Abstract
Under conditions of obesity and insulin resistance, the serine/threonine protein kinase Akt/PKB is required for lipid accumulation in liver. Two forkhead transcription factors, FoxA2 and FoxO1 have been suggested to function downstream of and to be negatively regulated by Akt and proposed as key determinants of hepatic triglyceride content. In this study, we utilize genetic loss of function experiments to show that constitutive activation of neither FoxA2 nor FoxO1 can account for the protection from steatosis afforded by deletion of Akt2 in liver. Rather, another downstream target positively regulated by Akt, the mTORC1 complex, is required in vivo for de novo lipogenesis and Srebp1c expression. Nonetheless, activation of mTORC1 and SREBP1c are not sufficient to drive postprandial lipogenesis in the absence of Akt2. These data show that insulin signaling through Akt2 promotes anabolic lipid metabolism independent of Foxa2 or FoxO1 and through pathways additional to the mTORC1-dependent activation of SREBP1c.
INTRODUCTION
Insulin is the primary hormone responsible for coordinating the metabolic response to nutrient intake. Following a meal, insulin suppresses the hepatic glucose production that provides the brain substrate during fasting and directs ingested nutrients into long-term energy stores. In particular, insulin promotes triglyceride (TG) synthesis and storage utilizing dietary fatty acids and induces de novo lipogenesis in liver and adipose tissue. Much of this synchronized metabolic response is thought to be mediated by hormone-dependent reduction in the transcription of gluconeogenic genes, such as glucose-6-phosphotase, catalytic subunit (G6pc) and cytosolic phosphoenolpyruvate carboxykinase 1 (Pck1), and activation of lipogenic gene expression via the master regulator sterol regulatory element binding transcription factor 1c (Srebp1c) (Argaud et al., 1996; Horton et al., 2002; O’Brien et al., 2001). Thus, the transcriptional targets of the insulin signaling pathway are of considerable interest.
The serine/threonine kinase Akt (also known as protein kinase B, PKB) is now well-established as a major mediator of the metabolic actions of insulin (Whiteman et al., 2002). Akt2 is the predominant isoform in the insulin responsive tissues like liver, muscle and adipose tissue (Cho et al., 2001). Akt2 knockout mice are “diabetic”, whereas liver-specific disruption of the Akt2 gene has mild effects on glycemia but more dramatic effects on lipogenic gene expression and steatosis in obese, insulin-resistant mice (Cho et al., 2001; Leavens et al., 2009). The canonical nuclear target of Akt, established in worms, flies as well as vertebrates, is the transcription factor Foxhead box O (FoxO) (Gross et al., 2009). Akt phosphorylates and inhibits the transcriptional activity of FoxO at least in part by promoting its exclusion from the nucleus (Durham et al., 1999; Guo et al., 1999; Nakae et al., 1999; Rena et al., 1999; Tang et al., 1999). There are now considerable data implicating FoxO1 as an intermediate in the pathway by which insulin through Akt suppresses hepatic gluconeogenic gene expression and glucose output (Haeusler et al., 2010b; Li et al., 2007; Liu et al., 2008; Matsumoto et al., 2007; Nakae et al., 2001; Puigserver et al., 2003; Qu et al., 2006).
In contrast to the consensus of opinion regarding the role of FoxO1 in the control of gluconeogenic gene expression, its contribution to regulation of hepatic lipid metabolism remains unclear. In some studies, expression of a constitutively active FoxO1 in liver induces Srebp1c expression and hepatic TG accumulation, though others have no fond this reponse; in addition, FoxO1 inhibits TG secretion (Matsumoto et al., 2006; Zhang et al., 2006). In contrast, Kamagate et al. reported that FoxO1 is necessary and sufficient to promote hepatic very-low-density lipoprotein associated TG (VLDL-TG) production and hypertriglyceridemia via its regulation of microsomal TG transfer protein (MTP), and further proposed that this pathway accounts for elevated serum lipids in insulin-resistant states (Kamagate et al., 2008). Loss-of-function approaches to understand the contribution of FoxO1 to lipogenic gene expression have yielded few striking results, though its ablation in streptozotocin-induced diabetes increases serum TG and VLDL-TG secretion (Haeusler et al., 2010a). Deletion of Foxo1 in insulin receptor substrate 1 and 2 (IRS1 and IRS2) double-knockout mouse livers partially rescues the deficit in VLDL-TG secretion (Dong et al., 2008). Ablation of all three FoxO isoforms in liver leads to hepatic steatosis, potentially through regulating the expression of nicotinamide phosphoribosyltransferase (Nampt) and consequently NAD+ levels (Tao et al., 2011). Thus, there persists considerable uncertainly concerning the physiological role of FoxO1 in controlling hepatic lipid metabolism.
Another forkhead family transcription factor, FoxA2, has been implicated in the control of hepatic lipid metabolism by insulin. Like FoxO, FoxA2 is phosphorylated by Akt, thereby leading to exclusion from the nucleus and inhibition of transcriptional activity, though some investigators have found that FoxA2 resides in nuclei constitutively and does not respond to different nutritional conditions (Wolfrum et al., 2004; Zhang et al., 2005). Expression of a constitutively active FoxA2 in liver of obese mice leads to a phenotype much like a deficiency of insulin signaling, i.e., increased fatty acid oxidation and TG secretion and reduced hepatic TG content (Wolfrum et al., 2004; Wolfrum and Stoffel, 2006). However, the role of FoxA2 in insulin action has been challenged; mice with liver-specific deletion of FoxA2 demonstrate impaired bile acid metabolism, but no alterations in hepatic or serum TG (Bochkis et al., 2008).
It has long been known that a major target of insulin signaling in the regulation of hepatic lipid metabolism is another transcription factor, SREBP1c, a member of the basic helix-loop-helix leucine-zipper family (Ferre and Foufelle, 2010; Horton et al., 2002). Both transcription and posttranslational processing of SREBP1c to its transcriptionally active form are stimulated by insulin signaling through Akt, contributing to postprandial increases in de novo lipogenesis (Chakrabarti et al., 2010; Fleischmann and Iynedjian, 2000; Leavens et al., 2009; Li et al., 2010; Porstmann et al., 2005; Porstmann et al., 2008). SREBP1c translocates to the Golgi complex where it is proteolyzed, liberating an amino-terminal fragment that activates its own expression as well as that of a set of lipogenic enzymes including fatty acid synthase (Fasn), acetyl-Coenzyme A carboxylase (Acc), ATP citrate lyase (Acly) and glycerol-3-phosphate acyltransferase (Gpat) (Horton et al., 2002; Postic and Girard, 2008). Insulin’s ability to promote Srebp1c expression depends on the mammalian target of rapamycin complex 1 (mTORC1), as knockdown of an essential component of mTORC1, Raptor (regulatory associated protein of mTOR), in multiple tissue culture cell lines significantly blunts insulin or Akt-stimulated expression of Srebp1c and its targets (Chakrabarti et al., 2010; Duvel et al., 2010; Porstmann et al., 2008). In primary hepatocytes, rapamycin prevents the insulin-dependent induction of Srebp1c, but not the reduction in Pck1, consistent with the idea that distinct transcriptional targets mediate the effect of insulin on gluconeogenic and lipogenic gene expression (Li et al., 2010).
The goal of the experiments reported herein is to test the dependency of these three transcriptional pathways as downstream targets linking insulin signaling to control of hepatic TG content and de novo lipogenesis in mice. We have taken a genetic approach to address the epistatic relationship between Akt and its downstream candidate nuclear targets. If inhibition of FoxO1 or FoxA2 were critical to insulin signaling, then the loss in hormone action that accompanies deletion of Akt2 in liver would be reversed by concomitant deletion of the target transcription factor. If Akt2 promotes de novo lipogenesis in liver via mTORC1, both its activity and Srebp1c expression would be decreased upon deletion Akt2 in liver. Thus we ablated Akt2 expression in liver by tissue-specific homologous recombination alone or simultaneously with either FoxA2 or FoxO1, and analyzed lipid metabolism. Neither FoxA2 nor FoxO1 is downstream of Akt2 in the control of hepatic lipid accumulation, but rather FoxO1 reduces hepatic TG content upstream of or via a parallel pathway to Akt2. We then tested the requirement and sufficiency of activation of mTORC1 for de novo lipogenesis. We found that mTORC1 was required for Srebp1c expression and de novo lipogenesis, but, when hepatic Akt2 was deleted, activation of mTORC1 and SREBP1c was insufficient to drive de novo lipogenesis following a high-carbohydrate meal.
RESULTS
Akt2 Regulates Hepatic TG Accumulation Independent of FoxA2
It has been proposed that insulin inhibits FoxA2 via Akt-dependent phosphorylation and nuclear exclusion, controlling lipid export, fatty acid oxidation and accumulation of TG in the liver (Wolfrum et al., 2004; Wolfrum et al., 2008; Wolfrum and Stoffel, 2006). We generated mice lacking both Akt2 and FoxA2 in liver by breeding Akt2(loxP/loxP);FoxA2(loxP/loxP) mice to AFP-Cre, which is expressed exclusively in liver (Zhang et al., 2005). As shown in Figure 1A, we achieved essentially complete deletion of Akt2 in both Akt2(loxP/loxP) as well as in Akt2(loxP/loxP);FoxA2(loxP/loxP) mice. FoxA2 protein levels were also great reduced in either FoxA2 single knockout or Akt2, FoxA2 double knockout hepatocytes (Figure 1A). Mice of all four genotypes were submitted to glucose tolerance tests, which showed indistinguishable rates of glucose disposal (Figure S1A). In addition, there were no significant changes in serum insulin or TG levels from either random-fed or overnight fasted mice lacking Akt2, FoxA2 or both in liver (Figure S1B and S1C). Adipose tissue mass was the same among the mice of four genotypes (Figure S1D).
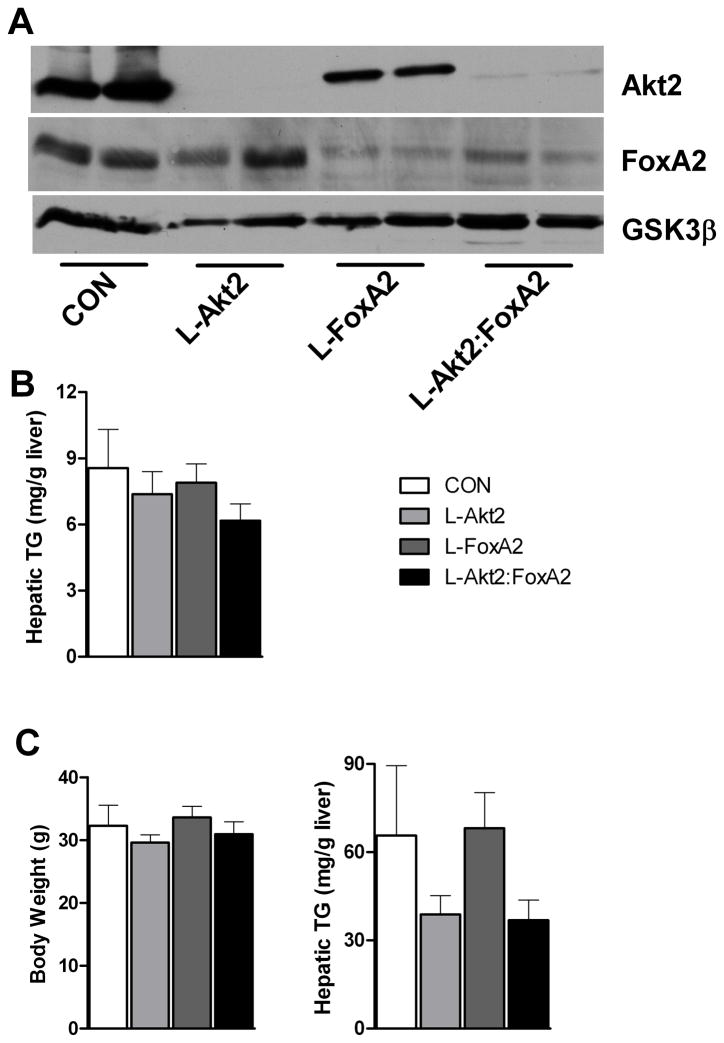
Figure 1. Akt2 Regulates Hepatic TG Accumulation Independent of FoxA2.
(A) Western blot for detecting deletion of Akt2 and FoxA2 in primary hepatocytes isolated from wild type controls (CON), AFP>Cre;Akt2(loxP/loxP) (L-Akt2), AFP>Cre;FoxA2(loxP/loxP) (L-FoxA2) and AFP>Cre;Akt2(loxP/loxP);FoxA2(loxP/loxP) (L-Akt2:FoxA2) mice. Two male mice for each genotype were 2-month-old and under random-fed condition when primary hepatocytes were isolated.
(B) Hepatic TG levels under random-fed conditions on normal chow in 2-month-old male mice, n=8–10 for each genotype.
(C) Body weight (left) and hepatic TG levels (right) under random-fed conditions on HFD. 1-month-old male mice were subject to 3-month HFD feeding, n=6–7 for each genotype. The body weight of control mice of comparable age but maintained on normal chow was 26.2 ± 0.4g. The variance of hepatic TG levels was significant among four different genotypes by one-way ANOVA, but did not achieve statistical significance upon pairwise comparison by Tukey’s post-test; error bars represents SEM.
The TG content in livers deficient in Akt2, FoxA2 or both was unchanged compared to wild type controls when assayed in mice under random, chow-fed conditions (Figure 1B). Akt2 is required for accumulation of liver TG under some situations that induce hepatic steatosis, and expression of a constitutively active FoxA2 reduces hepatic TG accumulation in leptin-deficient (ob/ob) mice (Leavens et al., 2009; Wolfrum et al., 2004; Wolfrum and Stoffel, 2006). To address the question of whether endogenous FoxA2 is responsible for the reduction in hepatic TG in livers deficient in Akt2, mice lacking Akt2, FoxA2 or both in liver were fed a HFD for 3 months. Glucose tolerance tests were undistinguishable among the four genotypes (Figure S2A), as were serum insulin and TG levels under both fasting and fed conditions (Figure S2B and S2C). Loss of Akt2 in livers trended to a decrease of hepatic TG accumulation relative to wild type controls after HFD feeding, and this was unaffected by deletion of FoxA2 (Figure 1C). These data show that, at least under conditions of diet-induced obesity (DIO), FoxA2 activity is unlikely to be the cause of the protection from steatosis rendered by removal of Akt2 from liver. These data are also consistent with previous findings challenging the notion of FoxA2 as a target of insulin signaling (Zhang et al., 2005).
Loss of Akt2 Suppresses Hepatic TG levels in FoxO1 Deficient Liver
FoxO1, a transcription factor negatively regulated by the insulin/Akt signaling pathway, promotes expression of gluconeogenic genes such as G6pc and Pck1 (Gross et al., 2009). To assess the function of FoxO1 as a downstream target of Akt2, we derived Akt2, FoxO1 and combined liver-specific double knockout mice. Akt2 and FoxO1 protein levels were significantly reduced in either Akt2 or FoxO1 single knockout livers, respectively, and in Akt2, FoxO1 double knockout livers (Figure 2A, left), and real-time PCR from either FoxO1 knockout livers or Akt2, FoxO1 double knockout livers showed reduction of FoxO1 mRNA levels compared to either wild type controls or Akt2 knockout livers (Figure 2A, right). Under conditions of normal chow feeding, both AFP>Cre;Akt2(loxP/loxP) and AFP>Cre;FoxO1(loxP/loxP) mice demonstrated normal glucose tolerance (Figure S3A). Moreover, serum insulin and TG levels were the same for all four genotypes when assayed under either fasting or fed conditions (Figure S3B and S3C). When challenged with pyruvate, AFP>Cre;FoxO1(loxP/loxP) mice trended to lower levels of blood glucose, as did in AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) mice. AFP>Cre;Akt2(loxP/loxP) mice did not differ from wild type controls (Figure 2B and 2C). These data are consistent with previously published results that support a model in which FoxO1 regulates glucose output and is downstream of Akt (Matsumoto et al., 2007).
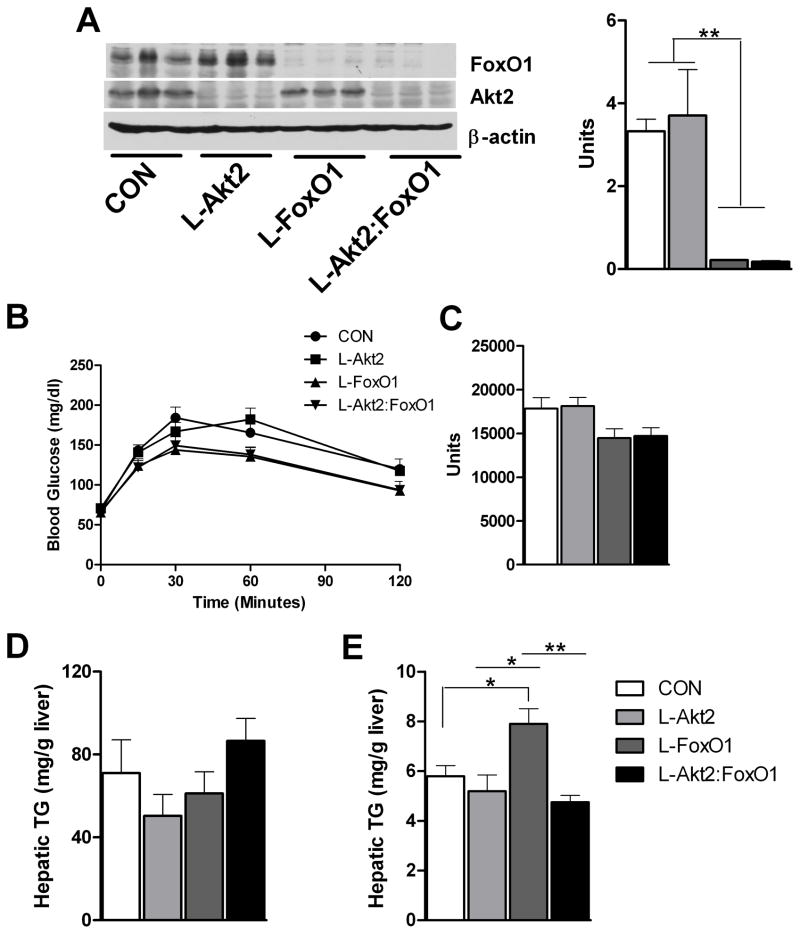
Figure 2. Loss of Hepatic Akt2 Decreases TG Accumulation in Liver Independent of FoxO1 under Normal Chow Feeding.
(A) Deletion of Akt2 and FoxO1 in liver from wild type controls (CON), AFP>Cre;Akt2(loxP/loxP) (L-Akt2), AFP>Cre;Akt2(loxP/loxP) (L-FoxO1) and AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) (L-Akt2:FoxO1) mice. Three male mice for each group were sacrificed under random-fed conditions on normal chow, and liver lysates were prepared to blot for Akt2, FoxO1 and β-actin (left). Realtime PCR was performed on cDNA prepared from RNA from 2-month-old male mouse livers under random-fed conditions, n=3–5 for each genotype (right).
(B) Pyruvate tolerance test (PTT). Two-month-old male mice were fasted overnight, and then injected with 2g/kg body weight pyruvate intraperitoneally. Blood glucose was determined at 0, 15, 30, 60 and 120 minutes after injection, n=4–8 for each genotype.
(C) Area under curve from PTT. The variance of AUC levels was significant among four different genotypes by one-way ANOVA, but did not achieve statistical significance by pairwise comparison using Tukey’s post-test.
(D) Hepatic TG levels (right) from two-month-old male mice fed normal chow after overnight fasting, n=5–7 for each genotype.
(E) Hepatic TG levels from random-fed, two-month-old male mice on normal chow, n=3–5 for each group. *, P < 0.05, **, P < 0.01 by one-way ANOVA followed by Tukey’s post-test; error bars represents SEM.
After an overnight fast, AFP>Cre;Akt2(loxP/loxP), AFP>Cre;FoxO1(loxP/loxP) and AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) mice had hepatic TG levels indistinguishable from that in wild type controls (Leavens et al., 2009; Matsumoto et al., 2007) (Figure 2D). Fed AFP>Cre;FoxO1(loxP/loxP) mice displayed increased hepatic TG levels compared to controls and AFP>Cre;Akt2(loxP/loxP) mice, though TG secretion was unaltered (Figure 2E and S4). Surprisingly, the accumulation of liver TG in the AFP>Cre;FoxO1(loxP/loxP) mice was suppressed by concomitant deletion of Akt2 (Figure 2E). Though deletion of FoxO1 lead to a phenotype opposite to that of deletion of Akt2, these data are inconsistent with a model in which Akt2 regulates lipid accumulation through suppression of FoxO1 activity.
Akt2 Regulates Hepatic TG Levels Independent of FoxO1 upon HFD Feeding
Suppression of FoxO1 by shRNA in livers from DIO mice decreases blood glucose and insulin levels, and improves insulin sensitivity as assessed by the euglycemic, hyperinsulinemic clamp (Samuel et al., 2006). To investigate the role of FoxO1 as a downstream mediator of Akt’s role in metabolism under pathological conditions, we placed the wild type controls, AFP>Cre;Akt2(loxP/loxP), AFP>Cre;FoxO1(loxP/loxP) and AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) mice on a high fat diet for 3 months. Glucose tolerance tests were indistinguishable in mice of all four genotypes (Figure 3A). Fasting and fed serum insulin and TG levels were also similar among these same mice (Figure 3B and 3C). However, under fed conditions, loss of hepatic Akt2 in HFD fed mice trended to a lower TG level in liver, whereas liver TG upon ablation of FoxO1 did not differ from wild type controls (Figure 3D). Deletion of FoxO1 concomitant with Akt2 did not reverse the protection from steatosis in AFP>Cre;Akt2(loxP/loxP) mice (Figure 3D), again militating against the idea that FoxO1 regulates lipid metabolism downstream of Akt2.
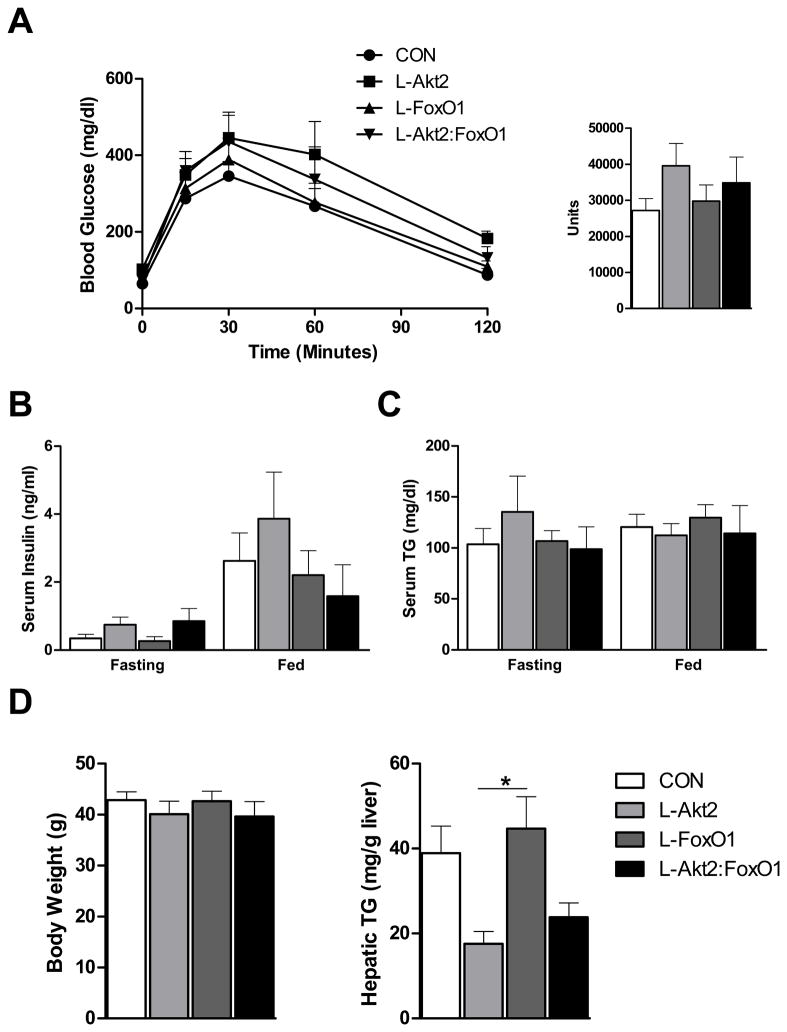
Figure 3. Loss of Hepatic Akt2 Decreases TG Accumulation in Liver Independent of FoxO1 under HFD Feeding.
(A) Glucose tolerance test (GTT). Four-month-old male mice fed a HFD for 3 months were fasted overnight, and injected intraperitoneally with 2g/kg glucose (left). Area under curve were taken from GTT (right), n= 4–5 for each genotype.
(B) Fasting and random-fed serum insulin levels, n=4–5 for each genotype.
(C) Fasting and random-fed serum TG levels, n=4–5 for each genotype.
(D) Body weight (left) and hepatic TG levels (right) under random-fed conditions on HFD, n= 4–6. Body weight from control mice that were of comparable age but maintained on normal chow was 31.5 ± 1.3g. The variance of hepatic TG levels was significant among four different genotypes by one-way ANOVA. *, P < 0.05 by one-way ANOVA followed by Tukey’s post-test; error bars represents SEM. Abbreviations are as indicated in Figure 2.
Loss of Akt2 Decreases Hepatic TG levels in FoxO1 Knockout Mice after Aurothioglucose Treatment
In DIO, deletion of Akt2 in liver reduces hepatic TG without altering lipogenesis, whereas in ob/ob mice protection from steatosis is accompanied by a concomitant reduction in lipogenesis and lipogenic gene expression (Leavens et al., 2009). To study the AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) mice under conditions that mimic ob/ob mice, we injected mice with aurothioglucose (gold-thioglucose, GTG), which causes hypothalamic damage, hyperphagia, and weight gain (Brecher et al., 1965; Marshall and Mayer, 1954). As shown in Figure 4A, eight weeks after GTG injection, mice of all genotypes displayed significantly increased body weight and hepatic TG levels compared to age-match controls (compare Figure 4B to Figure 2E). AFP>Cre;Akt2(loxP/loxP) mice trended to lower hepatic TG, while AFP>Cre;FoxO1(loxP/loxP) mice had significantly elevated TG in liver (Figure 4B). Interestingly, concomitant deletion of Akt2 and FoxO1 led to livers that accumulated TG levels comparable to those in AFP>Cre;Akt2(loxP/loxP) rather than AFP>Cre;FoxO1(loxP/loxP) mice (Figure 4B). This showed that the Akt2 mutant phenotype was dominant to that of FoxO1, which, again, did not support Akt2 as upstream of FoxO1, but rather suggested that Akt2 regulates hepatic TG levels downstream of or in parallel to FoxO1.
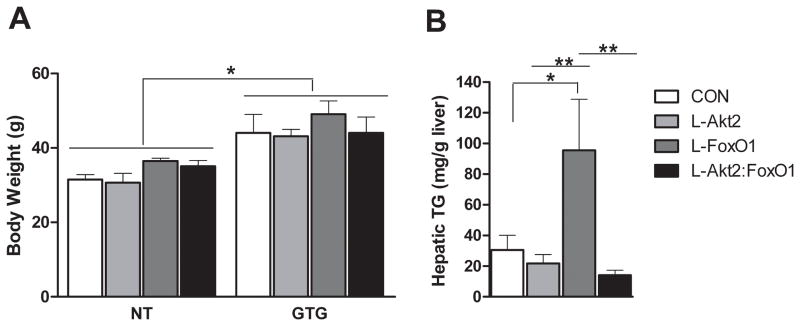
Figure 4. Loss of Hepatic Akt2 Decreases TG Accumulation in Liver Independent of FoxO1 After Aurothioglucose Treatment.
(A) Mice with aurothioglucose (GTG) injection gained more weight than age-match not-treated (NT) controls. Male mice were fasted overnight, and then injected with 0.3g/kg bodyweight GTG at 4–5-week-old, and maintained on normal chow for eight weeks before analysis, n=4–8 for each genotype; *, P < 0.05 by two-way ANOVA followed by Bonferroni’s post-test.
(B) Hepatic TG levels under random-fed conditions in mice injected with GTG as described above, n= 6–10 for each group. *, P < 0.05, **, P < 0.01 by one-way ANOVA followed by Tukey’s post-test; error bars represents SEM. Abbreviations are as indicated in Figure 2.
Akt2 is Required for Postprandial de novo Lipogenesis
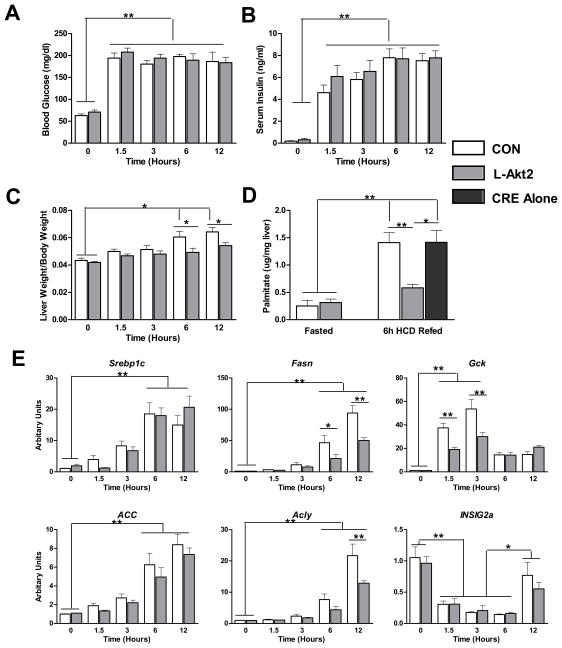
In order to address the pathways downstream of Akt2 that mediate lipid homeostasis, we sought to establish a model more physiological than hypothalamic obesity that would test the dependency of hepatic lipogenesis on Akt2. To accomplish this, mice were fasted overnight, and then refed a lipogenic high-carbohydrate diet (HCD). As expected, blood glucose and serum insulin levels increased by 1.5 hour after HCD refeeding, and remained high through the 12 hours animals were allowed continuous access to food (Figure 5A and 5B). Deletion of Akt2 in mouse liver did not alter the glucose or insulin levels comparing to wild type controls, indicating the minimal requirement for hepatic Akt2 in control of postprandial carbohydrate metabolism (Figure 5A and 5B). Liver weights normalized to body weight also increased significantly after 6-hour refeeding in wild type mice, but this was blunted in AFP>Cre;Akt2(loxP/loxP) mice (Figure 5C). To evaluate the rate of de novo lipogenesis under these conditions, we injected D2O at the third hour after feeding, and analyzed the incorporation of D2O into liver palmitate three hours later, i.e. 6-hours after feeding. During the labeling period, de novo lipogenesis increased about 5-fold compared to that during fasting, and this effect was blunted in the livers lacking Akt2 (Figure 5D). These data point to a discrepancy between the requirements for Akt2 in hepatic lipogenesis versus glycemia following a high carbohydrate meal. Hepatic mRNAs encoding lipogenic enzymes increased after refeeding a high-carbohydrate meal (Figure 5E). Surprisingly, the expression of Srebp1c and its targets Acly and Acc were only minimally if at all reduced in AFP>Cre;Akt2(loxP/loxP) mice, though there was a blunting in the induction of glucokinase (Gck) and Fasn (Figure 5E). HCD feeding suppressed insulin induced gene 2a (INSIG2a) expression for at least 6 hours independent of the presence of hepatic Akt2 (Figure 5E).
Figure 5. Akt2 is Required for de novo Lipogenesis after HCD Refeeding.
(A) Blood glucose levels after HCD refeeding. Mice were pre-fed with HCD for 2 days, fasted overnight, and refed with HCD. Blood glucose was measured at the indicated time points, n=4–6 for each condition.
(B) Serum insulin levels after HCD refeeding, n=4–6 for each condition.
(C) Liver weight to body weight ratio after HCD refeeding, n=4–6 for each condition.
(D) Newly synthesized palmitate. Mice treated as above were injected with 20ul/g bodyweight D2O three hours after HCD refeeding. 6h after refeeding, mice were sacrificed and incorporation of D2O into palmitate measured in liver, n=3–5 for each condition.
(E) Lipogenic gene expression after HCD refeeding. Mice were treated as described in (A), n=4–6 for each condition.
Con, wild type controls; L-Akt2, AFP>Cre;Akt2(loxP/loxP); *, P < 0.05, **, P < 0.01 by two-way ANOVA followed by Bonferroni’s post-test; error bars represents SEM.
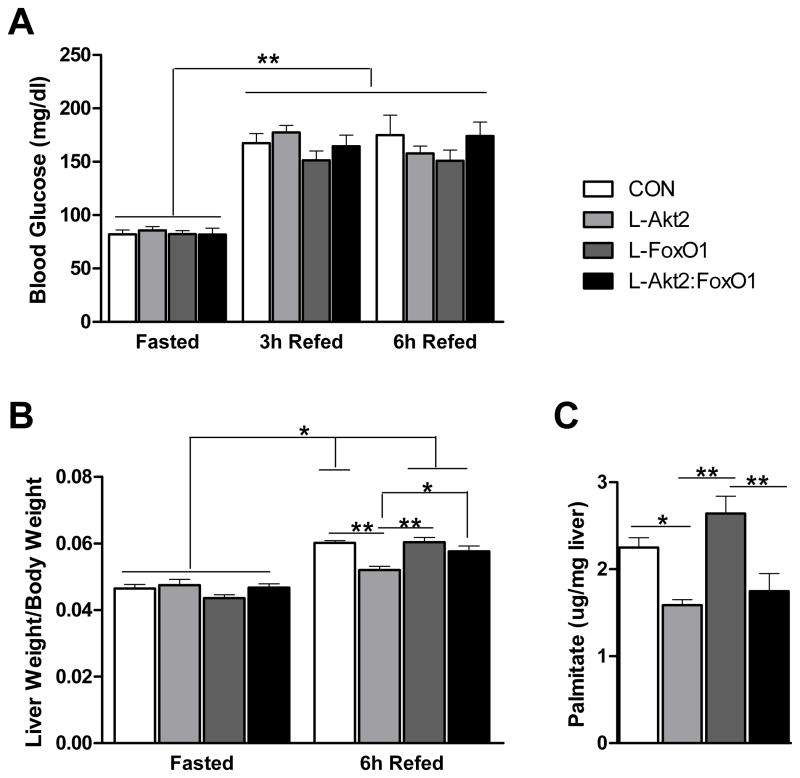
To test whether unrestrained activity of FoxO1 is the major factor responsible for the lack of postprandial de novo lipogenesis and hepatic weight gain in AFP>Cre;Akt2(loxP/loxP) mice, we measured fatty acid synthesis in livers from AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) mice. Elimination of FoxO1 in liver was without effect on the increase in blood glucose evident after ingestion of a HCD (Figure 6A). However, deletion of FoxO1 in liver appeared to reverse the prevention in hepatic weight gain evident in the AFP>Cre;Akt2(loxP/loxP) livers, much like the suppression of a similar defect in livers lacking IRS1 and IRS2 (Figure 6B) (Dong et al., 2008). Deletion of FoxO1 alone in liver led to a trend for increased de novo lipogenesis following a meal, consistent with the increase in hepatic TG under several conditions (Figures 2E, 4B and 6C). Unlike liver size, the decrease in lipid synthesis in livers lacking Akt2 was not reversed by concomitant removal of FoxO1; instead, AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) livers demonstrated rates of de novo lipogenesis virtually identical to those in AFP>Cre;Akt2(loxP/loxP) livers (Figure 6C). Delivery by adenovirus of a dominant-negative form of FoxO1 (Nakae et al., 2001) into AFP>Cre; Akt2(loxP/loxP);FoxA2(loxP/loxP) livers also failed to reverse the decreased lipogenesis in Akt2 null livers (data not shown). These data are consistent with those presented above in which Akt2 is epistatic to FoxO1 regarding the control of liver TG content.
Figure 6. Loss of Hepatic Akt2 Decreases de novo Lipogenesis Independent of FoxO1.
(A) Fasting and refed blood glucose levels. Two-month-old male mice were placed on HCD for three days, fasted overnight, and refed with HCD for 6 hours. Blood glucose levels were taken at fasting, 3h refeeding and 6h refeeding time points, n= 5–7 for each genotype, **, P < 0.01 by two-way ANOVA followed by Bonferroni’s post-test, error bars represents SEM.
(B) Liver weight to body weight ratio. Mice were as described above. 6h after refeeding, mice were sacrificed, and body weight and wet liver weight were determined, n=5–7 for each condition, *, P < 0.05, **, P < 0.01 by two-way ANOVA followed by Bonferroni’s post-test, error bars represents SEM.
(C) De novo lipogenesis, n=4–7 for each genotype, **, P < 0.01 by one-way ANOVA followed by Tukey’s post-test; error bars represents SEM. Abbreviations are as indicated in Figure 2.
mTORC1 is Required for de novo Lipogenesis in mouse liver
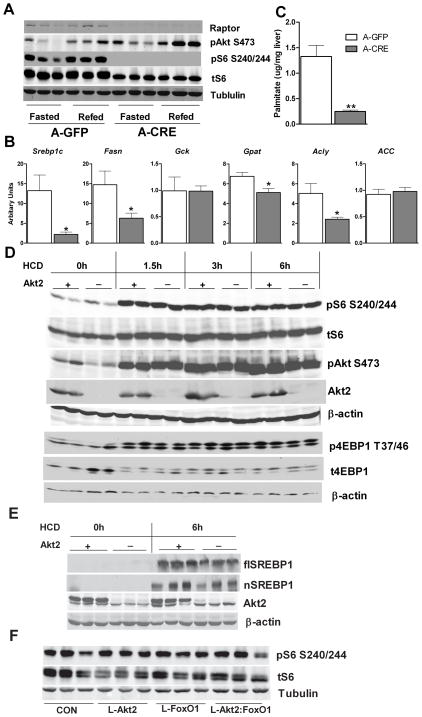
We next turned to the question: since FoxO1 and FoxA2 do not appear to function downstream of Akt2 in lipid homeostasis, what pathway could mediate Akt2’s effect on lipogenic gene expression? The most likely answer lies in the recent observation that in primary hepatocytes, insulin utilizes a pathway dependent on mTORC1 to stimulate the processing and expression of SREBP1c, a transcription factor that drives coordinated lipogenic gene transcription (Azzout-Marniche et al., 2000; Chakrabarti et al., 2010; Duvel et al., 2010; Fleischmann and Iynedjian, 2000; Li et al., 2010; Porstmann et al., 2008). To test the requirement for this pathway in vivo, we injected raptor(loxP/loxP) mice with an adeno-associated virus expressing liver-specific Cre recombinase (AAV>Cre) or green fluorescent protein (AAV>GFP), thus generating hepatic raptor knockout mice or controls. As shown in Figure 7A, raptor protein levels were substantially reduced in AAV>Cre;raptor(loxP/loxP) mice, and the phosphorylation of an mTORC1 target, ribosomal protein S6 (S6), was not detected, as assayed by reactivity with a phospho-specific antibody (Figure 7A). Expression of the lipogenic genes Srebp1c, Fas, Acly and Gpat were reduced in AAV>Cre;raptor(loxP/loxP) mice, though Acc and Gck were unchanged under random fed conditions (Figure 7B). Most notably, de novo lipogenesis following a high-carbohydrate meal was largely abrogated in liver from AAV>Cre;raptor(loxP/loxP) mice (Figure 7C).
Figure 7. Raptor Is Required for Lipogenic Gene Expression and de novo Lipogenesis.
(A) Western blot of Raptor knockout livers. RaptorloxP/loxP male mice were maintained on normal chow, and injected with either AAV-TBG-GFP (A-GFP) or AAV-TBG-Cre (AAV>Cre;raptor(loxP/loxP); A-CRE) at 6-weeks of age. Two weeks after virus injection, mice were fasted overnight (fasted), and then refed with normal chow for 4 hours (refed) before sacrifice.
(B) Lipogenic gene expression under random-fed conditions, n=6–7 for each condition, *, p < 0.05; **, p < 0.01 by student t-test; error bars represents SEM.
(C) De novo lipogenesis, n=6–7 for each condition, **, p < 0.01 by student t-test; error bars represents SEM.
(D) Western blot for liver samples from wild type controls and AFP>Cre;Akt2(loxP/loxP) mice after refeeding HCD. Mice were treated as described in Figure 5.
(E) Western blot for full-length SREBP1 (flSREBP1) and processed SREBP1 (nSREBP1). Liver samples from 24h fasted or 6h HCD refeeding control or AFP>Cre;Akt2(loxP/loxP) mice were used for blotting full-length SREBP1 and processed SREBP1.
(F) Western blot for liver samples from wild type controls (CON), AFP>Cre;Akt2(loxP/loxP) (L-Akt2), AFP>Cre;FoxO1(loxP/loxP) (L-FoxO1) and AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) (L-Akt2:FoxO1) mice 6h after HCD refeeding. Mice were treated as described in Figure 6C.
Having established the critical role of mTORC1 in de novo lipogenesis, we then asked whether defects in its expression and posttranslational processing of SREBP1c explained the disturbed lipid metabolism in AFP>Cre;Akt2(loxP/loxP) mice. Following a high carbohydrate meal, there was an increase in the phosphorylated form of protein S6 and eukaryotic translation initiation factor 4E-1A-binding protein 1 (4EBP1), which reflect activation of mTORC1 by nutrients and insulin. Surprisingly, this was unchanged in AFP>Cre;Akt2(loxP/loxP) mice (Figure 7D). Both the precursor and processed form of SREBP1c increased 6-hour after HCD feeding; induction of these proteins was only slightly reduced in AFP>Cre;Akt2(loxP/loxP) mice (Figure 7E), consistent with the minimal changes in lipogenic gene expression under these conditions (Figure 5E). These results indicated that the defective lipogenesis was unlikely to be attributed to lack of activation of mTORC1 and SREBP1c. Moreover, in the absence of Akt2, stimulation of mTORC1 and SREBP1c are not sufficient to promote de novo lipogenesis following a high carbohydrate meal (Figure 5D, 5E, 7D and 7E).
FoxO1 has been suggested to suppress mTORC1 activity by several mechanisms, e.g. stimulating the expression of mTORC1 inhibitors such as Sestrin3, Raptor independent companion of mTOR (Rictor), and possibly Tuberous sclerosis complex 1(TSC1) (Chen et al., 2010; Harvey et al., 2008). To ask whether such a mechanism accounts for the suppression of lipid accumulation by FoxO1, we assessed mTORC1 activity in animals lacking hepatic FoxO1. Six hours after ingesting a high-carbohydrate meal, hepatic phospho-S6 was not altered in AFP>Cre;FoxO1(loxP/loxP) or AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) mice (Figure 7E). The expression of Srebp1c, Gck, Fasn, Acc, Acly and Sestrin3 was also unchanged in livers from AFP>Cre;FoxO1(loxP/loxP) mice after HCD refeeding (Figure S5). These data argue against mTORC1 as the mediator of hepatic TG accumulation induced by deletion of FoxO1.
Insulin-simulated Phosphorylation of ATP Citrate Lyase is Blunted in Akt2-Deficient Livers
To explore other potential mechanisms utilized by Akt2 to regulate lipid metabolism, we examined two other pathways known to modulate hepatic lipogenesis activity responding to insulin signaling. ATP-Citrate lysases (ACLY) catalyzes the cleavage of cytosolic citrate to oxaloacetate and acetyl-CoA, the latter a precursor for fatty acid synthesis. The activity of ACLY is regulated transcriptionally as a target of SREBP1c, as well at the posttranslational level by Akt and protein kinase A phosphorylation (Berwick et al., 2002; Horton et al., 2002; Potapova et al., 2000). Insulin injection stimulated phosphorylation of Akt and ACLY, and both were blunted in livers from AFP>Cre;Akt2(loxP/loxP) mice (Figure S7A). To study this in more detail, hepatocytes were isolated form control and Akt2 KO livers. Again, insulin promoted Akt and ACLY phosphorylation in a dose dependent manner. Both were reduced in primary hepatocytes from Akt2-deficient livers, particularly at low concentrations of insulin (Figure S7B). Those data provide an additional pathway potentially dependent on Akt2 for the increase in de novo lipogenesis in liver.
Atypical PKCs (aPKCs) have been reported to be activated by insulin and to promote SREBP1c expression in liver (Farese et al., 2005; Matsumoto et al., 2003; Taniguchi et al., 2006). To ask whether aPKC is dependent on Akt2, we measured aPKC phosphorylation and activity in livers from control and AFP>Cre;Akt2(loxP/loxP) mice, but found the levels to be indistinguishable (data not shown). However, we were unable to detect increases in hepatic aPKC phosphorylation or activity following insulin injection of control or AFP>Cre;Akt2(loxP/loxP) mice (data not shown).
DISCUSSION
The metabolic disturbances associated with type 2 diabetes mellitus and the metabolic syndrome have been perceived as due to an inability of insulin to promote effectively its anabolic actions, a condition long referred to as insulin resistance. However, this view has been modified recently with the increasing appreciation that, while the “insulin-resistant” liver displays some features consistent with an inability of insulin to act, most notably an inappropriately high glucose output, its characteristic increased de novo lipogenesis and TG accumulation more closely resemble a state of augmented insulin sensitivity. The simplest, though by no means only explanation for this phenomenon is that insulin resistance occurs at a step distal to a bifurcation in insulin signaling, resulting in selective inhibition of the hormone’s effect on glucose output, while permitting signaling to lipid metabolism. Work from our laboratory as well as others suggests that this divergence occurs downstream of Akt, and thus the identification of the specific pathways that Akt2 utilizes to regulate metabolism has achieved particular importance (He et al., 2010; Leavens et al., 2009). In this study, we investigated the roles of two proposed Akt target transcription factors, FoxO1 and FoxA2, and an indirect route for control of a third, SREBP1c, which is regulated by mTORC1. Our data show that, whereas suppression of FoxO1 activity is downstream of Akt in the inhibition of hepatic glucose output, phosphorylation of neither FoxO1 nor FoxA2 is likely to mediate insulin’s effects on de novo lipogenesis and TG accumulation in liver. Our data also support the notion that mTORC1 is required for maintenance of Srebp1c expression and de novo lipogenesis, but its activation is not sufficient to reverse the reduction of de novo lipogenesis coincident with deletion of Akt2. Moreover, it is likely that the mechanism by which Akt2 induces lipogenesis after a high-carbohydrate meal is not based on regulation of gene expression by any of the three transcription factors assessed in this study.
Considerable data support a role for FoxO1 in the control of hepatic gluconeogenic genes expression, though there is much less clarity regarding its importance for glycemia and hepatic lipid metabolism. As has been reported, we found that under normal conditions, hepatic deletion of FoxO1 resulted in an impaired pyruvate tolerance test, in this study independent of Akt2 (Figure 2B and 2C) (Matsumoto et al., 2007). Inhibition of FoxO1 activity by various techniques in genetically obese and DIO mouse models decreases blood glucose and insulin levels, and reduces hepatic glucose production as assayed by hyperinsulinemic-euglycemic clamp; loss of hepatic FoxO1 ameliorates fasting hyperglycemia in the STZ-induced diabetic mice (Altomonte et al., 2003; Haeusler et al., 2010a; Kamagate et al., 2008; Nakae et al., 2002; Samuel et al., 2006). These studies suggest that FoxO1 activity contributes significantly to insulin resistance under pathological conditions. However, in our studies, AFP>Cre;FoxO1(loxP/loxP) mice did not demonstrate reduced blood glucose and insulin levels during DIO, perhaps due to the diet employed and/or the mouse strain used (Figure 3A and 3B).
We also found that livers deficient FoxO1 trended to increased hepatic TG content, as was reported previously after siRNA-mediated knockdown of FoxO1 or ablation of three FoxO isoforms in liver; these data suggest that FoxO1 normally suppresses lipid accumulation in liver (Kamagate et al., 2008; Tao et al., 2011). Nonetheless, the action of Akt2 to promote hepatic lipid accumulation is not mediated by a suppression of FoxO1, as concomitant deletion of both genes leads to a phenotype resembling loss of Akt2 alone (Figure 2E, 4B and 6C). Rather, FoxO1 signals to TG content by a pathway genetically upstream of or parallel to Akt2. FoxO1 regulates hepatic lipid content during fasting by accelerating TG secretion through increases in MTP (Kamagate et al., 2008). However, others have suggested FoxO1 inhibits VLDL-TG secretion in pathological conditions (Dong et al., 2008; Haeusler et al., 2010a; Matsumoto et al., 2007; Zhang et al., 2006). Fasting hepatic and serum TG levels were not changed in AFP>Cre;FoxO1(loxP/loxP) mice under normal conditions in this study and other’s (Figure 2D and S3C) (Matsumoto et al., 2007), and no change was found on the rate of TG secretion in AFP>Cre;FoxO1(loxP/loxP) mice (Figure S4). There were no alterations in AFP>Cre;FoxO1(loxP/loxP) mice of serum TG under obese conditions, and MTP expression was not changed in AFP>Cre;FoxO1(loxP/loxP) mice during hypothalamic obesity (Figure 3 and S6). These data suggest that changes in TG secretion are not responsible for hepatic TG accumulation in AFP>Cre;FoxO1(loxP/loxP) mice. FoxO1 also modulates mTORC1 activity, which regulates Srebp1c levels and lipogenesis (Chen et al., 2010; Duvel et al., 2010; Harvey et al., 2008). However deletion of Akt2, FoxO1 or both did not alter phosphorylation of ribosomal protein S6 after HCD refeeding (Figure 7E), suggesting that the pathway by which FoxO1 normally suppresses lipid accumulation is independent of mTORC1.
FoxA2 has been reported to stimulate fatty acid oxidation, increase TG secretion and reduce hepatic TG accumulation in obese animals (Wolfrum et al., 2004; Wolfrum et al., 2008; Wolfrum and Stoffel, 2006). Much of the data supporting this model are derived from experiments involving expression of constitutively-active FoxA2 in mouse liver, and thus have the potential of off-target effects. Other experiments took advantage of the reduction in nuclear FoxA2 associated with the obese state, during which the liver is likely to have numerous uncharacterized, complicating metabolic alterations. However, other studies found no change to the nuclear localization of FoxA2, even in the extreme case of elevated insulin levels in ob/ob mice (Zhang et al., 2005). In loss of function studies, liver-specific FoxA2 knockout mice displayed no abnormality in hepatic TG levels, consistent with the current results (Bochkis et al., 2008). Under normal chow feeding or in mice with DIO, loss of hepatic FoxA2 did not affect serum glucose and insulin or hepatic TG levels (Figure 1B, 1C, S1 and S2). These data are compatible with a model in which inhibition of FoxA2 is not required for Akt2 to regulate hepatic TG accumulation. These results are qualified by the possibility that, in other mouse strains, the requirements for hepatic steatosis might be different.
Recently, mTORC1 has been shown to enhance de novo lipogenesis by regulating Srebp1c expression and processing (Chakrabarti et al., 2010; Duvel et al., 2010; Li et al., 2010; Porstmann et al., 2008). The full activation of mTORC1 requires both nutrients and growth factor signaling, which determine cellular location and Akt-dependent activation of the mTORC1 complex, respectively (Sancak et al., 2010; Sancak et al., 2008; Sengupta et al., 2010b). In tissue culture cells and primary hepatocytes, insulin/Akt regulates SREBP1c expression and processing and de novo lipogenesis in a mTORC1 dependent manner (Azzout-Marniche et al., 2000; Chakrabarti et al., 2010; Duvel et al., 2010; Fleischmann and Iynedjian, 2000; Li et al., 2010; Porstmann et al., 2008). However, the model of an obligate role for mTORC1 in hepatic lipogenesis has not been tested genetically in vivo. Herein we report that conditional deletion of raptor in liver abolished mTORC1 activity, leading to a reduction in the expression of Srebp1c and its targets and decreased de novo lipogenesis (Figure 7A, 7B and 7C). Interestingly, even though AFP>Cre;Akt2(loxP/loxP) and AFP>Cre;Akt2(loxP/loxP);FoxO1(loxP/loxP) mice exhibit significantly decreased de novo lipogenesis following a high-carbohydrate meal, activation of mTORC1 and SREBP1c were largely unaffected (Figure 5D, 7D, 7E, and S5). These data indicate that mTORC1 activation is not sufficient to induce lipogenesis under these conditions. During the revision of this paper, Yecies et al. reported a mTORC1-independent pathway by which Akt2 signals through INSIG2a to promote SREBP1c processing (Yecies et al., 2011). We found no change in the normal decrease in hepatic INSIG2a mRNA in AFP>Cre;Akt2(loxP/loxP) mice after HCD refeeding and only a modest reduction in the nuclear form of SREBP1c or expression of its target genes (Figure 5E and 7E). Thus, the mTORC1-independent pathway revealed in this study by liver-specific deletion of Akt2 does not represent induction of INSIG2a and is unlikely to be related to SREBP1c processing or expression. Rather, we propose that the requirement for Akt2 is linked to its role in the posttranslational control of other targets such as ACLY.
Taking these data in aggregate, we suggest that Akt2 promotes hepatic lipid accumulation and de novo lipogenesis independent of FoxA2 and FoxO1. Moreover, activated mTORC1 and SREBP1c and increased lipogenic gene expression is required but not sufficient for postprandial lipogenesis. Thus, our data provide strong support that nutrients have a direct role in the liver to promote anabolic lipid metabolism by a process dependent on the presence of a functional mTORC1 complex as well as other Akt-dependent signaling pathways.
EXPERIMENTAL PROCEDURES
Animals
The AFP>Cre;Akt2(loxP/loxP), AFP>Cre;FoxO1(loxP/loxP), and RaptorloxP/loxP mice have been described previously (Leavens et al., 2009; Matsumoto et al., 2007; Sengupta et al., 2010a; Zhang et al., 2005). All animals were raised and treated with approval from the University of Pennsylvania IACUC in accordance with NIH guidelines. Mice were a 129/FVB/C57BL6 mixed background, and male littermates were used for all studies. In the FoxA2 experiments, the wild type control group included mice of genotypes AFP>CRE;Akt2loxP/+; FoxA2loxP/+; and Akt2loxP/LoxP; FoxA2loxP/loxP; mice labeled as AFP>Cre;Akt2(loxP/loxP) included the genotypes AFP>Cre;Akt2loxP/loxP; FoxA2loxP/+ and AFP>Cre;Akt2loxP/loxP; FoxA2+/+; mice labeled as AFP>Cre;FoxA2(loxP/loxP) included the genotypes AFP>Cre;Akt2loxP/+; FoxA2loxP/loxP and AFP>Cre;Akt2+/+; FoxA2loxP/loxP. In the FoxO1 experiments, wild type controls include genotypes AFP>Cre;Akt2loxP/+; FoxO1loxP/+ and Akt2loxP/LoxP; FoxO1loxP/loxP; mice indicated as AFP>Cre;Akt2(loxP/loxP) included the genotypes AFP>Cre;Akt2loxP/loxP; FoxO1loxP/+ and AFP>Cre;Akt2loxP/loxP; FoxO1+/+; mice labeled as AFP>Cre;FoxO1(loxP/loxP) included the genotypes AFP>Cre;Akt2loxP/+; FoxO1loxP/loxP and AFP>Cre;Akt2+/+; FoxO1loxP/loxP. In all cases there were no metabolic phenotypes among the pooled genotypes including Cre express alone. Mice were maintained on a normal chow diet (laboratory rodent diet 5001, LabDiet) unless otherwise noted under 12 hour light:dark cycle (7am:7pm) in a barrier facility. When fed with HFD (D12331i, Research Diets), mice were started at 1-month-old age, and remained for 3 months.
For Aurothioglucose (GTG, A0606, Sigma Aldrich) treatment, mice were maintained on normal chow. At 5–6-week-old, mice were fasted for 24 hours, and then injected intraperitoneally with one dose of 0.3g/kg GTG in PBS. After injection, mice were kept fasted for another 20 hours before normal chow diet was freely accessible for the following 2 months. This protocol has been suggested to reduce mortality caused by GTG injection in mice (Brecher et al., 1965).
For acute excision of Raptor in liver, the adeno-associated virus was generated by Viral Vector Core at University of Pennsylvania. GFP or Cre reconbimase was driven by a liver specific promoter, Thyroxine binding globulin (TBG) Promoter. Mice were injected postorbitally at a dosage of 1.0×1011gc virus diluted in PBS at 6-week-old age, and maintained on normal chow for another two weeks before analysis.
Metabolic Measurement
Blood glucose, serum insulin and hepatic and serum TG levels were measured as described before (Leavens et al., 2009). For experiments involving fasting, mice were deprived of food overnight. For “fed” measurements, mice were reared on designated diet ad libitum before blood and liver samples were taken during the morning between 8:00am and 10:00am. For glucose and pyruvate tolerance test, mice were fasted overnight followed by intraperitoneal injection of 2g/kg glucose or pyruvate, and then blood glucose levels were taken at designated time points.
De novo Lipogenesis
For de novo lipogenesis assay, mice were fed with HCD (D12450B, Research Diets) for two days, and then treated as described in the text. Whole blood and liver samples were taken 3 hours after D2O injection and frozen in liquid nitrogen. Palmitate was isolated and analyzed by using gas chromatography-electron impact ionization mass spectrometry by the Stable Isotope Tracer Kinetic Service Center in the Institute of Diabetes, Obesity and Metabolism. The absolute amount of newly made palmitate was assumed equivalent to the de novo lipogenesis rate.
TG Secretion Assay
TG secretion assay was performed as described previously (Millar et al., 2005). Mice were fasted overnight, and then injected I.P. with 1g/kg poloxamer 407 (kindly provided by Dr. John S. Millar, University of Pennsylvania). Blood samples were collected at indicated time points, and assayed for serum TG.
Statistics
All data are presented as mean ± SEM. As noted in figure legends, data were analyzed by using unpaired student t-test with two-tailed analysis, one-way ANOVA followed by Tukey’s post-test, or two-way ANOVA followed by Bonferroni post-test.
Supplementary Material
Acknowledgments
This work was supported by NIH grants RO1 DK56886, and PO1 DK49210 to M.J.B., and 1F30 DK081283 (K.F.L.). We would like to thank Dr. Domenico Accili of Columbia University for kindly providing us the FoxO1loxP/loxP mice; Dr. John S. Millar for suggestions on de novo lipogenesis assay and TG secretion assay. Deuterium labeled palmitate levels were measured by the Stable Isotope Tracer Kinetic Service Center in the Institute of Diabetes, Obesity and Metabolism, University of Pennsylvania. The Viral Vector Core of University of Pennsylvania supported by the Diabetes and Endocrinology Research Center (NIH DK-19525) provided viruses and valuable advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab. 2003;285:E718–728. doi: 10.1152/ajpendo.00156.2003. [DOI] [PubMed] [Google Scholar]
- Argaud D, Zhang Q, Pan W, Maitra S, Pilkis SJ, Lange AJ. Regulation of rat liver glucose-6-phosphatase gene expression in different nutritional and hormonal states: gene structure and 5’-flanking sequence. Diabetes. 1996;45:1563–1571. doi: 10.2337/diab.45.11.1563. [DOI] [PubMed] [Google Scholar]
- Azzout-Marniche D, Becard D, Guichard C, Foretz M, Ferre P, Foufelle F. Insulin effects on sterol regulatory-element-binding protein-1c (SREBP-1c) transcriptional activity in rat hepatocytes. Biochem J. 2000;350(Pt 2):389–393. [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14:828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher G, Laqueur GL, Cronkite EP, Edelman PM, Schwartz IL. The Brain Lesion of Goldthioglucose Obesity. J Exp Med. 1965;121:395–401. doi: 10.1084/jem.121.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59:775–781. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham SK, Suwanichkul A, Scheimann AO, Yee D, Jackson JG, Barr FG, Powell DR. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology. 1999;140:3140–3146. doi: 10.1210/endo.140.7.6856. [DOI] [PubMed] [Google Scholar]
- Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Sajan MP, Standaert ML. Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans. 2005;33:350–353. doi: 10.1042/BST0330350. [DOI] [PubMed] [Google Scholar]
- Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12(Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- Fleischmann M, Iynedjian PB. Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem J. 2000;349:13–17. doi: 10.1042/0264-6021:3490013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208–214. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- Haeusler RA, Han S, Accili D. Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. J Biol Chem. 2010a;285:26861–26868. doi: 10.1074/jbc.M110.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Kaestner KH, Accili D. FoxOs Function Synergistically to Promote Glucose Production. J Biol Chem. 2010b;285:35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Mattila J, Sofer A, Bennett FC, Ramsey MR, Ellisen LW, Puig O, Hariharan IK. FOXO-regulated transcription restricts overgrowth of Tsc mutant organs. J Cell Biol. 2008;180:691–696. doi: 10.1083/jcb.200710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Hou X, Kanel G, Zeng N, Galicia V, Wang Y, Yang J, Wu H, Birnbaum MJ, Stiles BL. The critical role of AKT2 in hepatic steatosis induced by PTEN loss. Am J Pathol. 2010;176:2302–2308. doi: 10.2353/ajpath.2010.090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, 3rd, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Mayer J. Energy balance in goldthioglucose obesity. Am J Physiol. 1954;178:271–274. doi: 10.1152/ajplegacy.1954.178.2.271. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, et al. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46:2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- O’Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA. Insulin-regulated gene expression. Biochem Soc Trans. 2001;29:552–558. doi: 10.1042/bst0290552. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova IA, El-Maghrabi MR, Doronin SV, Benjamin WB. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry. 2000;39:1169–1179. doi: 10.1021/bi992159y. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, Meseck M, Dong HH. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–5652. doi: 10.1210/en.2006-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, et al. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes. 2006;55:2042–2050. doi: 10.2337/db05-0705. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010a;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010b;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Tao R, Wei D, Gao H, Liu Y, Depinho RA, Dong XC. Hepatic FoxOs Regulate Lipid Metabolism via Modulation of Expression of the Nicotinamide Phosphoribosyltransferase Gene. J Biol Chem. 2011;286:14681–14690. doi: 10.1074/jbc.M110.201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Howell JJ, Ndungo E, Stoffel M. Foxa2 activity increases plasma high density lipoprotein levels by regulating apolipoprotein M. J Biol Chem. 2008;283:16940–16949. doi: 10.1074/jbc.M801930200. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt Stimulates Hepatic SREBP1c and Lipogenesis through Parallel mTORC1-Dependent and Independent Pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rubins NE, Ahima RS, Greenbaum LE, Kaestner KH. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2005;2:141–148. doi: 10.1016/j.cmet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.