SUMMARY
The Drosophila ecdysone receptor (EcR/Usp) is thought to activate or repress gene transcription depending on the presence or absence, respectively, of the hormone ecdysone. Unexpectedly, we found an alternative mechanism at work in salivary glands during the ecdysone-dependent transition from larvae to pupae. In the absense of ecdysone, both ecdysone receptor subunits localize to the cytoplasm, and the heme-binding nuclear receptor E75A replaces EcR/Usp at common target sequences in several genes. During the larval-pupal transition, a switch from gene activation by EcR/Usp to gene repression by E75A is triggered by a decrease in ecdysone concentration and by direct repression of the EcR gene by E75A. Additional control is provided by developmentally-timed modulation of E75A activity by NO, which inhibits recruitment of the co-repressor SMRTER. These results suggest a mechanism for sequential modulation of gene expression during development by competing nuclear receptors and their effector molecules, ecdysone and NO.
Keywords: ecdysone, nuclear receptors, co-activators, co-repressors, nuclear shuttling
INTRODUCTION
Nuclear receptors (NRs) play important roles in gene regulation. In general, steroid hormone NRs translocate from the cytoplasm to the nucleus in a hormone-dependent fashion to activate gene transcription (reviewed in (Hager et al., 2000). Other NRs such as the retinoid X receptor (RXR) and the all-trans retinoic acid receptor (RAR) reside in the nucleus and constitutively occupy their DNA response elements, activating and repressing transcription in the presence and absence of ligand, respectively (reviewed in (Glass and Rosenfeld, 2000). However, recent studies have blurred these distinctions. For example, in cultured cells, some non-steroidal NRs, such as RXR, were found in the cytoplasm in the absence of ligand (Cao et al., 2004). Molecular data for NR-dependent mechanisms in a developmental context is quite limited.
The steroid hormone 20-hydroxy-ecdysone (20HE, or simply ecdysone) plays a central role in Drosophila metamorphosis (Riddiford, 1993). The Drosophila ecdysone receptor complex, consisting of the ligand binding EcR and the DNA binding Usp, mediates its function. A peak of ecdysone in late 3rd instar larvae (Ashburner, 1972) activates transcription of ‘early’ genes in salivary glands (Huet et al., 1995), including Broad Complex (BR-C) and two homologs of mammalian Rev-Erb, E75A and E75B, which in turn activate a set of ‘late’ genes (reviewed in (Buszczak and Segraves, 2000). When the level of ecdysone diminishes at the prepupal stage, the unliganded EcR/Usp complex is thought to directly repress these and other ecdysone-inducible genes. While the role of EcR/Usp in ecdysone-dependent activation is well established, its ability to repress genes in the absence of ecdysone is less so. Analyses in imaginal discs showed that EcR/Usp target genes such as βFtz-F1, BR-C Z1 and EcR can be repressed (Schubiger et al., 2005) when Usp or EcR are removed genetically or reduced by RNAi. However, the molecular mechanism of repression is not known.
EcR/Usp, like mammalian NRs, recruits co-regulators. Once recruited, co-regulators modify histones, resulting in altered chromatin structure that affects access by transcription factors (reviewed in (Glass and Rosenfeld, 2000). For example, in the presence of ecdysone, the histone H3 lysine 4-specific histone methyltransferase Trithorax-related (TRR) interacts with EcR and Usp and assists in activating two target genes, hedgehog (hh) and BR-C (Sedkov et al., 2003). In the absence of hormone, a co-repressor SMRTER (silencing mediator of retinoid and thyroid hormone receptors-related ecdysone receptor-interacting factor) was shown to interact with EcR in two-hybrid assays and in vitro, and co-localize on salivary gland polytene chromosomes (Tsai et al., 1999).
The early ecdysone-induced gene E75 has been implicated genetically in repression of several genes in the ecdysone-triggered cascade (Hiruma and Riddiford, 2004; Horner et al., 1995). In line with this, E75 acts as a repressor in transient transfection assays (Reinking et al., 2005; Sullivan and Thummel, 2003; White et al., 1997). The E75A isoform can heterodimerize with DHR3, blocking its ability to activate (Hiruma and Riddiford, 2004; Reinking et al., 2005; Sullivan and Thummel, 2003; White et al., 1997). The mammalian homologues of E75, Rev-Erbα and Rev-Erbβ, recruit co-repressors SMRT and N-CoR (Yin and Lazar, 2005) suggesting that this may also be an alternative mechanism of repression by E75. E75 and Rev-Erb were recently shown to bind heme (Raghuram et al., 2007; Reinking et al., 2005; Yin et al., 2007), and heme was shown to facilitate recruitment by Rev-Erb of N-CoR (Raghuram et al., 2007).
Interestingly, NO can inhibit the repressive activities of E75 and Rev-Erb (Pardee et al., 2009; Reinking et al., 2005), at least in part by inhibiting function of co-repressors, including N-CoR (Pardee et al., 2009). In Drosophila, NO is thought to be important for organ development and tissue differentiation. The increased levels of NO at the end of the 3rd larval stage, in combination with the observed potential for NO to block cell proliferation, suggest that NO may serve as a growth arrest factor during the transition to metamorphosis (reviewed in Enikolopov et al., 1999).
Here, we investigate how the early ecdysone-induced gene BR-C is regulated in salivary glands by EcR/Usp and E75A, including when ecdysone titers fall at the transition from late larvae to prepupae (Emery et al., 1994; Huet et al., 1995). Our results suggest that activation and subsequent repression of BR-C involves an exchange of the activating EcR/Usp for the repressing E75A, along with their co-regulators. This depends on a decrease in ecdysone and on direct repression of EcR by E75A. Facilitating this exchange, EcR, Usp and TRR are localized to the cytoplasm in the absence of ecdysone. The repressive activity of E75A requires the co-repressor SMRTER. Recruitment of SMRTER by E75A is inhibited by NO at a particular stage, preventing premature repression of target genes. The opposing activities of EcR/Usp and E75A thus involve distinct ligands and effectors. This exchange process constitutes a distinct mechanism for modulating NR target gene expression during development.
RESULTS
Ecdysone-dependent switch in binding of activating and repressing NRs to common target genes
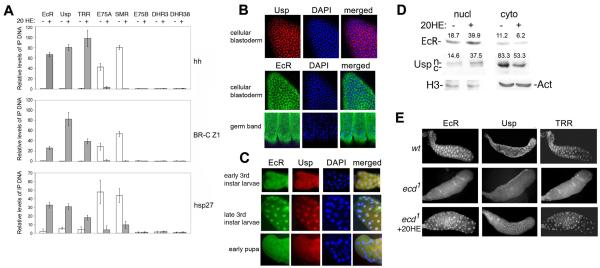
The EcR/Usp complex is thought to mediate both activation and repression of target genes in the presence and absence, respectively, of ecdysone. We found instead that occupancy in S2 cells by EcR and Usp of regulatory elements (EcREs) in 3 target genes, hh, BR-C (Sedkov et al., 2003) and hsp27 (Fig. 1A), along with their co-activator TRR, requires 20HE. Our results suggest that, at least in S2 cells, the ecdysone receptor is not involved in repression in the absence of ecdysone. To investigate whether other NRs might occupy these genes to block transcription, we used ChIP assays for NRs implicated in the late larval to pupal transition, DHR3, DHR38, E75B, and E75A. We found only one, E75A, associated with hh, BR-C and hsp27 specifically in the absence of 20HE (Fig. 1A). This suggests that E75A acts as a repressor of these genes. Rev-Erbα represses transcription through recruitment of N-CoR (Yin and Lazar, 2005), which is homologous to SMRTER. We detected SMRTER at the EcREs of hh, BR-C and hsp27 only in the absence of 20HE (Fig.1A), suggesting that an E75A/SMRTER complex represses these genes. Thus, a change in 20HE levels might lead to exchange between the E75A/SMRTER and EcR/Usp/TRR complexes.
Figure 1. Ecdysone-dependent changes in association with target genes and subcellular distribution of ecdysone receptor components.
(A) Relative amounts of EcR, Usp, TRR, E75A, SMR, E75B, DHR3, and DHR38 at the hh, BR-C Z1, and hsp27 promoters in S2 cells. Bars show means ± standard deviations above background from 3 ChIP experiments. Background was determined by omitting precipitating antibody. Primer set 4 (Fig. 4C) was used for BR-C Z1.
(B, C) Immunostaining with EcR and Usp antibodies of cellular blastoderm and germ band-retracted embryos (B), and salivary glands from early (1 day old), late 3rd instar larvae and early pupae (stages II and III, respectively, Fig. 4A).
(D) Western blots of EcR and Usp in extracts from nuclei and cytoplasm of S2 cells. Nuclear (n) and cytoplasmic (c) forms of Usp are indicated. Actin and histone H3 antibodies served as loading controls for cytoplasmic and nuclear extracts, respectively. Numbers in each column are the quantified band intensities, normalized to loading controls. The change in nuclear/cytoplasmic ratio with addition of 20HE is 3.88 for EcR and 4.02 for Usp.
(E) Salivary glands of wild-type (wt) and homozygous ecd1 3rd instar larvae immunostained with Usp, EcR and TRR antibodies. Wild-type and ecd1 larvae were grown at 29° C, (restrictive temperature), starting at the end of the 2nd larval instar.
Ecdysone-dependent cellular re-distribution of EcR, Usp and TRR
One possible reason for the lack of EcRE occupancy by EcR/Usp at low hormone titers is that its sub-cellular distribution is regulated. Indeed, we found that prior to the peak of 20HE that occurs in the middle of embryogenesis, EcR and Usp are mostly in the cytoplasm (Fig. 1B). At the peak of 20HE following germ band extension, EcR becomes more nuclear but is still detected in the cytoplasm (Fig. 1B, bottom). In the salivary glands of late 3rd instar larvae (stage II in Fig. 4A) when the level of 20HE is high, we found that both EcR and Usp are nuclear (Fig. 1C, middle). However, when 20HE is low, in both early 3rd instar larvae and early pupae, both proteins are also found in the cytoplasm in a significant number of cells (Fig. 1C, top and bottom). This suggests that their nuclear localization responds to changing levels of hormone throughout development.
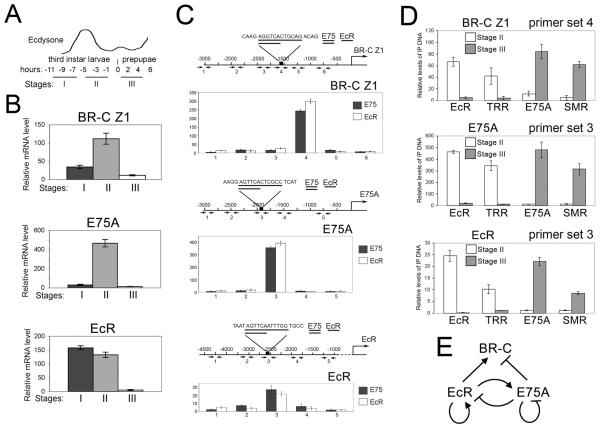
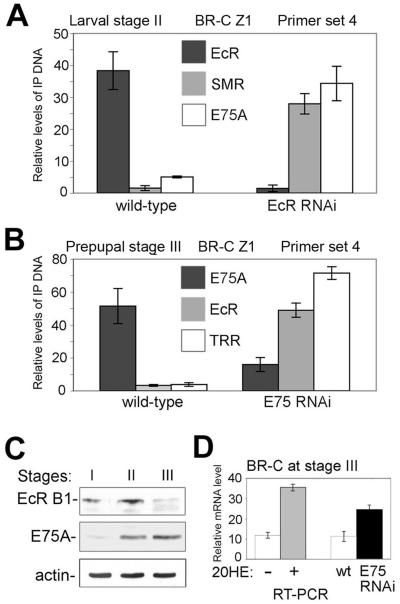
Figure 4. Association of EcR and E75A complexes correlates with activation and repression of target genes, respectively.
(A) Schematic of the larval-prepupal transition (adapted from (Huet et al., 1995).
(B) Relative levels of BR-C Z1, E75A and EcR mRNA at stages I, II and III, from quantitative RT-PCR analysis of staged salivary glands, as in Fig. 3.
(C) ChIP analysis of E75A and EcR in the BR-C Z1, E75A and EcR upstream regions of an equivalent mixture of salivary glands from stages II and III. Overlapping consensus binding sites for EcR and E75A in the upstream regions of BR-C Z1, E75A and EcR are shown in the maps above. Bars show means ± standard deviations. Note that the highest levels of both EcR and E75A are in the consensus binding site region.
(D) ChIP analysis of binding to BR-C Z1, E75A and EcR by EcR and E75A complex components in staged larvae. Bars show means and standard deviations (3 experiments) of EcR, TRR, E75A and SMRTER (SMR) signals (above background) in stage II larvae and stage III prepupae. Background was determined by omitting precipitating antibody. PCR primer sets span the consensus binding sites in C: primer set 4 for BR-C Z1, primer sets 3 for E75A and EcR.
(E) A scheme of cross-regulatory interactions between EcR and E75A.
We found that these sub-cellular patterns indeed depend on ecdysone. In S2 cells grown in steroid-depleted medium, we detected significant amounts of EcR and Usp in both nucleus and cytoplasm (Fig. 1D). Addition of 20HE led to their increase in the nucleus, and their decrease in the cytoplasm. We next examined whether nuclear localization of EcR and Usp is affected by lowering 20HE. We used the temperature-sensitive mutation ecd1 (Gaziova et al., 2004), a commonly used tool to manipulate 20HE in vivo, to decrease 20HE. Compared to wild-type salivary glands, the nuclear localization of EcR, Usp and TRR was strongly reduced in ecd1/ecd1 mutant glands of late 3rd instar larvae, with significant amounts present in the cytoplasm (Fig. 1E). Importantly, upon incubation of ecd1/ecd1 mutant glands with physiological concentrations of 20HE, all 3 proteins were predominantly seen in nuclei, similar to wild-type (Fig. 1E). Together, these results suggest that ecdysone regulates the nuclear localization of EcR, Usp and TRR in both embryos and salivary glands.
EcR and Usp are mutually required for nuclear localization and binding to target genes
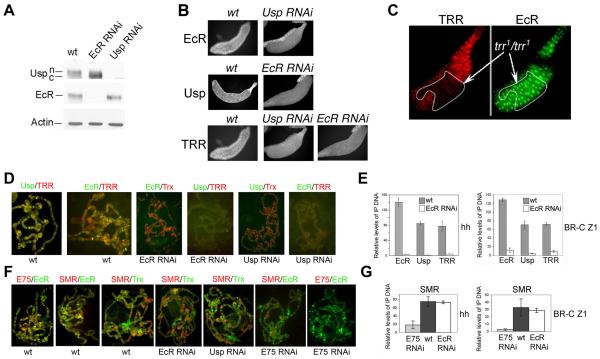
To assess mutual dependency of nuclear localization of EcR and Usp, we induced GAL4-dependent Usp and EcR RNAi in transgenic lines using a salivary gland-specific GAL4 driver. Induction of each RNAi cause a severe loss of the corresponding protein, while the levels of the other were unaffected (Fig. 2A), suggesting that absence of one protein does not affect the stability or synthesis of the other. In reciprocal experiments, we found that depletion of either EcR or Usp leads to a strong relocalization of the other to the cytoplasm (Fig. 2B). This was confirmed by Western blot analysis (Fig. 2A). In wild-type larvae, Usp is present mostly as a slower migrating phosphorylated form specific to the nucleus. PKC activity is necessary for the intracellular localization of USP (Sun et al., 2007). Following induction of EcR RNAi, Usp is detected almost exclusively as the faster migrating, cytoplasmic form (Fig. 2A). Interestingly, while TRR nuclear localization depends on EcR and Usp (Fig. 2B), EcR remains nuclear in trr1/trr1 null clones in salivary glands (Fig. 2C). These results show that both EcR and Usp are essential for nuclear localization of the entire complex.
Figure 2. Interdependence among EcR, Usp and TRR for nuclear localization and binding to target genes.
(A) Western blots of EcR and Usp in salivary gland lysates from wild-type (wt), Usp RNAi, and EcR RNAi 3rd instar larvae. Nuclear (n) and cytoplasmic (c) forms of Usp are indicated based on Fig. 1D.
(B) Immunostaining of wt, Usp RNAi, and EcR RNAi 3rd instar salivary glands with Usp, EcR and TRR antibodies (as indicated).
(C) Salivary glands dissected and double-immunostained with TRR (left) and EcR (right) antibodies. A trr1 null clone is outlined.
(D) Polytene chromosomes from wt, EcR RNAi or Usp RNAi 3rd instar salivary glands (Stage II, Fig. 4A). Note that EcR, Usp, and TRR almost completely co-localize in wt, binding of the control Trx is not affected in either RNAi line, while binding of EcR, Usp and TRR are almost completely abolished in both.
(E) Relative amounts of EcR, Usp and TRR detected by ChIP in the hh and BR-C Z1 upstream regions in wt and EcR RNAi. Bars show means ± standard deviations as in Fig. 1A.
(F) Polytene chromosomes from wt or Usp, EcR or E75 RNAi as in D. Note that E75A, EcR and SMR co-localize at most sites in wt, SMR and the control Trx in EcR and Usp RNAi lines are similar to wt; SMR signal is significantly reduced in E75 RNAi, while EcR signal is comparable to wt.
(G) Relative ChIP signals for SMR at hh and BR-C Z1 in E75 RNAi, wt, and EcR RNAi lines. Note that SMR in EcR RNAi is comparable to wt. Bars show means ± standard deviations as in Fig. 1A.
We asked whether chromosomal association of EcR and Usp is mutually dependent at common target sites, using 3rd larval instar salivary gland polytene chromosomes from EcR or Usp RNAi lines (Fig. 2D). Following induction of either RNAi, the structure of polytene chromosomes is comparable to that of wild-type (not shown), and binding of a control protein, Trithorax (Trx), is not affected (Fig. 2D). Expression of each RNAi leads to removal of the targeted protein from its binding sites. Importantly, detectable binding of EcR was abolished by depletion of Usp, and vice versa. Therefore, it seems unlikely that either EcR or Usp are involved in extensive binary interactions with other NRs, at least in the salivary gland. TRR co-localizes completely with EcR and Usp on polytene chromosomes (Sedkov et al., 2003). Detectable association of TRR with polytene chromosomes was also abolished by depletion of either EcR or Usp (Fig. 2D). To confirm these results, ChIP analysis was conducted for two ecdysone target genes, hh and BR-C, using chromatin prepared from wild-type and EcR RNAi salivary glands from late 3rd-instar larvae (stage II, Fig. 4A). Indeed, association of Usp and TRR with both hh and BR-C is significantly reduced in EcR RNAi salivary glands (Fig. 2E). Overall, our results suggest that EcR and Usp are mutually essential for binding to target genes on polytene chromosomes, and that TRR functions primarily or exclusively in conjunction with EcR/Usp.
SMRTER acts mainly as a co-repressor of E75A
Our data (Fig. 1) suggest that SMRTER may function in vivo as a co-repressor of E75A rather than of EcR/Usp. To investigate this further, we determined whether association of SMRTER with polytene chromosomes depends on EcR, Usp, or E75. EcR, SMRTER and E75A bind to similar sites on polytene chromosomes (Fig. 2F), suggesting that they regulate largely the same target genes. All detectable E75A on polytene chromosomes was lost in the E75 RNAi line, while EcR binding was not affected. Importantly, there is no apparent reduction of SMRTER binding upon removal of either EcR or Usp, while SMRTER signals are significantly decreased with removal of E75 (Fig. 2F). E75 RNAi targets E75A, B and C, and therefore the results of the experiments using this line pertain to all three E75 isoforms.
We conducted ChIP analysis at two ecdysone target genes, hh and BR-C, using chromatin from wild-type, EcR RNAi and E75 RNAi salivary glands at the prepupal stage (stage III, Fig. 4A), where the SMRTER binding signal is normally high. SMRTER association with both genes is significantly reduced in E75 RNAi salivary glands, while its association remains unchanged in the absence of EcR (Fig. 2G). In line with this, we found that while SMRTER efficiently co-immunoprecipitates with E75A in extracts from cells grown in the absence of ecdysone, no interaction between EcR and SMRTER is detected (below and Fig. 6D). We therefore conclude that recruitment of SMRTER to ecdysone-inducible genes is facilitated not by EcR/Usp, but by E75A.
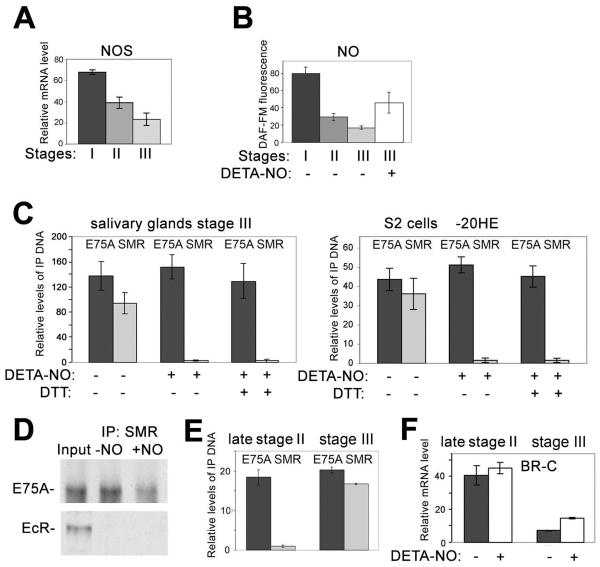
Figure 6. NO modulates the ability of E75A to repress BR-C.
(A) Quantitative RT-PCR analysis of the relative levels of NO synthase (NOS) mRNA in salivary glands at stages I, II, and III, performed as in Fig. 3.
(B) Relative levels of NO in salivary glands at stages I, II, and III, and III treated with DETA-NO (+), based on DAF-FM fluorescence. Bars show the averages (above background without DAF-FM) and standard deviations from at least 4 salivary glands for each stage.
(C) ChIP analysis (as in Fig. 1A) of the association of E75A and SMRTER (SMR) with the BR-C Z1 consensus binding site region (Fig. 4C) in wild-type stage III salivary glands (left) and in S2 cells in the absence of ecdysone (−20HE) (right), without (−) or with (+) DETA-NO and DTT, as indicated.
(D) Interaction of SMR with E75A and EcR in S2 cells grown in the absence of 20HE. Extract from S2 cells treated without (−NO) or with (+NO) DETA-NO was incubated with SMR antibody, and bound material was analyzed by western blotting with antibodies against E75A and EcR. Input, extract without IP.
(E) ChIP analysis (as in Fig. 1A) of the association of E75A and SMR with the BR-C Z1 consensus binding site region (Fig. 4C) in wild-type late stage II or stage III salivary glands.
(F) Quantitative RT-PCR analysis of the relative levels of BR-C Z1 mRNA in salivary glands at late stage II and stage III with (+) or without (−) incubation with DETA-NO, performed as in Fig. 3.
E75A is essential for gene repression at the larval-prepupal transition
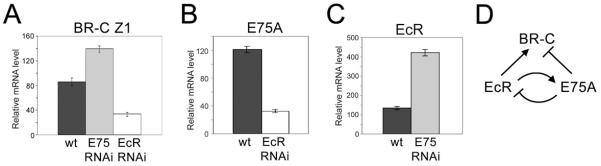
Our results (Figs. 1 and 2) suggest that E75A, rather than EcR/Usp, is a direct repressor of ecdysone-inducible genes in the absence of ecdysone. We tested this model by asking whether E75A or EcR is essential for repression of BR-C at stages that follow the ecdysone pulse at the end of the 3rd larval instar. As expected, BR-C and E75A mRNA levels were decreased in salivary glands of the EcR RNAi line from a mixture of 3rd instar larvae and prepupae (Fig. 3A, B, stages II and III in Fig. 4A). In contrast, BR-C RNA was increased at these stages in E75 RNAi glands (Fig. 3A). Surprisingly, we found that E75A also represses its own activator, EcR (Fig. 3C, D). This confirms our hypothesis that E75A, not EcR, is a direct repressor at these developmental stages.
Figure 3. E75A is involved in repression of BR-C Z1 and EcR in larvae and prepupae.
(A-C) Quantitative RT-PCR analysis of BR-C Z1 (A), E75A (B), EcR (C) mRNA in an equivalent mixture of salivary glands of 3rd instar larvae and prepupae from wild-type (wt, dark gray bars), E75 RNAi (light gray bars) and EcR RNAi lines (white bars). Rp49 is a non-target gene used to normalize results. Bars show means ± standard deviations from 3 experiments.
(D) Schematic of cross-regulatory interactions between EcR and E75A based on the results in A-C.
The above results suggest that repression of ecdysone-inducible genes is achieved by replacement of an NR-co-activator complex, EcR/Usp/TRR, by an NR-co-repressor complex, E75A/SMRTER. We investigated whether this mechanism is employed during the ecdysone-induced transition from larvae to prepupae (King-Jones and Thummel, 2005). We collected animals from 3 stages (Karim and Thummel, 1992): two 3rd instar larval stages with high levels, and prepupae with low levels, of ecdysone (stages I-III in Fig. 4A). The ecdysone pulse in 3rd instar larvae leads to an increase in the level of EcR mRNA at stages I and II (Fig. 4B, top). As EcR levels increase, early genes are activated, including E75A and BR-C (Huet et al., 1995). We also detected a high level of expression of these genes at stage II where the levels of EcR are still high (Fig 4B, middle, bottom). During stage III, when the levels of ecdysone and EcR mRNA decrease, expression of both genes is strongly reduced (Fig. 4B).
Based on the expression profile of BR-C, we selected stages II and III to examine the molecular events that accompany the transition from activation by EcR/Usp to potential repression by E75A. BR-C contains overlapping consensus binding sites for EcR and E75A (Fig. 4C, top graph). Consistent with this, in ChIP assays using an equal mixture of stage II and stage III salivary glands, EcR and E75A bind mostly to this region of BR-C (Fig. 4C, top). ChIP assays were then performed separately with stage II and stage III glands. At stage II, we saw strong signals for EcR and TRR (Fig. 4D, top), while both E75A and SMRTER are virtually undetected. In contrast, at stage III when BR-C is repressed, we detected both E75A and SMRTER but almost no EcR and TRR (Fig. 4D, top). This suggests that during the transition from larvae to prepupae, regulation of the BR-C occurs through an exchange of distinct nuclear receptor complexes with opposing activities.
E75A is an early ecdysone-inducible gene that is likely to be directly activated by EcR/Usp, and it has been proposed that EcR may autoactivate at low concentrations of ecdysone (Karim and Thummel, 1992). Expression of both genes is sharply decreased at stage III (Fig. 4B). Therefore, we wished to test whether these genes are also regulated by the same mechanism as BR-C. Consistent with this, we detected binding of EcR and E75A in the upstream DNA of both genes that contain overlapping consensus binding sites (Fig. 4C, middle, bottom). ChIP assays confirmed that activation of both EcR and E75A at stage II is accompanied by association of the EcR/TRR complex with these DNA elements (Fig. 4D, middle, bottom). At stage III, the same regions are occupied instead by the E75A/SMRTER complex. These results confirm our finding (Fig. 3C) that at low levels of ecdysone, EcR is repressed by E75A. Furthermore, these results suggest that E75A may be involved in its own repression. Thus, all 3 genes, BR-C, EcR and E75A, may be activated by EcR/Usp/TRR at high concentrations of ecdysone at the end of the 3rd larval instar, and then repressed by E75A/SMRTER when the level of ecdysone declines in prepupae.
These experiments reveal a complex cross- and auto-regulatory network between EcR and E75A at the transition from larvae to prepupae (scheme in Fig. 4E). E75A is activated by EcR/Usp shortly after an increase in the ecdysone titer during the 3rd larval instar. Following the decrease in ecdysone titer in prepupae, it represses one of its activators, EcR, as well as other genes that are activated by the ecdysone pulse, such as BR-C, and it may also be involved in auto-repression (Fig. 4E). The fact that EcR/Usp activates the repressor E75A, while E75A can in turn repress EcR, shows that genetic experiments alone do not readily distinguish which is the direct repressor. We chose a combination of in vivo approaches using salivary gland tissue to further understand the transcriptional switch mediated by these NRs.
A competitive exchange of EcR/Usp and E75A complexes
The exchange of activator and repressor NR complexes during the larval to prepupal transition described above occurs in specific regions of BR-C Z1, EcR and E75A, each of which contains overlapping binding sites for EcR and E75A (Fig. 4C). This suggests that the EcR/Usp and E75A complexes may compete for DNA occupancy in vivo. To examine this, we focused on the BR-C Z1 isoform that is expressed in salivary glands (Emery et al., 1994). The ChIP experiments shown in Figs. 1A, 2E, G and 4D used primer set 4, which spans the dual consensus binding site and detects the highest levels of both EcR and E75A in the 3 kb upstream region (Fig. 4C, top). Fig. 5A shows that while E75A and SMRTER are only weakly detected in wild-type larvae at stage II, elimination of EcR by RNAi leads to a strong increase in their association with this region. Note that while EcR induces E75A, EcR RNAi does not abolish expression of E75A (Fig. 3B). Reciprocally, association of EcR and TRR with this sequence is minimal in wild-type salivary glands at stage III, but is strongly increased following induction of E75 RNAi (Fig. 5B). This suggests that the EcR/Usp and E75A complexes compete for binding to BR-C Z1 DNA during the transition from activation to repression.
Figure 5. A switch in binding from EcR to E75A at BR-C is triggered by falling levels of ecdysone and EcR.
(A) ChIP analysis of EcR, SMRTER (SMR) and E75A at the consensus binding site region of BR-C Z1 in wild-type and EcR RNAi larvae at stage II. Bars show means ± standard deviations.
(B) ChIP analysis of E75A, EcR and TRR at the consensus binding site region of BR-C Z1 in wild-type and EcR RNAi prepupae (stage III). Bars show means ± standard deviations.
(C) Western blot analysis of EcR and E75A in staged larvae and prepupae. Actin served as a loading control.
(D) Quantitative RT-PCR analysis of the relative levels of BR-C Z1 mRNA in stage III salivary glands from wild-type without (−) or with (+) added 20HE, or from wild-type (wt) or E75 RNAi lines, performed as in Fig. 3.
We next investigated dynamic influences on the exchange of NRs. We found that at the prepupal stage, the amount of E75A is high, while the amount of EcR is strongly reduced (stage III, Fig. 5C), suggesting that EcR may be more short-lived than E75A. In addition, immunostaining (Fig. 1C) suggests that EcR is mainly cytoplasmic in prepupal salivary glands. Thus, the low levels of nuclear EcR and the higher levels of E75A may contribute to the exchange of EcR for E75A at the prepupal stage.
EcR is not completely absent from the nucleus, and the decrease in ecdysone titer also plays a role in this exchange, because incubation of stage III salivary glands with 20HE for 1 hr significantly increased the level of BR-C Z1 mRNA (Fig. 5D). Interestingly, the degree of activation of BR-C Z1 at stage III following incubation with ecdysone is similar to the level of de-repression observed following elimination of E75 by RNAi (Fig. 5D). These results suggest that E75A may outcompete the remaining nuclear EcR at low levels of ecdysone. We conclude that the low titer of ecdysone has a major effect on, while the reduced level of nuclear EcR/Usp may also contribute to, the switch from regulation by EcR/Usp to E75A during the larval-prepupal transition.
NO inhibits recruitment of the co-repressor SMRTER by E75A
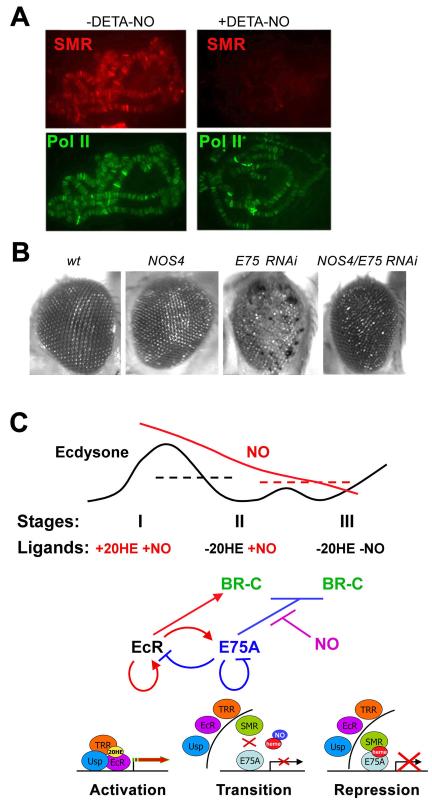
Since E75A is a heme-binding protein and a sensor of gases (Reinking et al., 2005), changing gas concentrations might affect E75A, contributing to the exchange of NR complexes. We first examined the levels of NO and nitric oxide synthase (NOS), an enzyme responsible for NO production, at 3 developmental stages. As shown in Fig. 6A and B, there is a gradual decrease in NOS expression and the concentration of NO as larvae approach puparium formation. Incubation of prepupal (stage III) salivary glands with the NO adduct DETA-NO, which produces NO in solution, leads to an increase of NO to a level comparable to that at larval stages I and II (Fig. 6B). DETA-NO treatment did not affect association of E75A with BR-C Z1 DNA in either salivary glands of stage III larvae or S2 cells (Fig. 6C). Importantly, SMRTER recruitment was almost completely abrogated (Fig. 6C), suggesting that NO blocks the ability of E75A to interact with its co-repressor. It was suggested previously that E75A is a sensor of gases through its heme group (Reinking et al., 2005), although it is possible that the effect of NO on recruitment of SMRTER is a result of S-nitrosylation of these proteins. To test this, we blocked S-nitrosylation by incubating salivary glands with 1mM DTT (Park et al., 2002). Addition of NO in the presence of DTT still prevented recruitment of SMRTER (Fig. 6C). While these results suggest that the effects of NO may indeed be mediated by the heme group of E75A, we cannot exclude the possibility that NO may induce other posttranslational modifications of these proteins. Fig. 6D shows that E75A, but not EcR, is associated with SMRTER in cell extracts grown in the absence of hormone. This is in line with our proposal that E75A recruits SMRTER to target genes. Importantly, association of SMRTER with E75A is decreased in cell extracts treated with DETA-NO (Fig. 6D). Compared to the more complete effect of DETA-NO on recruitment of SMRTER to BR-C DNA (Fig. 6C), the less complete inhibition of the E75A-SMRTER interaction in cell extracts may be because only part of the total pool of E75A in cells is the transcriptionally active nuclear protein that is associated with heme.
Based on our ChIP data, recruitment of E75A/SMRTER to DNA occurs between stages II and III (Fig. 4D). This may occur either simultaneously for both proteins, or E75A may be recruited first, followed by recruitment of SMRTER when NO decreases to a permissive level. To test this, we collected very late 3rd instar larvae just before pupariation (late stage II larvae), and tested whether E75A and SMRTER are associated with BR-C DNA. Fig. 6E shows that only E75A is associated. Consistent with the absence of SMRTER, addition of NO does not cause de-repression of BR-C Z1 in these late stage II larvae (Fig. 6F), suggesting that during the transition from stage II to stage III, E75A alone is not capable of repressing BR-C. In contrast, increasing the level of NO at stage III, when both E75A and SMRTER are associated with BR-C DNA, leads to an increase in BR-C Z1 transcription (Fig. 6F). This increase is comparable to the de-repression caused by induction of E75 RNAi (Fig. 3A). Taken together, these results suggest that during the transition from larvae to prepupae, NO can almost completely inhibit the repressor activity of E75A by preventing the recruitment of SMRTER. Thus, NO appears to be an important effector in the EcR/Usp- and E75A-mediated regulation of BR-C Z1 transcription during development.
The experiments above demonstrate the role of NO as an effector of the transcriptional regulation of BR-C, and probably other genes in the pathway, such as EcR and E75A (Figs. 3, 4). This mechanism may well function at other common target genes, as E75A and SMRTER show strong overlap in their multiple binding sites on salivary gland polytene chromosomes, and since elimination of E75A by RNAi leads to a significant loss of SMRTER signals from all sites on polytene chromosomes (Fig. 2F). We therefore tested whether NO has a more global role in regulating association of SMRTER with E75A target genes. Addition of NO to salivary glands resulted in an almost complete loss of SMRTER signals from all sites on polytene chromosomes, while binding of the control protein Pol II was not affected (Fig. 7A). Binding of E75A (not shown) was not affected, which is consistent with the results of ChIP experiments at BR-C (Fig. 6C). This suggests that NO can serve as a modulator of co-repressor recruitment to most E75A-regulated genes.
Figure 7. Inhibition of E75A repressive activity by NO may be a general feature of E75A function.
(A) Detection of SMRTER (SMR), and the control RNA Pol II phosphorylated at Ser 5 (Pol II), on polytene chromosomes of wild-type larval salivary glands with (+) and without (−) incubation with DETA-NO.
(B) Genetic interactions in the eye between NOS and E75. Mutant phenotypes were induced by the eye-specific GMR-driven expression of NOS4 and E75 RNAi. GMR-driven expression of E75 RNAi leads to a very strong rough eye phenotype with multiple necrotic areas. GMR-NOS4 rescues the E75 RNAi phenotype. Genotypes are (left to right): wt, GMR-GAL4/UAS-NOS4, GMR-GAL4/UAS-E75 RNAi, GMR-GAL4/UAS-NOS4/UAS-E75 RNAi.
(C) Model for the roles of EcR and E75A complexes and their effector molecules during the larval-pupal transition. Larval-pupal stages and changes in concentrations of the effector molecules ecdysone and NO are shown at the top. Dotted lines indicate functional threshold levels for ecdysone (black) and NO (red). Middle and bottom diagrams show regulatory and molecular events, respectively, that accompany the transition from activation to repression of ecdysone-induced genes.
To extend the generality of our findings, we examined whether NOS and E75 are linked in other physiological pathways. Expression of UAS-E75 RNAi using an eye-specific driver line (GMR-Gal4) leads to prominent defects in the eye ommatidia (Fig. 7B), suggesting that E75 plays a role in Drosophila eye development. When E75 RNAi and the dominant-negative form of NOS, NOS4, are both induced in the eye, we observed suppression of the E75 eye phenotype (Fig. 7B). This suggests that residual E75A repressor activity, which is repressed by NO, is increased when NOS activity is reduced. Thus, our results overall suggest that NO negatively regulates the activity of E75A in multiple tissues during development.
DISCUSSION
The main finding of this work is that two NRs, EcR/Usp and E75A, regulated by different ligands within the same pathway, confer activation and repression, respectively, of a common set of genes during Drosophila salivary gland development. This provides a model for how NRs regulate gene expression at multiple levels over time (scheme in Fig. 7C):
The first level involves cyclic changes in the concentration of one ligand, ecdysone. Increasing levels of ecdysone at the end of the 3rd larval instar lead to translocation of the ecdysone receptor complex, including EcR, Usp, and the co-activator TRR, to the nucleus and to target DNA. Consistent with previous models, this leads to activation of a number of genes, including a negative regulator, the heme-binding nuclear receptor E75A.
At the next developmental stage, E75A represses transcription of the common target genes BR-C and its own activator EcR. This provides negative feedback to limit the duration of the activation phase, and to solidify progression to the next stage. As E75A binds to target genes and represses expression, the EcR/Usp complex dissociates from DNA and exits the nucleus.
During this transition, repression of ecdysone-induced genes by E75A occurs through a two-step process. In the first step, E75A replaces EcR/Usp at target sites. Importantly, at this stage, although E75A is associated with DNA, it does not fully repress target genes, consistent with the absence of the co-repressor SMRTER on promoter DNA. The second step occurs at the transition to stage III, prepupae, when SMRTER is recruited, leading to full repression of target genes.
Recruitment of SMRTER at the intermediate late stage II is inhibited by NO, which binds to a heme prosthetic group of E75A. The role of NO appears to be to delay complete repression, presumably to allow time for the positive effectors of the pathway to carry out their functions. Our data suggest that this transcriptional cascade is of general significance, because its central aspects apply to the regulation not only of the genes investigated in detail here, but of numerous other genes in the developing salivary gland, as well as potentially in the eye.
Our results modify and extend the traditional view of cyclic activation and repression of target genes such as BR-C by EcR/Usp (King-Jones and Thummel, 2005), which was based in part on genetic studies in which loss of EcR or Usp leads to up-regulation of BR-C in imaginal discs (Ghbeish et al., 2001; Schubiger et al., 2005). This might be explained by an indirect effect of reduced expression of the direct repressor E75A (Figs. 3D and 4E). Other support for a repressive role for EcR/Usp came from transient transfection of reporter genes containing EcREs (Cherbas et al., 1991; Dobens et al., 1991) in which expression in the absence of ecdysone fell below the level seen without EcREs. However, the EcREs also contained consensus binding sites for E75A, allowing the possibility that E75A was the direct repressor, as suggested by the association of E75A and SMRTER with target genes in the absence of ecdysone (Fig. 1A). Nonetheless, given the described ability of SMRTER to associate with EcR (Tsai et al., 1999), it is possible that direct repression by EcR/Usp occurs in tissues or at developmental stages not examined here.
Our findings that EcR, Usp and TRR are predominately cytoplasmic at low levels of ecdysone, becoming nuclear following its increase (Fig. 1B-E), contrasts with previous conclusions that EcR and Usp are always nuclear (Koelle et al., 1991). A failure to detect EcR/Usp in the cytoplasm may be explained by the fact that only stages of development with relatively high ecdysone titers were examined. Indeed, while we also see a predominantly nuclear localization in 3rd instar larvae and in late embryos, both EcR and Usp are present in the cytoplasm in early 3rd instar larvae, in early pupae (Fig. 1C), and prior to the embryonic pulse of ecdysone (Fig. 1B). Similar to steroid NRs which shuttle between the nucleus and cytoplasm, EcR interacts with components of the MCH nuclear transport complex (Arbeitman and Hogness, 2000). Moreover, EcR is in the cytoplasm in Rhodnius prolixus (Hemiptera) at some stages (Vafopoulou and Steel, 2006), and EcR isoforms have both nuclear import and export signals (Gwozdz et al., 2007). The ecdysone-dependent relocalization of Usp is more unexpected, since Usp is a homolog of the prototypical non-steroidal NR RXR, although such mechanisms for RXR and Usp have been described in cultured cells (Cao et al., 2004; Lammerding-Koppel et al., 1998; Lin et al., 2004). Our analysis strongly supports the idea that non-steroidal receptors can use unconventional shuttling mechanisms in vivo. A related issue is the known ability of many NRs, including RXR, to interact with multiple partners, which can lead to a switch in the transcriptional activity of heterodimeric NRs. For example, it was suggested that EcR and Usp may interact with other nuclear receptors, Svp and DHR38, respectively, based on in vitro analysis (Baker et al., 2003; Zelhof et al., 1995). However, our data suggest that EcR and Usp are mutually required for both nuclear translocation and binding to common target genes in salivary glands, both of which are ecdysone-dependent. Thus, our results, obtained in a true physiological context, suggest that EcR and Usp act primarily as mutually dependent partners in gene activation induced by ecdysone.
This work shows that ecdysone and NO are involved in the same biological pathway, controlling transcriptional activities of competing NRs. We suggest that NO fine-tunes the ecdysone-controlled transition in salivary glands between 3rd instar larval stage II and prepupal stage III (Fig. 4A). At these two stages, falling levels of ecdysone and NO, respectively, control first the replacement of EcR/Usp/TRR by E75A at overlapping binding sites, and then recruitment of the co-repressor SMRTER (diagrammed in Fig. 7C). Consistent with this, we observed NO-dependent interaction of SMRTER with E75A at the BR-C and other common target genes (Figs. 6, 7). While a recent report shows that NOS is dispensible for normal development under laboratory conditions, there has clearly been selection during evolution for its activity (Yakubovich et al., 2010). We suggest that our assays provide a sensitive window into the role of NO, and that despite the ability of the organism to compensate for a lack of NOS at the gross phenotypic level, it nonetheless plays an important role in fine-tuning the molecular actions of E75A.
The repressor activity of the heme-bound gas sensor E75A is inhibited in vivo by an increased concentration of NO. Our results suggest that NO suppresses recruitment of SMRTER without affecting DNA binding by E75A (late stage II in Fig. 6E, F). This mechanism is in line with previous studies in cells, which suggested that NO can suppress the activities of both E75A and Rev-Erb (Pardee et al., 2009; Reinking et al., 2005). It was further shown that heme is essential for N-CoR recruitment to Rev-Erb (Raghuram et al., 2007), and that NO may inhibit either the recruitment or activity of N-CoR (Pardee et al., 2009). It is therefore possible that by binding to heme, NO affects heme’s ability to facilitate the interaction of E75A with SMRTER (and Rev-Erb with N-CoR). Overall, this work provides insight into gene regulation during development by NRs, governed by the changing titers of the steroid hormone ecdysone and NO.
EXPERIMENTAL PROCEDURES
Details of protocols, primers, and antibodies used are given in Supplementary Experimental Procedures. Immunostaining, western blotting, and co-IP were done using standard procedures.
Fly strains and genetic analyses
Fly strains were obtained from the Bloomington Stock Center unless otherwise indicated. Homozygous ecd1 flies were grown at room temperature (RT) until the early 3rd larval instar then transferred to 29°C (restrictive temperature) for 24 hrs. Genetic interactions used strains Oregon R, UAS-E75 RNAi (44851), GMR-Gal4 (BL1104), and GMR-NOS4 (gift from G. Enikolopov).
Cell culture
Drosophila S2 cells were grown in Schneider’s Medium (Gibco, Carlsbad, CA) supplemented with 12% charcoal/dextran-treated fetal bovine serum (Hyclone, Logan, Utah).
Chromatin immunoprecipitation
Chromatin prepared from either staged larval, prepupal, or RNAi-induced salivary glands, or S2 cells was processed using a ChIP assay kit as described by the manufacturer (Upstate Biotechnology, Charlottesville, VA).
DAF labeling protocols
Salivary glands from larval stages I-III and from stage III treated with DETA-NO for 1 hr were collected, transferred to Schneider’s medium, 10 μM DAF-FM diacetate (Invitrogen, Eugene, OR) added, incubated 10 min in the dark at RT, washed (Schneider’s medium), fixed (4% paraformaldehyde, 10 min, RT), washed 3X with medium and mounted on slides (Vectashield with DAPI, Vector, Burlingame, CA). Digital images of fluorescent salivary glands were processed using Adobe Photoshop. DAF-FM fluorescence was quantified in grayscale using Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Quantitative RT-PCR
RNA was prepared from salivary glands from Oregon R and induced E75 and EcR RNAi lines. 20 glands from each were collected in PBS, treated as described in figure legends, and RNA was extracted (High Pure RNA Isolation Kit, Roche). Reverse transcription used random hexamers and AMV reverse transcriptase (Roche). Amounts of BR-C Z1, EcR, E75A, or NOS RNA were normalized to signals using rp49-specific primers. Quantification used an Applied Biosystems StepOne Real-Time PCR system with SYBR Green and standard settings.
Polytene chromosome staining
Preparation and immunostaining of salivary gland polytene chromosome were performed as described (Sedkov et al., 2003) from Oregon R and induced Usp RNAi, EcR RNAi and E75 RNAi lines.
Histology and immunocytochemistry
trr1/ trr1 null clones in salivary glands were generated and marked as described previously (Sedkov et al., 2003).
Supplementary Material
ACKNOWLEDGEMENTS
We thank G. Enikolopov, N. Peunova and M. O’Connor for fly stocks and helpful discussions, C. Antoniewski for fly stocks, and C. Thummel, H. Krause, and the Developmental Studies Hybridoma Bank for antibodies. This work was supported by NIH GM075141, NIH CA050507, and March of Dimes 6-FY06-346 to A.M. and NIH GM050231 and NSF MCB-0818118 to J.B.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arbeitman MN, Hogness DS. Molecular chaperones activate the Drosophila ecdysone receptor, an RXR heterodimer. Cell. 2000;101:67–77. doi: 10.1016/S0092-8674(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Ecdysone induction of puffing in polytene chromosomes of Drosophila melanogaster. Effects of inhibitors of RNA synthesis. Exp Cell Res. 1972;71:433–440. doi: 10.1016/0014-4827(72)90313-8. [DOI] [PubMed] [Google Scholar]
- Baker KD, Shewchuk LM, Kozlova T, Makishima M, Hassell A, Wisely B, Caravella JA, Lambert MH, Reinking JL, Krause H, et al. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell. 2003;113:731–742. doi: 10.1016/s0092-8674(03)00420-3. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Segraves WA. Insect metamorphosis: out with the old, in with the new. Curr Biol. 2000;10:R830–833. doi: 10.1016/s0960-9822(00)00792-2. [DOI] [PubMed] [Google Scholar]
- Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, Han YH, Dawson MI, Zhang XK. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24:9705–9725. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L, Lee K, Cherbas P. Identification of ecdysone response elements by analysis of the Drosophila Eip28/29 gene. Genes Dev. 1991;5:120–131. doi: 10.1101/gad.5.1.120. [DOI] [PubMed] [Google Scholar]
- Dobens L, Rudolph K, Berger EM. Ecdysterone regulatory elements function as both transcriptional activators and repressors. Mol Cell Biol. 1991;11:1846–1853. doi: 10.1128/mcb.11.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery IF, Bedian V, Guild GM. Differential expression of Broad-Complex transcription factors may forecast tissue-specific developmental fates during Drosophila metamorphosis. Development. 1994;120:3275–3287. doi: 10.1242/dev.120.11.3275. [DOI] [PubMed] [Google Scholar]
- Enikolopov G, Banerji J, Kuzin B. Nitric oxide and Drosophila development. Cell Death Differ. 1999;6:956–963. doi: 10.1038/sj.cdd.4400577. [DOI] [PubMed] [Google Scholar]
- Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 2004;131:2715–2725. doi: 10.1242/dev.01143. [DOI] [PubMed] [Google Scholar]
- Ghbeish N, Tsai CC, Schubiger M, Zhou JY, Evans RM, McKeown M. The dual role of ultraspiracle, the Drosophila retinoid X receptor, in the ecdysone response. Proc Natl Acad Sci U S A. 2001;98:3867–3872. doi: 10.1073/pnas.061437798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- Gwozdz T, Dutko-Gwozdz J, Nieva C, Betanska K, Orlowski M, Kowalska A, Dobrucki J, Spindler-Barth M, Spindler KD, Ozyhar A. EcR and Usp, components of the ecdysteroid nuclear receptor complex, exhibit differential distribution of molecular determinants directing subcellular trafficking. Cell Signal. 2007;19:490–503. doi: 10.1016/j.cellsig.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Hager GL, Lim CS, Elbi C, Baumann CT. Trafficking of nuclear receptors in living cells. J Steroid Biochem Mol Biol. 2000;74:249–254. doi: 10.1016/s0960-0760(00)00100-x. [DOI] [PubMed] [Google Scholar]
- Hiruma K, Riddiford LM. Differential control of MHR3 promoter activity by isoforms of the ecdysone receptor and inhibitory effects of E75A and MHR3. Dev Biol. 2004;272:510–521. doi: 10.1016/j.ydbio.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Horner MA, Chen T, Thummel CS. Ecdysteroid regulation and DNA binding properties of Drosophila nuclear hormone receptor superfamily members. Dev Biol. 1995;168:490–502. doi: 10.1006/dbio.1995.1097. [DOI] [PubMed] [Google Scholar]
- Huet F, Ruiz C, Richards G. Sequential gene activation by ecdysone in Drosophila melanogaster: the hierarchical equivalence of early and early late genes. Development. 1995;121:1195–1204. doi: 10.1242/dev.121.4.1195. [DOI] [PubMed] [Google Scholar]
- Karim FD, Thummel CS. Temporal coordination of regulatory gene expression by the steroid hormone ecdysone. Embo J. 1992;11:4083–4093. doi: 10.1002/j.1460-2075.1992.tb05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Lammerding-Koppel M, Spindler-Barth M, Steiner E, Lezzi M, Drews U, Spindler KD. Immunohistochemical localization of ecdysteroid receptor and ultraspiracle in the epithelial cell line from Chironomus tentans. Tissue Cell. 1998;30:187–194. doi: 10.1016/s0040-8166(98)80067-0. [DOI] [PubMed] [Google Scholar]
- Lin XF, Zhao BX, Chen HZ, Ye XF, Yang CY, Zhou HY, Zhang MQ, Lin SC, Wu Q. RXRα acts as a carrier for TR3 nuclear export in a 9-cis retinoic acid-dependent manner in gastric cancer cells. J Cell Sci. 2004;117:5609–5621. doi: 10.1242/jcs.01474. [DOI] [PubMed] [Google Scholar]
- Pardee KI, Xu X, Reinking J, Schuetz A, Dong A, Liu S, Zhang R, Tiefenbach J, Lajoie G, Plotnikov AN, et al. The structural basis of gas-responsive transcription by the human nuclear hormone receptor REV-ERBβ. PLoS Biol. 2009;7:e43. doi: 10.1371/journal.pbio.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Yoon HJ, Lee HB, Hooper NM, Park HS. Nitric oxide inhibits the shedding of the glycosylphosphatidylinositol-anchored dipeptidase from porcine renal proximal tubules. Biochem J. 2002;364:211–218. doi: 10.1042/bj3640211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinking J, Lam MM, Pardee K, Sampson HM, Liu S, Yang P, Williams S, White W, Lajoie G, Edwards A, et al. The Drosophila nuclear receptor e75 contains heme and is gas responsive. Cell. 2005;122:195–207. doi: 10.1016/j.cell.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormone receptors and the regulation of insect metamorphosis. Receptor. 1993;3:203–209. [PubMed] [Google Scholar]
- Schubiger M, Carre C, Antoniewski C, Truman JW. Ligand-dependent de-repression via EcR/USP acts as a gate to coordinate the differentiation of sensory neurons in the Drosophila wing. Development. 2005;132:5239–5248. doi: 10.1242/dev.02093. [DOI] [PubMed] [Google Scholar]
- Sedkov Y, Cho E, Petruk S, Cherbas L, Smith ST, Jones RS, Cherbas P, Canaani E, Jaynes JB, Mazo A. Methylation at lysine 4 of histone H3 in ecdysone-dependent development of Drosophila. Nature. 2003;426:78–83. doi: 10.1038/nature02080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AA, Thummel CS. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Mol Endocrinol. 2003;17:2125–2137. doi: 10.1210/me.2002-0430. [DOI] [PubMed] [Google Scholar]
- Sun Y, An S, Henrich VC, Sun X, Song Q. Proteomic identification of PKC-mediated expression of 20E-induced protein in Drosophila melanogaster. J Proteome Res. 2007;6:4478–4488. doi: 10.1021/pr0705183. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Kao HY, Yao TP, McKeown M, Evans RM. SMRTER, a Drosophila nuclear receptor coregulator, reveals that EcR-mediated repression is critical for development. Mol Cell. 1999;4:175–186. doi: 10.1016/s1097-2765(00)80365-2. [DOI] [PubMed] [Google Scholar]
- Vafopoulou X, Steel CG. Ecdysteroid hormone nuclear receptor (EcR) exhibits circadian cycling in certain tissues, but not others, during development in Rhodnius prolixus (Hemiptera) Cell Tissue Res. 2006;323:443–455. doi: 10.1007/s00441-005-0076-1. [DOI] [PubMed] [Google Scholar]
- White KP, Hurban P, Watanabe T, Hogness DS. Coordination of Drosophila metamorphosis by two ecdysone-induced nuclear receptors. Science. 1997;276:114–117. doi: 10.1126/science.276.5309.114. [DOI] [PubMed] [Google Scholar]
- Yakubovich N, Silva EA, O’Farrell PH. Nitric oxide synthase is not essential for Drosophila development. Curr Biol. 2010;20:R141–142. doi: 10.1016/j.cub.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Lazar MA. The orphan nuclear receptor Rev-erbα recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–1459. doi: 10.1210/me.2005-0057. [DOI] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- Zelhof AC, Yao TP, Chen JD, Evans RM, McKeown M. Seven-up inhibits ultraspiracle-based signaling pathways in vitro and in vivo. Mol Cell Biol. 1995;15:6736–6745. doi: 10.1128/mcb.15.12.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.