Abstract
The mechanosensitive channel of large conductance (MscL) from E. coli serves as an emergency release valve allowing the cell to survive acute osmotic downshock. It is one of the best studied mechanosensitive channels and serves as a paradigm for how a protein can sense and respond to membrane tension. Two MscL crystal structures of the orthologs M. tuberculosis and S. aureus have been solved showing pentameric and tetrameric structures, respectively. Several studies followed to understand whether the discrepancy in their stoichiometry was a species difference or a consequence of the protein manipulation for crystallization. Two independent studies now agree that the full-length S. aureus MscL is actually a pentamer, not tetramer. While detergents appear to play a role in modifying the oligomeric state of the protein, a cytoplasmic helical bundle has also been implicated. Here, we evaluate the role of the C-terminal region of S. aureus MscL in the oligomerization of the channel in native membranes by using an in vivo disulfide-trapping technique. We find that the oligomeric state of S. aureus MscLs with different C-terminal truncations, including the one used to obtain the tetrameric S. aureus MscL crystal structure, are pentamers in vivo. Thus, the C-terminal domain of the S. aureus protein only plays a critical role in the oligomeric state of the SaMscL protein when it is solubilized in detergent.
Keywords: mechanosensitive channel, oligomerization, detergent solubilization, stoichiometry, disulfide trapping
Introduction
The E. coli mscL was the first gene definitively shown to encode a mechanosensitive channel activity.1 The encoded mechanosensitive channel of large conductance (MscL) protein (EcoMscL) is one of the best studied mechanosensitive channels, serving as a paradigm for how a protein can sense membrane tension.2 A crystal structure from M. tuberculosis MscL (MtMscL) was first obtained by Douglas Rees' group in 1998, depicting what appeared to be a closed state of the channel.3,4 It was a homopentamer with each subunit containing two transmembrane domains and a cytoplasmic C-terminal α-helical bundle. The same group recently obtained a second crystal structure from a C-terminal truncated S. aureus MscL (SaMscL) in what they speculated to be an expanded intermediate state; surprisingly, this was a tetramer.5 A different oligomeric state was unexpected given that the two orthologs are highly conserved [Fig. 1(A)], as is the entire MscL family.6–8 To further investigate the discrepancy between the stoichiometry of these two channels, several studies have been performed. In one study, we developed an in vivo disulfide-trapping assay to determine the state of the S. aureus channel when in its native membrane environment; it was unambiguously a pentamer, demonstrating that the crystal did not reflect a native state of the protein.9 Thus, two possibilities existed: either the truncation of the C-terminal bundle altered channel stoichiometry to tetramer, or the detergent solubilization did. Using crosslinking, sedimentation equilibrium centrifugations and light scattering, we concluded that on solubilization with the detergent n-dodecyl-N,N-dimethylamine-N-oxide (LDAO), which was used to obtain the crystal structure, the channel reversibly reorganizes as a tetramer; in contrast, TritonX-100 and pentaethylene glycol monooctyl ether (C8E5) detergents did not alter stoichiometry.9 However, the influence of protein truncation was not assessed. Indeed, one previous study of in vitro translated and n-Octyl-Beta-D-Glucopyranoside (OG) solubilized EcoMscL implied that the C-terminal end of the protein played a critical role in assembly.10 Recently, using a new technique coined oligomer characterization by addition of mass (OCAM), the Rees' group confirmed that both MtMscL, and full length (FL) SaMscL are pentamers when solubilized in n-Dodecyl β-D-maltoside (DDM); but they also showed that a SaMscL with the C-terminal deletion, as used in the crystallographic study, was heterogeneous in its oligomeric state, existing as both pentamers and tetramers. Thus, it appeared that the C-terminal helical bundle does influence the oligomeric state of the protein. However, because both of these studies used detergent-solubilized proteins, it was unclear if the C-terminal region of the protein played a role in MscL stoichiometry in vivo. Here, we utilize our in vivo disulfide-trapping assay to address this issue.
Figure 1.
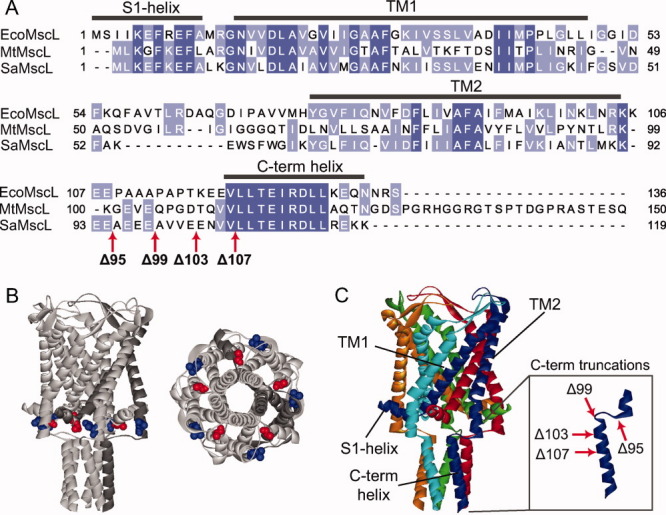
Alignment of MscL homologs and protein modifications. A: Sequence alignment of three MscL homologs from E. coli (EcoMscL), M. tuberculosis (MtMscL), and S. aureus (SaMscL) showing the regions corresponding to the different protein domains. The degree of conservation is color coded with dark blue residues indicating identity and light blue similarity. The red arrows at the C-terminal end of SaMscL sequence indicate the sites were the stop codons were added to generate the different C-terminal truncated constructs. B: Localization of residues A10 (red) and L97 (blue) in M. tuberculosis MscL, corresponding to L10 and M91 in SaMscL. These residues were substituted to cysteines for the in vivo disulfide trapping experiments. A lateral view (left) and bottom view (right) are shown. C: Crystal structure of M. tuberculosis MscL, showing a pentameric stoichiometry. The inset shows a detail of the C-terminal domain and the location of the C-terminal truncations.
To evaluate the role of the c-terminal region of SaMscL in its oligomeric state, we have generated multiple C-terminal truncated constructs of different lengths that have two cysteine mutations (L10C/M91C; Fig. 1). As can be seen in Figure 1(B), these two sites are predicted to be in close proximity in the closed state3 and have been shown previously to generate the most efficient in vivo crosslinking.9 We investigated the FL channel as well as four different C-terminal deletions: Δ95 (which was the truncation crystallized as a tetramer), Δ99, Δ103, and Δ107. Two of these truncations, Δ95 and Δ99, are located in the linker between TM2 and the cytoplasmic helix and the other two are in the cytoplasmic helix itself [Fig. 1(A,C)]. As previously described, bacteria expressing the different constructs were given an osmotic shock in the presence of copper phenanthroline; this removes cellular reducing agents such as glutathione while adding an oxidant. The treated cells were pelleted, resuspended in sodium dodecyl sulphate (SDS) loading buffer, and the MscL protein visualized by SDS polyacrylamide gel electrophoresis (PAGE) and western blot. Note that the protein is trapped in its native membrane environment because no solubilization or protein handling is involved before SDS PAGE. As shown in Figure 2 (left and center panels), SaMscL exists almost exclusively as a pentamer regardless of the length of the C-terminal truncation. Although a ladder of monomer through pentamer can be observed for 10C/91C Δ95 SaMscL in the no-shock/nontreated protein, in the presence of an oxidizer the pentamer becomes the prevalent band in a time dependant manner (Fig. 2, right panel). Note that for the SaMscL single cysteine mutants under the same treatment, no other species than monomer was observed for M91C but a small proportion of L10C existed as dimmers (Supplemental information). Dimerization of L10C was expected as the equivalent mutant from EcoMscL, M12C, also form dimmers under similar treatment.11 Finally, we show by patch clamp of native membranes that both FL and Δ95 truncated 10C/91C SaMscL respond similarly to changes in the redox potential of the bath (Fig. 3). In the presence of the reducing agent dithiothreitol (DTT), both channels are functional and activity can be observed in response to negative pressures applied to the membrane patch (left traces). After perfusion with a 3% peroxide solution, channel activity could no longer be elicited even under high negative pressures (right traces). These results are consistent with the channels being locked closed after disulfide trapping, suggesting that in native membranes the FL and truncated channels are structural and functionally similar. Hence, in vivo the C-terminal helical bundle plays no detectable role in proper pentameric assembly.
Figure 2.
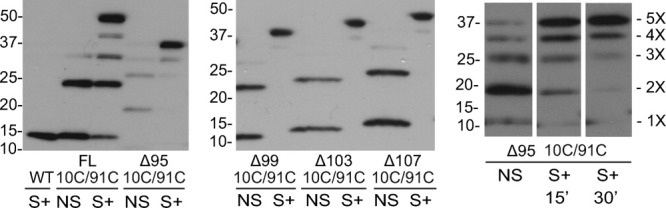
Disulfide trapping experiments show that C-terminally truncated SaMscL protein exists as a pentamer in vivo. Western blot analysis of WT SaMscL, double cysteine mutant 10C/91C SaMscL FL and with Δ95 truncation (left panel). All samples were either nonshocked (NS) or shocked (S+) in the presence of the oxidizing agent copper phenanthroline. When shocked in the presence of copper phenanthroline the majority of 10C/91C exists as a pentamer, independent of the length of the C-terminal truncation (left and central panels). A ladder of monomer through pentamer is already present in the nonshock sample; pentamer becomes the major band when osmotically downshocked in the presence of copper phenanthroline; this occurs in a time-dependent manner (right panel). Note that no detergent solubilization before resuspension in Laemmli sample buffer is performed in these experiments.
Figure 3.
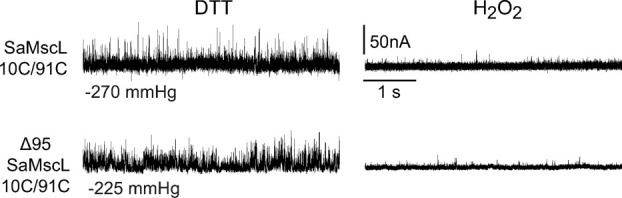
The FL and truncated SaMscL 10C/91C channels are functionally similar. The electrophysiological data shows single-channel activities of both SaMscL 10C/91C FL and the truncated construct Δ95 SaMscL 10C/91C. Channel openings are seen as upward deflections. Both channels respond equally to changes in the redox potential of the bath. In the presence of the reducing agent DTT, the channels are functional and activity can be observed at the indicated pressures (left traces), but after perfusing a peroxide solution in the bath, the channel activity is lost (right traces).
Many previous studies have concluded that MtMscL and EcoMscL are also pentamers. A study using crosslinking, sedimentation equilibrium, and size-exclusion chromatography showed EcoMscL as a pentamer;12 another study using crosslinking and electron microscopy reached the same conclusion.10 The crystal structure of MtMscL as well as the recent OCAM analysis also showed a pentameric structure for this ortholog.13 Finally, when studied by using our in vivo disulfide trapping in native membranes, all three MscL homologs have shown pentameric stoichiometry (Supplementary Information). Hence, there appears to be no variability in the oligomeric state of these MscL orthologs.
Collectively, the data suggest that the C-terminal domain of the SaMscL channel only plays a critical role in the oligomeric state of the protein when it is solubilized in detergent. Different detergents seem to have different abilities to alter MscL oligomeric structure. It appears that LDAO is the harshest detergent in this sense, leading to oligomeric rearrangement of the full-length SaMscL protein.9 DDM and perhaps other detergents can lead to rearrangements of only the truncated, not FL, SaMscL.13 However, there also appear to be species differences; from the OCAM study, the FL EcoMscL appears to form hexamers as well as pentamers when solubilized in DDM. In addition, the same study demonstrated that truncation of the MtMscL did not destabilize the pentameric organization when solubilized in DDM.13 Hence, detergents alone can cause specific protein complexes to rearrange their oligomeric state while others appear stable. As detergent solubilization is a critical and necessary step to obtain membrane proteins for crystallization and other biophysical and structural studies, where possible, a complementary set of data assessing the oligomeric state in vivo seems advisable.
Methods
Molecular biology
FL S. aureus MscL and the FL L10C/M91C S. aureus MscL mutant were cloned into pET21a vector, which has a His-tag at the c-terminal end. Truncated S. aureus MscL mutants were produced by polymerase chain reaction (PCR) by adding stop codons at residues A96 (Δ95), A100 (Δ99), E104 (Δ103), and L108 (Δ107). Constructs were cloned into pet15b vector at NdeI/BamHI and transformed into PB116s.14 All constructs from the pet15b vector have a his-tag at the N-terminal.
In vivo disulfide trapping
Overnight cultures were diluted 1:100 and grown 1 h at 37°C in Luria-Bertani (LB) media (∼410 mOsmolar). LB with 1M NaCl was then added for a final concentration of 0.5M. Cultures were then induced with 1 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) for 1 h when an optical density (OD) 600 of 0.2 was reached. Cultures were either Mock shocked (0.5M NaCl LB) or shocked (water with 1.5 μM copper phenanthroline) at a 1:20 dilution for 15 min at 37°C. Samples were spun down at 4000g for 20 min and immediately resuspended in nonreducing sample buffer, adjusted for final OD, and run on a 4–20% gel (Bio-Rad) for western blot analysis.14 Note that in less than 5 min, the samples are collected and loaded on the gel. The primary antibody anti-Penta His (Qiagen) was used at 1:4000 and the secondary Goat anti-Mouse horseradish peroxidase (HRP) (Bio-Rad) at 1:100,000. Blots were developed using HRP substrate (Millipore) and exposed to film.
Electrophysiology
E. coli giant spheroplasts were generated and used in patch-clamp experiments as described previously.15 Excised, inside-out patches were examined at room temperature under symmetrical conditions using a buffer containing 200 mM KCl, 90 mM MgCl2, 10 mM CaCl2, and 5 mM HEPES pH 6 (Sigma, St. Louis, MO). To study redox effects in channel activity H2O2 1–3% v/v or DTT 1-10 mM (Sigma, St. Louis, MO) were added to the bath. A previous study has demonstrated that this treatment has no detrimental effect on channel function; in fact, one cysteine mutant was shown to increase activity.16 Recordings were performed at −20 mV (positive pipette). Data were acquired at a sampling rate of 20 kHz with a 5-kHz filter using an AxoPatch 200B amplifier in conjunction with Axoscope software (Axon Instruments, Union City, CA). A piezoelectric pressure transducer (World Precision Instruments, Sarasota, FL) was used to monitor the pressure throughout the experiments. Data analysis was performed using Clampfit9 from Pclamp9 (Axon Instruments).
Glossary
Abbreviations:
- C8E5
pentaethylene glycol monooctyl ether
- LDAO
n-dodecyl-N,N-dimethylamine-N-oxide
- DDM
n-Dodecyl β-D-maltoside
- OG
n-Octyl-beta-D-glucopyranoside
- EcoMscL
E. coli MscL
- MtMscL
M. tuberculosis MscL
- SaMscL
S. aureus MscL
- SDS
sodium dodecyl sulphate
- DTT
dithiothreitol
- OCAM
oligomer characterization by addition of mass
Supplementary material
REFERENCES
- 1.Blount P, Sukharev SI, Moe PC, Schroeder MJ, Guy HR, Kung C. Membrane topology and multimeric structure of a mechanosensitive channel protein of Escherichia coli. EMBO J. 1996;15:4798–4805. [PMC free article] [PubMed] [Google Scholar]
- 2.Blount P, Iscla I, Moe PC, Li Y. MscL: the bacterial mechanosensitive channel of large conductance. In: Owen PH, editor. Current topics in membranes. San Diego: Academic Press; 2007. pp. 201–233. [Google Scholar]
- 3.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 4.Steinbacher S, Bass R, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. In: Owen PH, editor. Current topics in membranes. San Diego: Academic Press; 2007. pp. 1–24. [Google Scholar]
- 5.Liu Z, Gandhi CS, Rees DC. Structure of a tetrameric MscL in an expanded intermediate state. Nature. 2009;461:120–124. doi: 10.1038/nature08277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, Saier MH. Two families of mechanosensitive channel proteins. Microbiol Mol Biol R. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer J, Elmore D, Lester H, Dougherty D. Comparing and contrasting Escherichia coli and Mycobacterium tuberculosis mechanosensitive channels (MscL). New gain of function mutations in the loop region. J Biol Chem. 2000;275:22238–22244. doi: 10.1074/jbc.M003056200. [DOI] [PubMed] [Google Scholar]
- 8.Balleza D, Gomez-Lagunas F. Conserved motifs in mechanosensitive channels MscL and MscS. Eur Biophys J. 2009;38:1013–1027. doi: 10.1007/s00249-009-0460-y. [DOI] [PubMed] [Google Scholar]
- 9.Dorwart MR, Wray R, Brautigam CA, Jiang Y, Blount P. S. aureus MscL is a pentamer in vivo but of variable stoichiometries in vitro: implications for detergent-solubilized membrane proteins. PLoS Biol. 2010;8:e1000555. doi: 10.1371/journal.pbio.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura K, Usukura J, Sokabe M. Gating-associated conformational changes in the mechanosensitive channel MscL. Proc Natl Acad Sci USA. 2008;105:4033–4038. doi: 10.1073/pnas.0709436105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iscla I, Wray R, Blount P. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys J. 2008;95:2283–2291. doi: 10.1529/biophysj.107.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukharev SI, Schroeder MJ, McCaslin DR. Stoichiometry of the large conductance bacterial mechanosensitive channel of E. coli. A biochemical study. J Membr Biol. 1999;171:183–193. doi: 10.1007/s002329900570. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi CS, Walton TA, Rees DC. OCAM: a new tool for studying the oligomeric diversity of MscL channels. Protein Sci. 2011;20:313–326. doi: 10.1002/pro.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wray R, Eaton C, Blount P. An open-pore structure of the mechanosensitive channel MscL derived by determining transmembrane domain interactions upon gating. FASEB J. 2009;23:2197–2204. doi: 10.1096/fj.09-129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 1999;7:420–424. doi: 10.1016/s0966-842x(99)01594-2. [DOI] [PubMed] [Google Scholar]
- 16.Iscla I, Levin G, Wray R, Blount P. Disulfide trapping the mechanosensitive channel MscL into a gating-transition state. Biophys J. 2007;92:1224–1232. doi: 10.1529/biophysj.106.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


