm; Plastics One, Roanoke, VA) aimed at either the BNST, CeA, or the BLA. One end of the i.v. catheter was inserted into the right jugular vein (secured in place by silk sutures) and the other end passed subcutaneously to a threaded cannula (Item 313G, Plastics One) that exited though a small hole on the midline of the animal’s back. The cannula was secured with dental cement to a 2-cm square of Mersilene surgical mesh (Bard; Warwick, RI) that was laid flat against the subdermal tissue
Stainless steel intracranial guide cannula was stereotaxically aimed 1 mm above the BNST, CeA, or BLA using the following coordinates relative to bregma and the skull surface (based on Paxinos and Watson 1998): for the BNST, AP −0.6, ML ±3.5, and DV −6.2 with a lateral inclination of 15°; for the CEA, AP −2.4, ML ±4.0, and DV −6.4; and for the BLA, AP −2.7, ML ±5.2, DV −6.8. Cannula were secured with dental cement and four stainless steel screws that anchored the assembly to the skull. An obdurator was placed into each guide cannula to seal the opening and thus maintain patency and reduce the risk of infection.
During surgery, each rat received an injection of atropine (0.2 mg/kg in 0.06 ml/kg i.m.) to reduce respiratory congestion, the non-opiate analgesic flunixin meglumine (FluMeglumine; Phoenix Pharmaceuticals, Belmont, CA) to relieve pain (1.3 mg/kg in 0.06 ml/kg s.c.), and an injection of 3.0 ml of 0.9% physiological saline (i.p.) to prevent dehydration. Additionally, i.v. infusion of the antibiotic ticarcillin–clavulanate (50 mg in 0.25 ml i.v.) followed by heparinized saline (6.0 IU heparin in 0.1 ml 0.9% physiological saline i.v.) helped defend against microbial infection and promote catheter patency. Following surgery, catheter patency was maintained via daily i.v. injections of ticarcillin–clavulanate (20 mg in 0.1 ml) followed by 0.1 ml of the heparinized saline. Catheter patencywas confirmed weekly by assessing the loss of the righting reflex following an i.v. injection of the fast-acting barbiturate, methohexital (2.0 mg in 0.1 ml). Animals were allowed to recover for 7 days after surgery prior to the start of behavioral testing.
Drugs
Cocaine hydrochloride (NIDA, Baltimore, MD) was dissolved in 0.9% physiological saline and delivered in a dose of 1.0 mg/kg i.v. in 0.1 ml over a period of 4.3 s via a 10-ml syringe seated in a motorized syringe pump (Razel Scientific Instruments, St. Albans, VT). This dose of cocaine was selected on the basis of previous work demonstrating its optimal reinforcing value in the runway as reflected by the fastest start and run times (e.g., Raven et al. 2000). Lidocaine (Sigma Aldrich, St. Louis, MO) was dissolved in a vehicle solution of artificial cerebrospinal fluid (CSF) composed of 147 mmol/l sodium chloride, 1.53 mmol/l calcium chloride, 2.68 mmol/l potassium chloride, and 2.1 mmol/l magnesium chloride dissolved in Nanopure water. All rats received the same dose of lidocaine (20 μg/0.5 μl/side) administered over 90 s via a 10-μl Hamilton syringe seated in a motorized syringe pump. This dose of lidocaine has been successfully employed to produce reversible functional lesions of the same target sites as those being examined in the current study (e.g., Liang et al. 2001; Waraczynski 2003; Woods and Ettenberg 2004). Vehicle injections involved the same volume of CSF fluid without lidocaine.
Runway apparatus
Two straight-arm runways (155 cm L×15 cm W×40 cm H) served as the test apparatus. On opposite ends of the straight alley were identically sized start and goal boxes (24×25×40 cm) separated from the runway by retractable doors. The floor of each apparatus was composed of small diameter steel rods aligned perpendicular to the side walls of the alley. Along the interior length of each alley were 13 infrared photodetector-emitter pairs positioned in the walls approximately 16 cm apart and 5 cm above the floor. Input from these photocells was fed through a custom ANY-maze interface (Stoelting Co., Wood Dale, IL) to a desktop computer that recorded the subjects’ location in the runway in real time throughout each trial.
Above and running along the entire length of each runway were two magnetic tracks positioned in parallel 3 cm apart. Positioned between the tracks was a flow-through plastic swivel (375-22PS; Instech Laboratories Inc., Plymouth Meeting, PA) that was connected via PE20 tubing to the animal’s i.v. catheter on one end, and to a cocaine-filled 10-ml syringe seated in a syringe pump on the other end. A plastic disc attached to the midsection of the swivel prevented it from falling through the opening between the two magnetic tracks. Affixed to the underside of the disc was a pot magnet whose polarity was arranged to repel the charge of the magnetic tracks. The resulting magnetic repulsion between the swivel and the tracks permitted the swivel to float slightly above the tracks thereby providing a low-friction mechanism that allowed the rat to move and turn freely throughout the alley (for a description of the runway apparatus, see Geist and Ettenberg 1990).
Locomotor apparatus
Locomotor activity was measured in 12 identical Plexiglas chambers (each 20 cm L×40 cm W×20 cm H; Kinder Scientific, San Diego, CA). Located 8 cm above the floor of each chamber were 15 infrared photodetector-emitter pairs evenly spaced along the long axis and 7 more along their narrow axis. Movement within the chamber produced infrared-beam interruptions that were recorded by a desktop computer running custom software (Kinder Scientific). Locomotor data (distances traveled) were collected in real time over 3-h test sessions.
Runway self-administration procedure
Rats were habituated to the runway on a single 10-min trial with the goal door closed. Runway testing began 24 h later. Each rat was infused i.c. with either lidocaine or the CSF vehicle delivered into the BNST. Infusions were accomplished with a 28-gauge internal cannula (Plastics One) that was inserted into and protruded 1.0 mm beyond the tip of the indwelling guide cannula. The infusion cannula was connected by PE tubing to a 10-μl syringe seated in a Razel syringe pump. The pump was then activated and infusions slowly applied over a 90-s period. An additional 60 s was provided to permit the solutions to diffuse away from the cannula tip before the internal cannula were removed. The animal was then returned to its home cage for 15 min, followed by a single runway trial for i.v. cocaine.
On each trial, the rat was connected to the drug delivery system by inserting the internal infusion cannula (which was attached by PE tubing to the flow-through swivel) into the guide cannula on the animal’s back. The rat was then placed into the start box and, 5 s later, the start door was opened and the trial initiated. Once the rat entered the goal box, the goal door was closed (to prevent retracing) and a single i.v. infusion of cocaine (1.0 mg/kg) was administered. After 5 min in the goal box, the animals was removed from the apparatus and returned to its home cage. Testing consisted of 14 single daily trials during which three dependent measures were recorded. Start latency—the time required for the animal to leave the start box once the start door was opened; run time—the time required for the rat to enter the goal box after it had left the start box; and retreats—the number of times an animal halted its forward motion, turned and retreated back at least the length of two photocells in the runway (approx. 30 cm).
Upon completion of the BNST experiment, a second experiment was conducted in which lidocaine or CSF was applied to the CeA of two new groups of animals. A third and final experiment was then conducted to investigate the impact of BLA inactivation in an additional set of animals. All procedures were conducted in an identical manner for all three experiments.
Locomotor procedure
One week following the completion of runway testing, the impact of lidocaine and CSF-vehicle infusions on spontaneous locomotor behavior was assessed (in the same subjects) to determine whether group differences in runway behavior could be accounted for by treatment-induced changes in the motor capacity of the animals. Testing was conducted in the activity apparatus (described above) over the course of two days 48 h apart during which the distance traveled by each subject (in centimeters) served as an index of locomotor activity. On each day, the animals were habituated to the chamber for 2 h after which they were removed from the apparatus and treated with lidocaine or vehicle. Half the animals received bilateral i.c. infusions of lidocaine (20 μg/0.5 μl/per side) into the BNST, CeA, or BLA and the remaining half an equal volume of the CSF vehicle. After 15 min (i.e., the same time course employed in the runway phase of the study), the animals were returned to their locomotor chamber for a 1-h test session. Day 2 of locomotor testing was conducted in the identical manner as day 1, except that those animals infused with i.c. lidocaine on day 1 now received infusions of CSF vehicle, and vice versa.
Histology
At the conclusion of each experiment, intracranial cannula placements were identified from 40-μm frozen coronal sections stained with cresyl violet and viewed under magnification.
Results
Start latency criterion
In traditional self-administration studies, animals that do not learn to lever press for the drug reinforcer are simply removed from the study. With the runway protocol, this screening is accomplished by examining the change in start latencies over trials. When the goal box contains positive (including mixed positive+negative) stimuli, start latencies decrease as trials progress (Ettenberg 2009). In the current study, those animals whose average start latency over the final three trials was not shorter (reflecting faster responding) than their average over the initial three trials were removed from the study on the basis that learning to associate the goal box with the reinforcing drug could not be verified. Based upon this criterion, 15 rats were removed from the study. Separate analyses of variance computed on start latency data from the remaining 78 rats confirmed that the latencies to initiate responding decreased (got faster) as trials progressed in each of the three experiments [BNST F(13, 195)=1.945, p<0.03; CeA F(13, 247)=2.28, p<0.01; BLA F(13, 208)=3.003, p<0.001].
Cannula placement
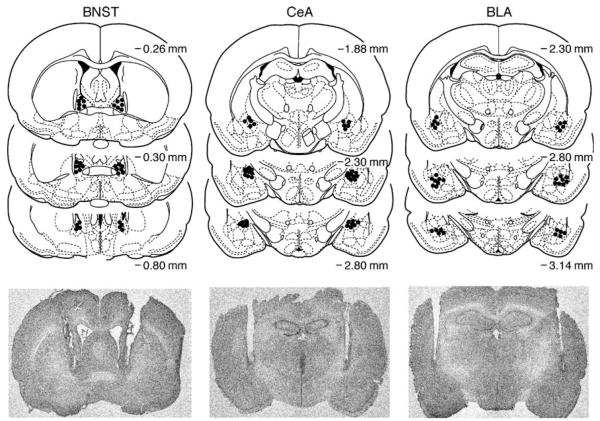
The results of histological analyses are depicted in Fig. 1. The figure includes a representative photomicrograph of bilateral cannula placements for a lidocaine-treated animal from each of the three targeted brain sites. Note that the internal injection cannula would have protruded 1.0 mm beyond the end of the cannula tracts that are visible in the figure. While there was some mild necrosis of the tissue around the tips of the cannula guides, this was true in each experiment for both the CSF vehicle and lidocaine groups and hence cannot explain differences in behavior due to treatment nor differences in the impact of inactivating the three target brain sites. Any significant damage beyond that shown in the figure resulted in removal of the subject from the data analysis. Additionally, because of the adjacency of the BLA and CeA, we conservatively removed subjects whose cannula was located at the border of the two regions. A total of 22 subjects were excluded from the study either due to indications of necrosis at the injection site or misplacement of one or both of the cannula guides. Ultimately, 56 rats successfully completed the study with sample sizes as follows: BNST+lidocaine, n=9; BNST+CSF, n=8; CeA+lidocaine, n=12, CeA+CSF, n=9; BLA+lidocaine, n=9; and BLA+CSF, n=9.
Fig. 1.
Histological analyses of cannula placements. The dots on the panels represent the location of bilateral infusion cannula tip placements within the BNST, CeA, or BLA plotted on coronal sections with distance from bregma (in millimeters) denoted beside each section. Adapted from Paxinos and Watson (1998). A representative photomicrograph identifies the location of the cannula tract for a lidocaine-treated animal in each group
Runway self-administration
Run times
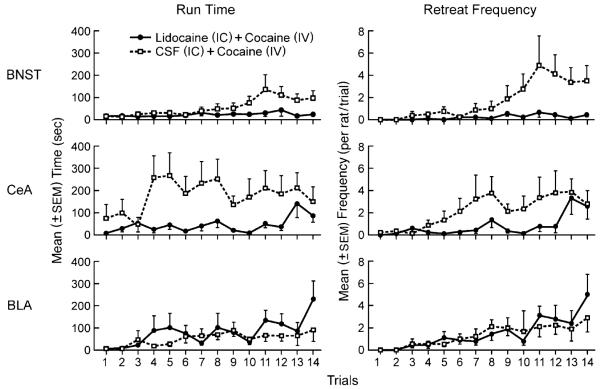
Figure 2 depicts the mean (±SEM) run times and retreat frequencies for the groups in each of the three experiments. A mixed three-factor (group×brain region×trial) ANOVA was computed on the run times (the left panels of Fig. 2), and another on the retreat scores (the right panels of Fig. 2). For run times, the ANOVA identified a main effect of trial [F(13, 650)=4.37, p<0.001]—i.e., when averaged across all animals, run times slowed as testing progressed. There was also a significant main effect of brain region [F(2, 50)=4.44, p<0.02], which appears to stem from the behavior of the CeA control (CSF) subjects who were slower to reach the goal box than rats in the BNST or BLA groups. The main effect for group (lidocaine vs CSF) was also significant when averaged across all three brain regions [F(1, 50)=5.10, p<0.03] and, or particular relevance to the current study, there was a significant group×brain region×trial interaction [F(26, 650)=1.56, p<0.04] and a group×trial interactions [F(2, 50)=5.58, p< 0.01] indicating that the lidocaine and CSF groups behaved differently across trials and brain regions. To explore the potential source(s) of these interactions, three separate two-factor (group×trial) ANOVAs were computed on the data depicted in each of the three left panels of Fig. 2. These analyses confirmed that the effects of lidocaine differed for each of the three brain regions. While both BNST-lidocaine and CeA-lidocaine animals produced faster run times than their vehicle controls [main effect for group; F(1, 15)=5.101, p<0.04 and F(1, 19)=8.17, p<0.02], intra-BLA lidocaine had no appreciable effect on run time (p>0.05). The run times of subjects in each of the three experiments also differed over trials. As expected (see Fig. 2), when averaged across lidocaine and CSF treatments, run times tended to increase as testing progressed (i.e., as retreats developed) in all three experiments: main effects of trial for BNST [F(13, 195)=3.43, p<0.001], CeA [F(13.247)=2.89, p<0.02], and BLA [F(13, 208)=3.06, p<0.001]. Finally, the effect of lidocaine administration differentially changed over trials in the three experiments, with a significant group×trial interaction only observed in the BNST animals [F(13, 195)=2.00, p<0.03].
Fig. 2.
Mean run times and retreat frequencies. The left three panels depict the mean (±SEM) run times and the right panels the mean retreat frequencies of rats traversing an alley for a single i.v. cocaine infusion (1.0 mg/kg/injection in 0.1 ml) following i.c. injection of either lidocaine (solid lines) or artificial cerebrospinal fluid (dashed lines) into either the BNST (top), CeA (middle), or BLA (bottom)
Retreat frequency
The mean frequency (±SEM) of approach–avoidance retreat behaviors for each group is shown in the right panels of Fig. 2. A mixed three-factor (group×brain region×trial) ANOVA was computed on the data from all three experiments. The ANOVA identified a main effect of both trial [F(13, 650)=12.22, p<0.001] and group [lidocaine vs CSF; F(1, 50)=4.98, p<0.04] but no effect of brain region (p>0.05). Thus, the retreats exhibited by animals in the BNST, CeA, and BLA experiments (when averaged across CSF and lidocaine treatments) were statistically equivalent. There was, however, a significant group×trial interaction [F(13, 650)=1.71, p=0.05] and a marginal group×brain region×trial interaction [F(26, 650)=1.40, p=0.08], suggesting that lidocaine and CSF may have produced different effects across the three experiments. To examine this possibility more closely, three separate two-factor (group×trial) ANOVAs were computed on the data shown in each of right panels of Fig. 2. These analyses identified significant effects of trial (increased retreats over time when performance was averaged across the two groups in each experiment): BNST [F(13, 195)=3.760, p<0.001], CeA [F(13, 247)=4.517, p<0.001], and BLA [F(13, 208)=6.808, p<0.001]. However, only BNST animals showed a significant main effect of group [F(1, 15)=6.559, p<0.03] and a significant group×trial interaction [F(13, 195)=2.506, p=0.003]. Indeed, only in the BNST did lidocaine suppress the development of retreats throughout the two-week testing regimen. Inactivation of the CeA appeared to retard the development/onset of retreat behaviors (Fig. 2, middle/right panel) although this did not result in a statistically significant group × trial interaction [F(13, 247) = 1.33, p>0.05]. Lidocaine delivered into the BLA had no discernible effect relative to vehicle controls.
Locomotor activity
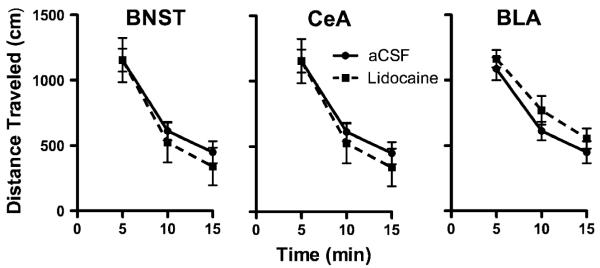
The mean (±SEM) locomotor distances traveled when subjects were pretreated 15 min prior to testing with either i.c. lidocaine or CSF are depicted for each brain region in Fig. 3. Although there were no treatment differences across the entire test 1-h session, only the first 15 min is depicted here to correspond with the time course employed in the runway phase of the study and in recognition of the fact that the dose of lidocaine employed here has been shown to produce neuronal inhibition only up to 30 min post-infusion (Lomber 1999; Tehovnik and Sommer 1997). A three-factor (treatment×brain region×time) ANOVA computed on these data identified a highly significant main effect for time [F(2, 38)=79.8, p<0.001], but no main effects for brain region (BNST vs CeA vs BLA) or treatment (lidocaine vs CSF) and no significant interaction effects (p>0.5). Thus, there is no evidence that inactivation of the BNST, CeA, or BLA produced alterations in spontaneous locomotor behavior (relative to CSF-vehicle treatment) that could account for the results observed in the runway phase of the study.
Fig. 3.
Mean (+SEM) distance traveled (in centimeters) of rats during 15 min of locomotor testing beginning 15 min after i.c. injection of either lidocaine or artificial CSF infused into either the BNST, CeA, or BLA. While locomotor behavior decreased over time in all three groups, lidocaine inactivation produced no significant effects on locomotor activity (relative to CSF vehicle) when applied to any of the three brain regions
Discussion
As previously reported, vehicle-treated animals running an alley for i.v. cocaine exhibited an approach–avoidance conflict about goal box entry as reflected in the development of retreat behaviors over trials (e.g., Ettenberg and Geist 1991, 1993; Wakonigg et al. 2003). Retreats reflect a form of approach–avoidance behavior that results from mixed positive and negative associations with the goal box related to the dual and opposing properties of cocaine (Ettenberg 2004). Indeed, the same pattern of behavior is observed in animals traversing a runway for a goal box containing known positive and negative stimuli (e.g., food+foot shock, Cohen et al. 2009; Geist and Ettenberg 1997; Miller 1944) and, like other forms of conflict behavior, cocaine-induced retreats can be reduced by anxiolytic drugs such as diazepam (Ettenberg and Geist 1991), buspirone (Ettenberg and Bernardi 2006), and ethanol (Knackstedt and Ettenberg 2005). Others have similarly shown that pretreatment with an anxiolytic agent enhances cocaine self-administration and reduces the latency for subjects to make the first operant response (Maier et al. 2008; but see Goeders et al. 1993).
As is the case in human chronic drug users, where a euphoric “high” is followed by an anxiogenic “crash” (Anthony et al. 1989; Resnick et al. 1977; Williamson et al. 1997; Rohsenow et al. 2007), the dual effects of acute cocaine in rats also appear to be temporally dissociated. The immediate effects of the drug produce conditioned place preferences while the effects present 15 min post i.v. injection produce conditioned place aversions (Ettenberg et al. 1999; Knackstedt et al. 2002; Ettenberg and Bernardi 2007). This delayed onset of cocaine’s negative properties inherently weakens their associability with the goal box, thereby accounting for the development of retreats only after multiple goal box–cocaine experiences (Ettenberg 2004).
In an attempt to understand the underlying neurobiology of cocaine’s opponent processes, the current study examined the impact of bilateral reversible inactivation of the BNST, CeA, or BLA on the development of cocaine-induced retreat behaviors in the runway. Intracranial lidocaine administration produces temporary functional lesions of discrete brain areas via blockade of Na+ channels (Catterall 1980). The concentration and volume utilized in the present study have been shown to produce neuronal inhibition for up to 30 min within a spherical boundary surrounding the infusion cannula tip with a radius of 0.5 mm (Lomber 1999; Tehovnik and Sommer 1997). Therefore, the size of the drug bolus and the time course of lidocaine’saction ensured for adequate inactivation of the target sites throughout the duration of each runway trial. The use of lidocaine, like that of permanent lesioning techniques, reflects a broad brush approach intended to assess the impact of inactivating both cell bodies and possible fibers of passage in the target areas. In that context it is a useful first step for identifying critical brain regions worthy of additional investigation and has been successfully employed by others to investigate the functional roles of BNST, CeA, and BLA in other behavioral paradigms (Kantak et al. 2002; Waraczynski 2003; Woods and Ettenberg 2004).
The targeted brain regions in the current study were selected on the basis that all three contain monoamine synapses at which cocaine acts, exhibit elevated levels of CRF in response to cocaine, and have been implicated in the behavioral responses to negative emotional, anxiogenic, and/or fearful stimuli (see “Introduction” for references). It was therefore hypothesized that inactivation of one or more of these brain regions would attenuate the anxiogenic response to cocaine and thereby reduce the frequency, and/or retard the development, of cocaine-induced approach–avoidance behaviors in the runway. Results showed that inactivation of the BNST prevented the slowing of run times and the development of retreats over trials in rats running for i.v. cocaine. CeA inactivation reduced the slowing of run times but only retarded and did not reverse the onset of retreats, while BLA inactivation had no appreciable effects on runway behavior. Note that these differences in the runway performance of the three lesioned groups cannot be easily accounted for by treatment-induced differences in locomotor behavior. As Fig. 3 clearly shows, 15 min after i.c. infusions (a time course comparable to that employed in the runway portion of the study), lidocaine-induced inactivation of the BNST, CeA, and BLA produced no changes in the locomotor activity of subjects relative to their own performance following CSF infusions.
The development of retreats requires the presence of both positive (approach) and negative (avoidance) associations with the goal box. Hence, the BNST data could conceivably stem from a diminution in the negative effects of cocaine, an enhancement of cocaine’s positive effects, or both. Although the current results do not conclusively identify which of these actions produced the behavioral changes associated with BNST inactivation, we note that inactivation of each of the three brain areas did not prevent the improvement in start latencies over trials nor were the start latencies of lidocaine- and CSF-treated rats different from one another. Hence the “approach” component of the runway behavior was unaffected by the lidocaine treatments. This also suggests that inactivation of the three brain areas did not disrupt the subjects’ ability to associate the goal box with cocaine administration. This is particularly relevant to the BLA experiment since this nucleus has been implicated in the consolidation of stimulus–reward associations and cue-induced reinstatement (Gabriele and See 2010; Théberge et al. 2010). Indeed, the development of normal approach and avoidance behavior in BLA-inactivated animals clearly indicates that these subjects were able to associate the goal box with the mixed consequences of cocaine administration.
The authors therefore conclude that the behavioral/runway effects of BNST inactivation, and to a lesser degree CeA inactivation, are best accounted for by a reduction in the aversive and/or anxiogenic response to self-administered cocaine. The BLA does not appear to be critical in this context. We note that the variability in the retreat data of the CeA CSF-group was relatively high and may have compromised the ability to obtain statistically significant effects (run times were reliably reduced by intra-CeA lidocaine, but not retreat behaviors, and there were no significant group×trial interactions for either run times or retreats). However, an examination of the data from Fig. 2 suggests that the pattern of runway behaviors differed between the lidocaine-treated groups across the three experiments. While the BNST-inactivated group did not develop retreats throughout the test period, and the BLA animals exhibited a progressive and slow increase in retreats over the course of testing, the CeA group exhibited very few retreats until the final two to three trials of the experiment when retreat frequencies rose dramatically and reached levels comparable to those of the BLA animals. This is consistent with the significant interactions (group×trial×brain region and group×brain region) revealed by the three-factor overall ANOVA. We therefore hypothesize that BNST inactivation blocked the anxiogenic response to cocaine, while CeA inactivation retarded the development of retreats due to a diminution (but not blockade) of that response.
There is a considerable body of work on the relative importance of the BNST (compared to other brain sites) in the behavioral response to anxiogenic stimuli. For example, in reviewing the findings of dozens of acoustic startle studies, Davis and colleagues conclude that while the CeA and BLA are involved in the response to acute fearful stimuli, longer lasting and more diffuse anxiogenic states are related to activity within the BNST (Davis 2006; Davis et al. 2010; Davis and Shi 1999; Walker et al. 2003). Indeed, reversible inactivation or electrolytic lesions of the BNST have been shown to reduce the anxiogenic response of animals placed in an environment previously paired with aversive stimuli (Resstel et al. 2008; Sullivan et al. 2004), but had no effect in the same animals on the fear response to the presentation of a discrete tone predictive of foot shock (Sullivan et al. 2004). Several investigators have now suggested that the BNST may be primarily involved in anxiety and less so in the response to discrete aversive stimulus (fear, e.g., Treit et al. 1998; Waddell et al. 2006).
In the drug self-administration literature, numerous studies have implicated the BNST in the stress-induced reinstatement of drug-seeking behavior after a period of cocaine withdrawal (Buffalari and See 2010; Erb and Stewart 1999; Leri et al. 2002). Such reinstatement appears to be related to the release of CRF within structures of the extended amygdala including the BNST (see review by Shaham et al. 2003). For example, withdrawal from chronic drug use has been shown to correlate with increased levels of CRF within the CeA (Ambrosio et al. 1997; Erb et al. 2004; Richter and Weiss 1999; Sarnyai et al. 1995), which sends dense CRF projections to the BNST (Sakanaka et al. 1986). Additionally, infusion of norepinephrine (NE) antagonists into the CeA or BNST attenuated stress-induced reinstatement of cocaine seeking (Leri et al. 2002). Given that NE terminals have been shown to synapse on CRF neurons within the BNST (Phelix et al. 1994) and binding of NE to β-noradrenergic receptors can increase CRF release (Tsagarakis et al. 1988), Leri and colleagues (2002) have postulated that stress-induced increases in NE within the CeA and BNST cause a release of CRF in both structures that is ultimately responsible for the “state” that induces animals to reinstate cocaine seeking. Since cocaine blocks the NE transporter (Rothman et al. 2001) and thereby increases extracellular NE levels within the CeA and BNST, the acute anxiogenic effects of self-administered cocaine may well be mediated by the same CRF mechanisms within the extended amygdala. The relative importance of the BNST compared to the CeA is suggested by several findings. For example, CRF antagonism within the BNST (but not the CeA) completely blocked stress-induced reinstatement of cocaine seeking (Erb and Stewart 1999), while disruption of the CRF projection from the CeA to BNST only partially disrupted response reinstatement (Erb et al. 2001). Also consistent with the current results are reports that lesions of either the CeA or BNST, but not BLA lesions, disrupt drug withdrawal-induced conditioned place aversions (Watanabe et al. 2002; Nakagawa et al. 2005) and BLA lesions did not reduce CRF levels in the BNST (Sakanaka et al. 1986).
In conclusion, while the BNST has received a great deal of attention for its role in anxiety and particularly its putative involvement in stress-induced reinstatement of drug-seeking behavior, the current results suggest that the involvement of this brain region is not limited to its actions during drug withdrawal. Indeed, it would seem that the motivation to seek cocaine during acute self-administration involves both positive and negative consequences of the drug whose ingestion is, therefore, likely maintained by both positive (reward–approach) and negative (anxiety–avoidance) reinforcement processes.
Acknowledgments
This research was supported by grant DA05041 from the National Institute of Drug Abuse awarded to AE. The authors wish to thank Dr. Skirmantas Janusonis for the assistance with creating the histological photomicrographs.
References
- Ambrosio E, Sharpe LG, Pilotte NS. Regional binding to corticotropin releasing factor receptors in brain of rats exposed to chronic cocaine and cocaine withdrawal. Synapse. 1997;25(3):272–276. doi: 10.1002/(SICI)1098-2396(199703)25:3<272::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Tien AY, Petronis KR. Epidemiologic evidence on cocaine use and panic attacks. Am J Epidemiol. 1989;129(3):543–549. doi: 10.1093/oxfordjournals.aje.a115166. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:L1–L118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Kaawaloa JN, Hebert MA, Blanchard DC. Cocaine produces panic-like flight responses in mice in the mouse defense test battery. Pharmacol Biochem Behav. 1999;64(3):523–528. doi: 10.1016/s0091-3057(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2010;213(1):19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Ann Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar O, Ettenberg A. Anxiolytic effects of nicotine in a rodent test of approach–avoidance conflict. Psychopharmacology. 2009;204(3):541–549. doi: 10.1007/s00213-009-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas M, Roncero C, Casas M. Corticotropin releasing factor and neuroplasticity in cocaine addiction. Life Sci. 2010;86:1–9. doi: 10.1016/j.lfs.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Mora MP, Gallegos-Cari A, Arizmendi-García Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: structural and functional analysis. Prog Neurobiol. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Erb S. Evaluation of the relationship between anxiety during withdrawal and stress-induced reinstatement of cocaine seeking. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):798–807. doi: 10.1016/j.pnpbp.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19(20):RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158(4):360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Borkowski S, Watson SJ, Akil H. Effects of chronic cocaine exposure on corticotropin-releasing hormone binding protein in the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroscience. 2004;123(4):1003–1009. doi: 10.1016/j.neuroscience.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27(8):721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behav. 2006;85:393–399. doi: 10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103(4):455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Regulation of norepinephrine release from the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1997;50:1040–1046. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1040::AID-JNR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Gabriele A, See RE. Reversible inactivation of the basolateral amygdala, but not the dorsolateral caudate putamen, attenuates consolidation of cocaine-cue associative learning in a reinstatement model of drug-seeking. Eur J Neurosci. 2010;32(6):1024–1029. doi: 10.1111/j.1460-9568.2010.07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. A simple method for studying intravenous drug reinforcement in a runaway. Pharmacol Biochem Behav. 1990;36:703–706. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. Concurrent positive and negative goal-box events produce runway behaviors comparable to those of cocaine-reinforced rats. Pharmacol Biochem Behav. 1997;57:145–150. doi: 10.1016/s0091-3057(96)00300-0. [DOI] [PubMed] [Google Scholar]
- Goeders NE, McNulty MA, Guerin GF. Effects of alprazolam on intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1993;44:471–474. doi: 10.1016/0091-3057(93)90493-d. [DOI] [PubMed] [Google Scholar]
- Guzman D, Ettenberg A. Runway self-administration of intracerebroventricular cocaine: evidence of mixed positive and negative drug actions. Behav Pharmacol. 2007;18(1):53–60. doi: 10.1097/FBP.0b013e3280144ac9. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Ettenberg A. Ethanol consumption reduces the adverse consequences of self-administered intravenous cocaine in rats. Psychopharmacology. 2005;178(2–3):143–150. doi: 10.1007/s00213-004-1996-2. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav. 2002;72:931–936. doi: 10.1016/s0091-3057(02)00764-5. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic homeostatic dysregulation as a driver of drug-seeking behavior. Drug Discov Today Dis Models. 2008;5(4):207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Chen HC, Chen DY. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol. 2001;44:33–43. [PubMed] [Google Scholar]; Chin J Physiol. 44:151. Erratum. [Google Scholar]
- Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods. 1999;86:109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Maier E, Ledesma RT, Seiwell AP, Duvauchelle CL. Diazepam alters cocaine self-administration, but not cocaine-stimulated locomotion or nucleus accumbens dopamine. Pharmacol Biochem Behav. 2008;91:202–207. doi: 10.1016/j.pbb.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57:571–599. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- Miller NE. Experimental studies of conflict. In: McV Hunt J., editor. Personality and behavior disorders. The Ronald Press Company; New York: 1944. pp. 431–465. [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134(1):9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th edn Academic Press; New York: 1998. [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull. 1994;33:109–119. doi: 10.1016/0361-9230(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol. 2000;8:117–124. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Kestenbaum RS, Schwartz LK. Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science. 1977;195:696–698. doi: 10.1126/science.841307. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Alves FH, Reis DG, Crestani CC, Corrêa FM, Guimarães FS. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience. 2008;154:869–876. doi: 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Richelson E, Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepi-nephrine uptake. Eur J Pharmacol. 1984;104:277–286. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Eaton CA, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. 2007;68:641–648. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Bíró E, Gardi J, Vecsernyés M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur J Neurosci. 2003;18:2343–2350. doi: 10.1046/j.1460-9568.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Théberge FR, Milton AL, Belin D, Lee JL, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Aujla H, Menard J. Does the bed nucleus of the stria terminalis mediate fear behaviors? Behav Neurosci. 1998;112:379–386. doi: 10.1037//0735-7044.112.2.379. [DOI] [PubMed] [Google Scholar]
- Tsagarakis S, Holly JM, Rees LH, Besser GM, Grossman A. Acetylcholine and norepinephrine stimulate the release of corticotropin-releasing factor-41 from the rat hypothalamus in vitro. Endocrinology. 1988;123(4):1962–1969. doi: 10.1210/endo-123-4-1962. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Wakonigg G, Sturm K, Saria A, Zernig G. Opioids, cocaine, and food change runtime distribution in a rat runway procedure. Psychopharmacology. 2003;169:52–59. doi: 10.1007/s00213-003-1488-9. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Waraczynski M. Lidocaine inactivation demonstrates a stronger role for central versus medial extended amygdala in medial forebrain bundle self-stimulation. Brain Res. 2003;962:180–198. doi: 10.1016/s0006-8993(02)04033-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamoto R, Maeda A, Nakagawa T, Minami M, Satoh M. Effects of excitotoxic lesions of the central or basolateral nucleus of the amygdala on naloxone-precipitated withdrawal-induced conditioned place aversion in morphine-dependent rats. Brain Res. 2002;958:423–428. doi: 10.1016/s0006-8993(02)03468-6. [DOI] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Woods VE, Ettenberg A. Increased amphetamine-induced locomotion during inactivation of the basolateral amygdala. Behav Brain Res. 2004;149:33–39. doi: 10.1016/s0166-4328(03)00212-2. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]
- Young AM, Rees KR. Dopamine release in the amygdaloid complex of the rat, studied by brain microdialysis. Neurosci Lett. 1998;249:49–52. doi: 10.1016/s0304-3940(98)00390-5. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]