SUMMARY
Adipocyte differentiation is characterized by an increase in mitochondrial metabolism. However it is not known whether the increase in mitochondrial metabolism is essential for differentiation or a byproduct of the differentiation process. Here, we report that primary human mesenchymal stem cells undergoing differentiation into adipocytes display an early increase in mitochondrial metabolism, biogenesis, and ROS generation. This early increase in mitochondrial metabolism and ROS generation was dependent on mTORC1 signaling. Mitochondrial targeted antioxidants inhibited adipocyte differentiation which was rescued by the addition of exogenous hydrogen peroxide. Genetic manipulation of mitochondrial complex III revealed ROS generated from this complex is required to initiate adipocyte differentiation. These results indicate that mitochondrial metabolism and ROS generation are not simply a consequence of differentiation, but are a causal factor in promoting adipocyte differentiation.
Keywords: obesity, mTOR, superoxide, PI3K, stem cells
INTRODUCTION
Adipocytes are dynamic reservoirs for energy homeostasis in mammals. There are two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT)(Gesta et al., 2007). White adipocytes function primarily to store triglycerides as energy (Spiegelman and Flier, 2001) while brown adipocytes generate heat energy at the expense of ATP generation due to their high expression of the uncoupling protein UCP1 (Cannon and Nedergaard, 2004). Mesenchymal stem cells give rise to distinct white and brown adipocyte precursors (Park et al., 2008). Brown adipocytes share precursors with muscle cells, but not with white adipocytes (Seale et al., 2008). The transcription factors PPARγ and C/EBPα drive white adipocyte differentiation while the transcriptional regulators PRDM16 and PPARγ drive brown fat differentiation (Tontonoz and Spiegelman, 2008). Although much progress has been made in understanding the transcriptional program that induces adipogenesis, the signaling mechanisms underlying the activation of transcriptional machinery are not fully understood.
During adipogenesis, autophosphorylation of insulin/IGF-1 receptor tyrosine kinases in the presence of insulin initiates glucose transport, glucose metabolism, proadipogenic gene transcription, and de novo lipid synthesis (Saltiel and Kahn, 2001). Akt activation downstream of insulin signaling makes multiple contributions to adipocyte differentiation, and is required for the process (Peng et al., 2003). Mammalian target of rapamycin (mTOR) complex 1 is one output of Akt signaling required for adipogenesis (Gagnon et al., 2001). Rapamycin-mediated inhibition of mTORC1 diminishes protein levels of PPARγ and C/EBPα and adipogenesis in vitro (Kim and Chen, 2004). Depletion of Raptor by RNAi results in diminished mTORC1 activity and reduced adipogenesis (Polak et al., 2008). By contrast, hyper-activation of mTORC1 due to loss of TSC2 increases PPARγ and C/EBPα mRNA and protein expression to promote adipogenesis in vitro (Zhang et al., 2009). mTORC1 dependent protein translation is one likely mechanism that contributes to the control of adipogenic transcriptional machinery (Carnevalli et al., 2010). Nevertheless, our current understanding of how mTORC1 controls the adipogenic transcriptional machinery is still incomplete. One output of activated mTORC1 is to positively regulate mitochondrial oxygen consumption in mammalian cells (Schieke et al., 2006). Mitochondrial oxygen consumption and overall ROS levels both increase during adipogenesis (Imhoff and Hansen, 2010; Wilson-Fritch et al., 2003). However, it is not known whether these increases are causal for promoting adipocyte differentiation. In the present study, we tested whether mitochondrial ROS are required to initiate the PPARγ transcriptional machinery downstream of mTORC1activation during differentiation of human mesenchymal stem cells (MSCs) into adipocytes.
RESULTS
Mitochondrial targeted antioxidants diminish adipocyte differentiation
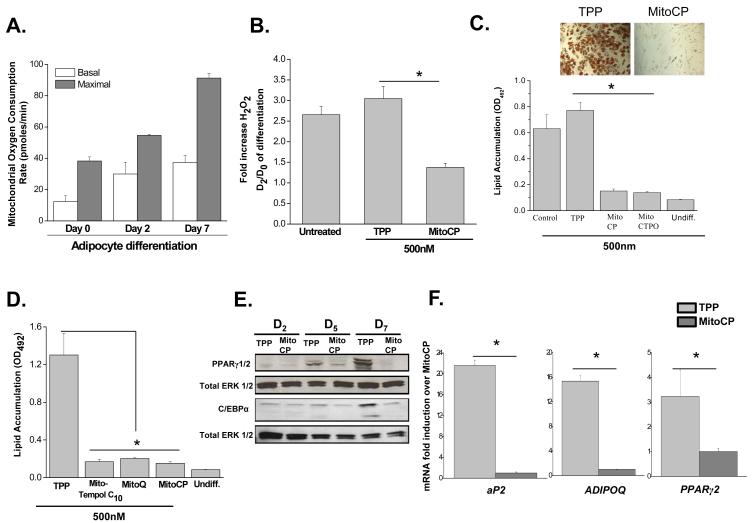
Primary human mesenchymal stem cells isolated from bone marrow display robust lipid accumulation within 21 days when exposed to a combination of indomethacin, dexamethasone isobutylmethylxanthine (IBMX), and insulin (INS) (Pittenger et al., 1999). We observed a gradual increase in the basal and maximal oxygen consumption rate (OCR) of human mesenchymal stem cells exposed to adipogenic medium over 7 days (Figure 1A). Most of the basal oxygen consumption rate was coupled to the generation of ATP synthesis indicating these cells display hallmarks of white adipocytes (data not shown). We hypothesized that an increase in mitochondrial OCR would be accompanied by an increase in ROS levels. Indeed, an intracellular increase of H2O2 was observed at Day 2 of differentiation compared to Day 0 control human MSCs (Figure 1B). The increase in intracellular H2O2 was attenuated in the presence of the mitochondrial targeted antioxidant MitoCP (Figure 1B). This nitroxide based antioxidant is targeted to the mitochondria by the covalent coupling of its nitroxide moiety to a triphenylphosphonium cation (TPP), which serves as a control compound (Dhanasekaran et al., 2005). These results indicate that an increase in mitochondrial oxygen consumption and an increase in intracellular ROS are early events during human MSC differentiation into adipocytes.
Figure 1. Mitochondrial targeted antioxidants diminish adipocyte differentiation.
(A) Mitochondrial oxygen consumption rates of human MSCs at day 0 (four hours), 2, and 7 of adipocyte differentiation. Cells were treated with FCCP (10 μM) to obtain maximal oxygen consumption rates. N=4 ± SEM.
(B) Intracellular H2O2 levels of human MSCs at Day 0 (4 hours) and Day 2 of differentiation. Cells were treated with 500 nM of TPP or MitoCP for 4 hours prior to measurement on day 2. N=4 ± SEM
(C) Mitochondrial targeted antioxidants MitoCP and MCTPO attenuate lipid accumulation. Human MSCs were treated with 500nM of TPP control and mitochondrial targeted antioxidants MitoCP or MitoCTPO starting at day 2 of differentiation. Subsequently, optical density (OD) values of Oil Red O were assessed at day 21. N=3± SEM.
(D) Mitochondrial targeted antioxidants MitoQ and Mito-Tempol diminish lipid accumulation. Human MSCs were treated starting at day 2 of differentiation with 500 nM of Mito-Tempol C10, Mito-Q, Mito CP or TPP control. Subsequently, at day 21 of differentiation cells were stained with Oil Red O and optical density (OD) values were assessed. N=3 ± SEM.
(E) Western blot analysis of PPARγ and C/EBPα protein at days 2, 5, and 7 of differentiation in human MSCs treated with 500 nM of MitoCP or TPP starting at day 2. Day 2 cells were lysed after 4 hours of MitoCP or TPP treatment.
(F) Gene expression of PPARγ2 and its target genes at day 7 of differentiation in human MSCs treated with 500 nM MitoCP or TPP starting at day 2. N=3 ± SEM.
To test whether the increase in mitochondrial generated ROS are required for adipocyte differentiation, human MSCs were treated with the control TPP and mitochondria-targeted antioxidants MitoCP or MitoCTPO starting at Day 2 of differentiation. We began adipocyte treatment with mitochondrial targeted antioxidants starting at day 2 to avoid interference with mitotic clonal expansion previously noted during adipocyte differentiation (Gagnon et al., 2001). This allows us to examine whether mitochondrial ROS regulate adipocyte differentiation independent of early proliferative signaling. A significant reduction in lipid accumulation was observed in adipocytes treated with mitochondrial antioxidants (500 nM) (Figure 1C). The decrease in lipid accumulation by MitoCP and MitoCTPO was concentration dependent (Figure S1A). Lipid accumulation was also inhibited in adipocytes treated with other well characterized mitochondria-targeted antioxidants Mito-Q and Mito-Tempol C10 (Figure 1D). In conjunction with lipid accumulation, protein levels of major adipogenic transcription factors CCAAT/enhancer-binding protein alpha (C/EBPα) and peroxisome proliferator activated receptor gamma 2 (PPARγ2) were decreased in the presence of MitoCP compared to TPP controls (Figure 1E). PPARγ2 target genes, fatty acid-binding protein-4 (aP2), adiponectin (ADIPOQ), as well as PPARγ2 mRNA were also significantly decreased in the presence of MitoCP at day 3, 5 (Figure S1B) and day 7 of differentiation after treatment with MITOCP (Figure 1F). Similar results were observed in the well established 3T3L1 model of adipocyte differentiation (Figure S2). These results demonstrate that mitochondrial ROS are required for activation of the transcriptional machinery that regulates adipocyte differentiation.
Exogenous H2O2 rescues adipocyte differentiation in the presence of mitochondrial antioxidants
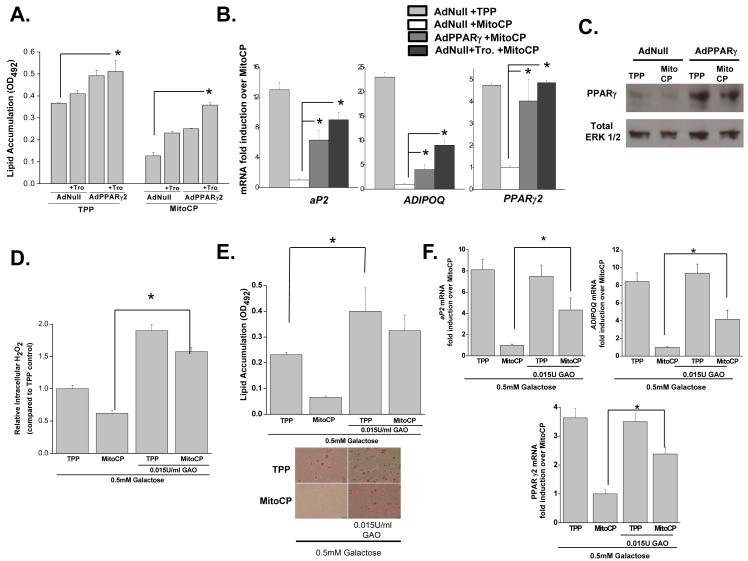
If mitochondrial ROS are indeed upstream of transcriptional programming during adipocyte differentiation then overexpression of PPARγ2 should circumvent the inhibition of adipocyte differentiation observed in adipocytes treated with MitoCP. PPARγ2 is known as the master transcriptional regulator of adipocyte differentiation (Rosen et al., 2000). Adenovirus expressing PPARγ2 (AdPPARγ2) or control null adenovirus (AdNull) were infected into human MSCs at day 1. The PPARγ agonist troglitazone (Tro.) was also added to adipogenic media to boost the efficacy of AdPPARγ2 mediated transcription. Human MSCs infected with AdPPARγ2 or AdNull were treated with MitoCP or TPP control starting at day 2 of differentiation. AdPPARγ2 + troglitazone was effectively able to increase lipid accumulation and induction of adipogenic genes in the presence of MitoCP (Figure 2A-C). Hydrogen peroxide is the major form of ROS that triggers redox dependent signaling in the cytosol (Rhee, 2006). Mitochondrial targeted antioxidants MitoCP and MitoCTPO detoxify superoxide within mitochondria thus preventing their release and subsequent accumulation of H2O2 within the cytosol. We reasoned that if H2O2 was the major form of ROS required for adipocyte differentiation then we should be able to rescue the diminished adipocyte differentiation induced by MitoCP by administering exogenous peroxide that would rapidly enter the cytosol. In the presence of D-galactose, galactose oxidase (GAO) produces hydrogen peroxide in the culture media which rapidly enters the cytosol (Wang et al., 1998). Human MSCs treated with D-galactose (0.5 mM) and GAO (0.015U/ml) to continuously generate exogenous H2O2 starting at day 2 displayed an increase in intracellular H2O2 in the presence of MitoCP at day 3 of differentiation (Figure 2D). Importantly, this increased both lipid accumulation and induction of adipogenic genes in the presence of MitoCP (Figure 2E and 2F). Collectively our data suggest that mitochondrial electron transport chain generates superoxide which is converted to H2O2 to initiate the PPARγ transcriptional machinery that regulates adipocyte differentiation.
Figure 2. Exogenous PPARγ or H2O2 rescues adipocyte differentiation in the presence of mitochondrial targeted antioxidants.
(A) Human MSCs were infected with AdPPARγ2 or AdNull at day 1 and treated with TPP (500nM) or MitoCP (500nM) +/− 5 μM troglitazone (Tro) starting at day 2. Subsequently, at day 21 of differentiation cells were stained with Oil Red O and optical density (OD) values were assessed. N=3 ± SEM.
(B) Gene expression of PPARγ2 and its target genes at day 7 of differentiation in human MSCs infected with AdPPARγ2 or AdNull at day 1 and treated with TPP (500nM) or MitoCP (500nM) +/− 5 μM troglitazone (Tro.) starting at day 2. N=3 ± SEM.
(C) Western blot analysis of PPARγ protein at Day 5 of adipocyte differentiation. Human MSCs were infected with AdNull or AdPPARγ2 at day 1 and administered 500 nM of MitoCP or TPP control starting at day 2.
(D) Intracellular H2O2 levels of human MSCs treated with galactose (0.5mM) with or without galactose oxidase (GAO, 0.015U/ml) in the presence of 500 nM of TPP or MitoCP starting at Day 2. N=3 ± SEM.
(E) Human MSCs were treated with galactose (0.5mM) with or without GAO (0.015U/ml) plus 500 nM of TPP or MitoCP starting at day 2. Subsequently, at day 21 of differentiation cells were stained with Oil Red O and optical density (OD) values were assessed. N=3 ± SEM.
(F) Human MSCs were treated with galactose (0.5mM) with or without GAO (.015U/ml) plus 500 nM TPP or MitoCP starting at day 2. Gene expression was analyzed at day 7 of differentiation. N=3 ± SEM.
Mitochondria complex III ROS is required for activation of adipogenic transcriptional machinery
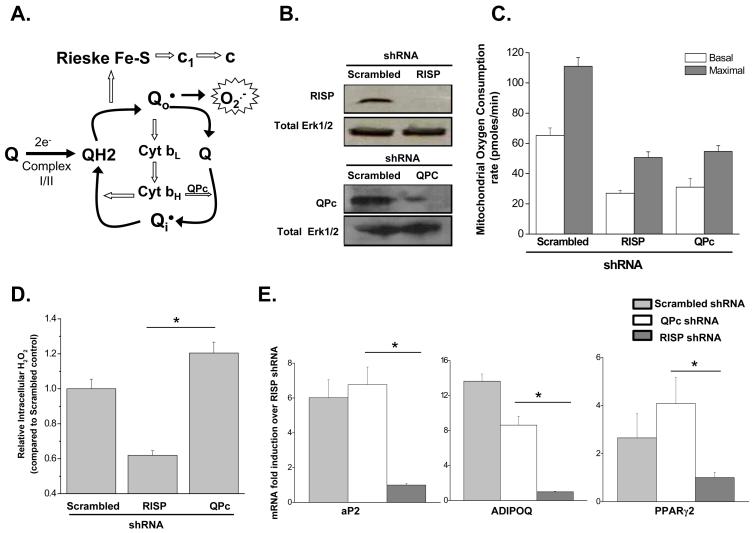
Mitochondria can generate ROS at complexes I, II and III. Complex I and II produce superoxide within the matrix (Brand, 2010). Complex III has the ability to generate superoxide within the matrix or intermembrane space (Brand, 2010). Complex III generated superoxide in the intermembrane space can escape into the cytosol. Complex III has 10 subunits including Rieske iron sulfur protein (RISP) and ubiquinone binding protein (QPc) and generates superoxide as it conducts the ubiquinone (Q) cycle. RISP is required for superoxide generation during the Q cycle while the function of the QPc is downstream of superoxide generation (Figure 3A). Lentiviral infection of shRNA targeting RISP and QPc diminished protein levels (Figure 3B) and as expected decreased both basal and maximal oxygen consumption rate to similar levels (Figure 3C). Knocking down RISP diminished ROS production while knocking down QPc maintained ROS production in human MSCs (Figure 3D). PPARγ mRNA and PPARγ dependent gene targets were significantly attenuated in RISP shRNA expressing human MSCs undergoing adipocyte differentiation compared to the QPc shRNA expressing cells and control infected cells (Figure 3E). Similar results were obtained with a second shRNA targeting RISP and QPc (Figure S3A-C). Pharmacologic inhibition with the complex III inhibitor antimycin, which is known to diminish oxygen consumption but maintains superoxide production from complex III (Turrens et al., 1985), also did not attenuate PPARγ dependent gene targets (Figure S3D). Importantly, mitochondrial targeted antioxidants abolished the increase in PPARγ mediated transcription in the presence of antimycin A (Figure S3E). These results indicate that PPARγ dependent transcription during adipocyte differentiation is dependent on mitochondrial complex III generated superoxide but independent of oxidative phosphorylation.
Figure 3. Mitochondrial complex III ROS are required for adipocyte differentiation.
(A) Mitochondrial complex III generates ROS through the ubiquinone (Q) cycle. The Q cycle generates superoxide through the donation of an electron from ubisemiquinone (Q.) to oxygen. Loss of RISP subunit abolishes the generation of Q. thus superoxide. By contrast, loss of the QPc subunit maintains superoxide production since it functions downstream from the formation of Q. and superoxide.
(B) Western blot showing efficacy of stably transfected shRNA targeted against RISP or QPc in human MSCs.
(C) Mitochondrial oxygen consumption rate is decreased in RISP and QPC knockdown MSCs. Cells were treated with FCCP (10μM) to obtain maximal oxygen consumption rates. N=3 ± SEM.
D) Intracellular H2O2 measured at Day 2 of differentiation in human MSCs infected with RISP or QPc shRNA. N=5 ± SEM.
E) Real time PCR analysis of PPARγ2 and its target genes at day 7 of differentiation in human MSCs inflected with scrambled, RISP or QPc shRNA. N=3 ± SEM.
mTORC1 is required for the early increase in ROS during adipocyte differentiation
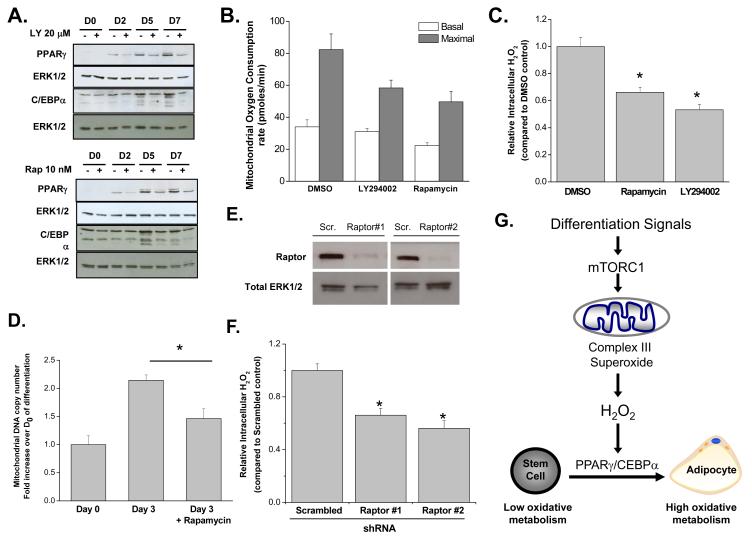
PI3-kinase signaling pathway and its downstream effector mTORC1 are well characterized regulators of lipogenesis and PPARγ dependent transcription during adipocyte differentiation (Laplante and Sabatini, 2009). In concordance with previous studies in human MSCs (Yu et al., 2008), PPARγ and C/EBPα protein levels were decreased at days 5 and 7 after treatment with PI3K inhibitor LY-294002 or mTORC1 inhibitor rapamycin (Figure 4A). Next, we investigated whether PI3K and mTORC1 signaling results in an increase in mitochondrial oxygen consumption and ROS during adipocyte differentiation. Human MSCs undergoing adipocyte differentiation treated with LY-294002 or rapamycin starting at day 2 displayed diminished mitochondrial oxygen consumption at day 7 compared with DMSO control (Figure 4B). Rapamycin diminished intracellular H2O2 production at day 3 (Figure 4C). Also, we observed an increase in mitochondrial biogenesis assessed by the ratio mitochondrial DNA (COXI gene) to nuclear DNA (PPRC1 gene) at day 3 compared to day 0, which was in part diminished by rapamycin (Figure 4D). We genetically confirmed the positive regulation of mTORC1 on mitochondrial ROS by knocking down raptor, an essential component of mTORC1. Diminishing raptor levels in human MSCs decreases ROS production indicating that mTORC1 is required for the increase in ROS production during adipocyte differentiation (Figure 4E and 4F).
Figure 4. mTORC1 positively regulates ROS production during adipocyte differentiation.
(A) Western blot analysis for PPARγ expression and C/EBPα expression of human MSCs treated with LY294002 (20 μM) or rapamycin (10 nM) starting at day 2 of differentiation. Day 2 cells were treated for four hours prior to protein isolation.
(B) Mitochondrial oxygen consumption rate was analyzed at day 7 of adipocyte differentiation in human MSCs treated with DMSO, LY-294002 (20 μM) or rapamycin (10nM) at day 2. Cells were treated with FCCP (10 μM) to obtain maximal oxygen consumption rates. N=4 ± SEM.
(C) Amplex Red analysis of intracellular H2O2 assessed at Day 3 in human MSCs treated with DMSO, rapamycin (10 nM), and LY294002 (20 μM) starting at day 2. N=4 ± SEM.
(D) Mitochondrial copy number as assessed by the ratio of mitochondrial gene COXI to nuclear gene PPRC1 at Day 0 (4 hours) and Day 3 of differentiation. Rapamycin (10 nM) was added at starting at day 2. N=6 ± SEM.
(E) Western blot showing efficacy of transfected shRNA targeted against raptor in human MSCs.
(F) Intracellular H2O2 measured at Day 2 of differentiation is decreased in human MSCs infected with raptor shRNA. N=5 ± SEM.
(G) mTORC1 dependent increase in mitochondrial complex III ROS is required for PPARγ dependent transcription during adipocyte differentiation.
A likely contributor to the increase in mitochondrial ROS early during adipocyte differentiation is mTORC1-dependent protein translation which increases cellular ATP demand and thereby oxygen consumption. Cyclohexamide inhibition of protein translation inhibition slightly diminished mitochondrial ROS generation during differentiation (Figure S4A). It is possible that mTORC1 might directly affect mitochondrial function as we observed a pool of raptor in the mitochondrial fraction (Figure S4B) indicating the proximity of this complex to mitochondria. Interestingly, adenoviral PPARγ infection did not rescue adipogenesis in the presence of PI3K or mTORC1 inhibition (Figure S4C and D) in contrast to the rescue observed in presence of mitochondrial antioxidants (Figure 2 A-C). Thus, effectors other than ROS are also activated by mTORC1 which are also required for adipocyte differentiation.
DISCUSSION
Chronic elevated levels of ROS within a cell have been attributed to diseases including obesity and diabetes (Furukawa et al., 2004; Houstis et al., 2006). Much of these previous studies have suggested ROS as initiators of cellular damage. We and others have proposed that low levels of ROS can serve as signaling molecules to regulate physiology such as oxygen sensing while at higher levels ROS cause damage (Finkel, 2011). Furthermore, our knowledge of ROS has become more complicated in the past decade as the type of ROS and the compartmentalization of ROS are likely to regulate distinct biological outcomes (Hamanaka and Chandel, 2010).
In our current study we uncovered a physiologic role of mitochondrial-generated ROS in regulating adipocyte differentiation. We observed that early during adipocyte differentiation there is an mTORC1 dependent increase in mitochondrial metabolism and biogenesis. A consequence of this increase is the production of mitochondrial complex III ROS resulting in the induction of PPARγ transcriptional machinery required to initiate adipocyte differentiation (Figure 4G). Although we observe a positive correlation between mTORC1 and mitochondrial metabolism and ROS signaling, recent studies from Shadel and colleagues demonstrate that reduced TORC1 signaling leads to an increase in respiration and ROS signaling in yeast (Bonawitz et al., 2007; Pan et al., 2011). This could reflect metabolic difference between mammalian cells and yeast, primarily the strong induction of the Crabtree effect observed in yeast. Nevertheless, these studies in yeast and our current study in mammalian cells conceptually advance the idea that TORC1 can control mitochondrial ROS to initiate signaling.
It has been noted that stem cells undergo an increase in mitochondrial biogenesis and metabolism upon differentiation (St John et al., 2005). This has led to the hypothesis that an increase in mitochondrial metabolism is required to meet the energy demands of differentiation. Our results indicate that an increase mitochondrial metabolism is not only necessary for energetic demands but is causal in promoting differentiation through the production of ROS. The broader implication of our findings is that increased ROS levels provide a permissive oxidative environment for signaling that initiates cellular differentiation. Mitochondrial ROS have been linked to differentiation during hematopoiesis in Drosophila (Owusu-Ansah and Banerjee, 2009). Future studies will shed light as to whether an increase in mitochondrial ROS is a general requirement for cellular differentiation.
EXPERIMENTAL PROCEDURES
Cell culture and reagents
Human mesenchymal stem cells were obtained from Lonza and cultured according to manufacturers’ instructions. To induce adipocyte differentiation, cells were cultured in high-glucose (25 mM) Dulbecco’s Modified Eagles Medium (DMEM-HG) medium supplemented with 5% Fetal Bovine Serum, 2% HEPES, 1% Penicillin-Streptomycin. The adipogenic differentiation media also contained 200μM Indomethacin (INDMT), 1μM dexamethasone (DEX), 10μg insulin (INS), and 0.5mM isobutylmethylxanthine (IBMX) which were all purchased from Sigma-Aldrich USA. Rapamycin, LY-294002, FCCP, antimycin, rotenone were purchased from Sigma.
Oil Red O assays to determine lipid accumulation
Human MSCs were incubated in adipogenic differentiation media for 21 days. Subsequently, cells were stained with Oil Red O using the adipogenesis assay kit (Millipore) as per manufacturer’s instructions and quantified at 492 nm using the Multiskan MCC plate reader (Fisher).
Real-time RT-PCR Analysis
RNA was extracted using TRIzol Reagent (Invitrogen) according to manufacturers’ instructions. 2 μg of RNA per sample was made into cDNA using MMLV reverse transcriptase (Applied Biosystems). Prepared cDNA was amplified using the Biorad iCycler iQ system (Biorad Laboratories) and analyzed using the SYBR Green PCR Master Mix (Applied Biosystem). PCR primers were designed using Beacon Designer 3.01 software (Premier Biosoft international) and are listed in Supplementary text. Cycle Threshold (Ct) values were normalized for amplification using mitochondrial ribosomal protein L19 and the data was analyzed using the ΔCt method.
Immunoblot Analysis
Protein levels were analyzed in whole cell lysates obtained using cell lysis buffer (Cell Signaling) and samples were resolved on a SDS polyacrylamide gel. Gels were analyzed by immunoblotting with antibodies for p44/p42 MAPK, raptor, PPARγ, and C/EBPα obtained from Cell Signaling; RISP monoclonal antibody (MitoSciences) and QPc (Proteintech).
Mitochondrial Assays
Seahorse bioscience extracellular flux (XF24) analyzer was utilized to measure oxygen consumption rates. Cells were plated in 24 well plates custom designed for XF24 analysis at a density of 2-3 × 104. Mitochondrial oxygen consumption was calculated by subtracting the residual rate after antimycin (1 μM) + rotenone (1 μM) treatment. Mitochondrial biogenesis was assessed by measuring mitochondrial DNA copy number as previously described (Li et al., 2005).
Adenoviral infection of human MSCs
Adenovirus PPARγ was obtained from Vector BioLabs and adenovirus null was obtained from ViraQuest Inc. Human MSCs were infected with adenovirus 24 hours prior to treatment with antioxidants using GeneJammer Transfection reagent (Stratagene) as previously described (Bosch and Stice, 2007).
shRNA and generation of stable cell lines
The pLKO.1 validated lentiviral vectors were obtained from Sigma to express shRNA targeting human RISP, QPc and raptor. Stable cell lines were generated by infection using viral supernatants from 293FT packaging cells followed by puromycin selection. For raptor shRNA delivery, constructs were transfected using GeneJammer Transfection reagent (Stratagene) according to manufacturer’s instructions. The shRNA sequences are provided in the supplemental text.
ROS Measurement
Intracellular ROS was measured using Amplex Red (Invitrogen) in lysed cell as previously described (Bell et al., 2007).
Statistical analysis
One-way analysis of variance was performed in Origin 7 to determine the presence of significant differences in the data. When analysis of variance indicated that a significant difference was present, two-sample Student’s t-tests were performed to compare experimental data with appropriate controls. Statistical significance was determined at a value of P<0.05 and is represented with an asterisk. Error bars defined as standard error of the mean (SEM).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a NIH Grant R01GM060472-10 to N.S.C and RO1CA152810-02 to B.K. KVT was supported by pre-doctoral NRSA grant 5F31CA142164-02. R.H. was supported by a post-doctoral training grant 1F32HL099007-02. E.M. was supported by Government of Navarra, Spain.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental information includes 4 figures and real time PCR primers and shRNA sequences which can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bell E, Klimova T, Eisenbart J, Moraes C, Murphy M, Budinger G, Chandel N. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch P, Stice S. Adenoviral transduction of mesenchymal stem cells. Methods Mol Biol. 2007;407:265–274. doi: 10.1007/978-1-59745-536-7_18. [DOI] [PubMed] [Google Scholar]
- Brand M. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carnevalli L, Masuda K, Frigerio F, Le Bacquer O, Um S, Gandin V, Topisirovic I, Sonenberg N, Thomas G, Kozma S. S6K1 plays a critical role in early adipocyte differentiation. Dev Cell. 2010;18:763–774. doi: 10.1016/j.devcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi S, Thomas S, Joseph J, Kalyanaraman B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39:567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon A, Lau S, Sorisky A. Rapamycin-sensitive phase of 3T3-L1 preadipocyte differentiation after clonal expansion. J Cell Physiol. 2001;189:14–22. doi: 10.1002/jcp.1132. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Hamanaka R, Chandel N. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010 doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Imhoff BR, Hansen JM. Extracellular redox environments regulate adipocyte differentiation. Differentiation. 2010;80:31–39. doi: 10.1016/j.diff.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chen J. regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53:2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini D. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046–1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of Yeast Chronological Life Span by TORC1 via Adaptive Mitochondrial ROS Signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Halperin DS, Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metab. 2008;8:454–457. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Peng X, Xu P, Chen M, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen W, Crawford S, Coleman K, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M, Mackay A, Beck S, Jaiswal R, Douglas R, Mosca J, Moorman M, Simonetti D, Craig S, Marshak D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige J, Auwerx J, Rüegg M, Hall M. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Rhee S. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rosen E, Walkey C, Puigserver P, Spiegelman B. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- Saltiel A, Kahn C. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Schieke S, Phillips D, McCoy JJ, Aponte A, Shen R, Balaban R, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells. 2005;7:141–153. doi: 10.1089/clo.2005.7.141. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Turrens J, Alexandre A, Lehninger A. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, DuBois JL, Hedman B, Hodgson KO, Stack TD. Catalytic galactose oxidase models: biomimetic Cu(II)-phenoxyl-radical reactivity. Science. 1998;279:537–540. doi: 10.1126/science.279.5350.537. [DOI] [PubMed] [Google Scholar]
- Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Chen Z, Zhang J, Zhang L, Ke H, Huang L, Peng Y, Zhang X, Li S, Lahn BT, et al. Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol Cell Biochem. 2008;310:11–18. doi: 10.1007/s11010-007-9661-9. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Huang J, Düvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.