Abstract
The metabolic programming effects of nutritional modifications in the immediate postnatal life are increasingly recognized to independently contribute to the development of metabolic syndrome in later life. Adjustment of litter size in rodents has been used to induce either under- or overnourishment in the immediate postnatal life of the offspring. While undernourishment led to growth retardation in the offspring, overnourishment produced increased body weight gains, hyperinsulinemia and hyperleptinemia. Overnourishment during the suckling period induced several adaptations in the energy circuitry in the hypothalamus of the offspring predisposing them for the onset of obesity later in life. Another approach for a nutritional modification in the immediate postnatal period is the artificial rearing of newborn rat pups on a high-carbohydrate (HC) milk formula without changes in the total calorie availability. Hyperinsulinemia, immediately evident in the HC pups, persisted in the post-weaning period even after withdrawal of the HC milk. Significant alterations in pancreatic islets supported chronic hyperinsulinemia in the HC rats. Alterations in the gene expression of hypothalamic neuropeptides predisposing to hyperphagia were evident during the period of the HC dietary modification. The persistence of these hypothalamic adaptations supported the obese phenotype in adult HC rats. A transgenerational effect gave rise to the development of chronic hyperinsulinemia and adult-onset obesity in the offspring of the HC female rats. Other studies have shown that lactation by a diabetic, obese or malnourished mother resulted in predisposition for the onset of metabolic disorders in the offspring. These observations from animal studies on the metabolic programming effects due to altered nutritional experiences in the immediate postnatal life strongly suggest that altered feeding practices for infants (formula feeding and early introduction of infant foods) could contribute to the rising incidence of overweight/obesity in children and adults.
Key Words: Hyperinsulinemia, Hyperphagia, Hypothalamic energy homeostasis, Increased carbohydrate intake, Nutritional experiences, Obesity, Overnourishment, Suckling period
Key Messages
• Altered nutritional experience during the lactational period can impact on adult health in the offspring.
• Overnourishment and increased carbohydrate intake during the suckling period predisposes to adult-onset obesity and related metabolic diseases.
• Is there a link between altered feeding practices for infants and adult-onset obesity?
Introduction
Obesity is one of the most daunting health challenges of this century. In 2007–2008, the estimate for the prevalence of obesity in adults aged 20–70 years in the United States was 33.8% and for the combined prevalence of overweight and obesity the estimate was 68% [1]. Moreover, in the pediatric age group, the prevalence of obesity has nearly tripled over the past 30 years [2]. The prevailing obesity epidemic is a major health concern because obesity significantly increases the risk for a variety of chronic metabolic diseases, e.g. type 2 diabetes, hypertension, hypercholesterolemia and/or heart disease [3].
In addition to genes, poor eating habits and sedentary life styles are generally recognized as the major contributors to the obesity epidemic. However, the enormity of the problem indicates that other factors must also play a role in the etiology of the obesity epidemic. The pioneering epidemiological studies by Barker et al. [4] on the long-term effects of a malnourished pregnancy were pivotal for the recognition that the adult phenotype of the offspring can be determined to a large extent by the maternal nutritional status during gestation. The hypothesis of fetal origins of adult-onset diseases introduced by Barker et al. [4] has been corroborated by other cross-sectional studies and by observations from animal models for fetal programming. The reader is referred to the excellent reviews on this topic [5,6,7,8,9,10,11,12].
In most mammalian species, organ development is not complete at birth and continues in the immediate postnatal period (suckling period). For example, maturation of pancreatic islets and development of neuronal systems in the hypothalamus continue in the suckling period in the rat [13,14]. Therefore, a nutritional insult or stimuli encountered during the suckling period alone can function as an independent cue for induction of lasting programming effects. Developmental plasticity during early periods in life affords the offspring the ability to respond to an altered nutritional environment to enable its short-term survival. In the long run, such responses are detrimental as they predispose the organism for adult-onset metabolic disorders.
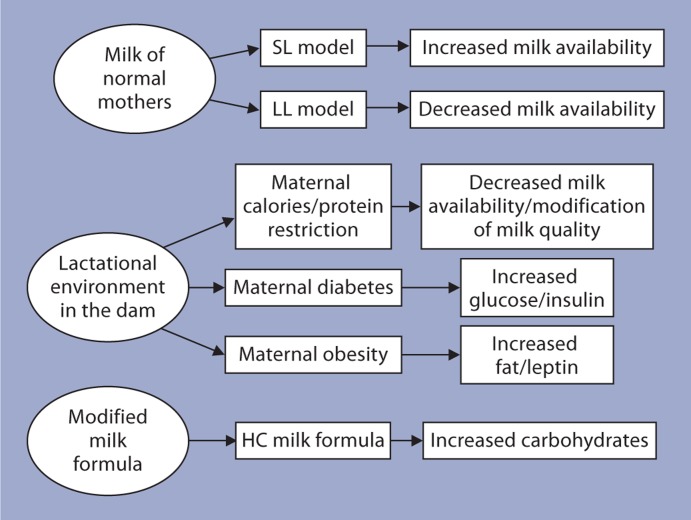
Compared to studies on fetal programming, studies on the programming effects due to alternations in the nutritional experiences during the suckling period are few. The focus of this article is on the long-term consequences for the offspring due to nutritional alterations in the immediate postnatal life (suckling period). Figure 1 indicates the nutritional stimuli/insults investigated in the immediate postnatal life in animal models. Adjustment of litter size is commonly used to investigate the lasting effects of either under- or overnourishment during the suckling period. The long-term effects of a modified milk formula (carbohydrate-enriched) without alterations in the total calorie intake in newborn rats is the focus of research in our laboratory. Other studies include investigations on the enduring effects for the offspring nursed by diabetic, obese or malnourished mothers.
Fig. 1.
The various animal models for altered nutritional experiences in the immediate postnatal life. The changes in the quality or quantity of milk received by the offspring are also indicated.
Under- or Overnourishment in the Immediate Postnatal Life
Undernourishment
The long-term consequences for the offspring due to undernourishment during the suckling period have been investigated by raising rat pups in large litters (LL; 14–24 pups/dam) [15,16]. Due to the increased competition for milk, the total calories available for each pup are reduced. LL pups demonstrated lower body weights, as well as hypoinsulinemia and hypoleptinema in the immediate postnatal life followed by catch-up growth in the post-weaning period. Changes in the hypothalamic neuropeptidergic system such as increases in the number of neuropeptide Y (NPY) neurons were observed in the arcuate (ARC) and paraventricular nuclei in LL rats [17,18] with a concomitant increase in the mRNA levels of NPY in the ARC of these rats. These observations have been attributed to reduced leptin signaling due to hypoleptinemia in these rats [19]. Programmed effects on pancreatic β-cell function, insulin sensitivity, glucose intolerance and adipose tissue metabolism have also been reported in adult LL rats [15,16].
Overnourishment
The common approach for induction of overnourishment in newborn rat pups is to reduce the litter size for newborn rat pups to 3 pups/dam (small litter; SL). Due to the increased consumption of milk, SL rats were overweight as well as hyperinsulinemic, hyperleptinemic and hyperglycemic during the suckling period compared to normal litter pups (10 pups/dam) [17,18,20,21]. In the post-weaning period, SL rats demonstrated hyperphagia and maintained increased body weight gain throughout life [22].
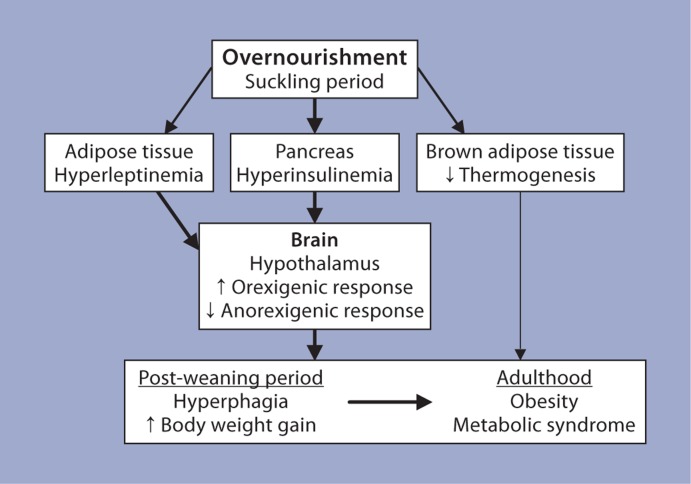
The hypothalamus is the primary center in the brain for regulation of food intake and body weight homeostasis [23]. Since SL rats were hyperphagic and overweight, several studies have focused on alterations in the hypothalamic energy homeostasis mechanism in these rats (fig. 2). Electrophysiological studies performed on coronal slices obtained from brains of SL and age-matched normal litter rats revealed significant changes in the response to various stimuli/inhibitors. Some of these observations include: (a) leptin and insulin resistance in the ARC which are typically observed in the obese condition [24,25]; (b) inhibition of neurons in the paraventricular nuclei and the ventromedial nucleus by NPY, agouti-related protein, corticotrophin-releasing factor and dopamine favoring feeding and reduced energy expenditure [26,27]; (c) reciprocal response in the ARC and ventromedial nucleus by melanin-concentrating hormone, supporting increased food intake and reduced energy expenditure [28,] and (d) altered neuronal wiring with the GABAergic neurons in SL rats which could contribute to a persistently reduced negative feedback of the adiposity signals [29]. Further, morphometric studies indicated that the number of NPY neurons in the ARC was increased while cholecystokinin-positive neurons were reduced [18,30]. Additionally, a marked reduction in the thermogenic capacity of the brown adipose tissue in SL further supported the increased body weight gain in SL rats [31] (fig. 2).
Fig. 2.
The possible mechanisms supporting the development of obesity/metabolic syndrome in the SL model. It is postulated that the altered hormonal levels observed during the suckling period ‘malprogram’ the energy homeostatic mechanism in the SL (overnourished) rats resulting in hyperphagia and increased body weight gains in the post-weaning period and development of obesity and metabolic syndrome in adulthood. The thickness of arrows outside the boxes indicates the relative contributions to metabolic programming. The shorter arrows in the boxes indicate the direction of the changes.
As reported for SL rats, overnourishment in mice during the suckling period resulted in metabolic programming effects. Mice raised in SL (3 pups/dam) developed marked obesity and insulin resistance in response to a high-fat diet in the post-weaning period. Leptin resistance in the ARC of suckling SL mice was implicated to contribute to the development of this phenotype [32].
Other reports on SL rats include observation of a reduction in the insulin-stimulated glucose transport in adipocytes in adulthood; this defect was attributed to the low expression of insulin signaling molecules in the adipose tissue of these rats [33]. Pancreatic islets from SL rats secreted a reduced amount of insulin to a glucose stimulus [34].
Modification of Milk Composition
The High-Carbohydrate Rat Model
Newborn rat pups depend on maternal milk in the immediate postnatal period for nourishment. Studies on the long-term consequences due to alterations in the milk composition for newborn rat pups are few because of difficulties in raising them away from the dam. By adopting the artificial rearing technique described by Hall [35], the long-term effects of a high-carbohydrate (HC) milk formula are being investigated in our laboratory. In this formula, the major source of calories is carbohydrate. This is in contrast to rat milk wherein the major source of calorie is fat. Artificial rearing of rat pups on the HC milk formula (without changes in total calorie intake) for 3 weeks in their immediate postnatal life led to chronic hyperinsulinemia and adult-onset obesity in these rats [36]. The observation that rat pups artificially raised on a high-fat milk formula do not develop the obese phenotype indicated that the artificial rearing protocol per se does not play a role in the development of this phenotype. The immediate and lasting metabolic programming effects induced by the HC dietary modification are described below.
Effects Induced by Increased Carbohydrate Intake (HC Milk Formula) in the Immediate Postnatal Period
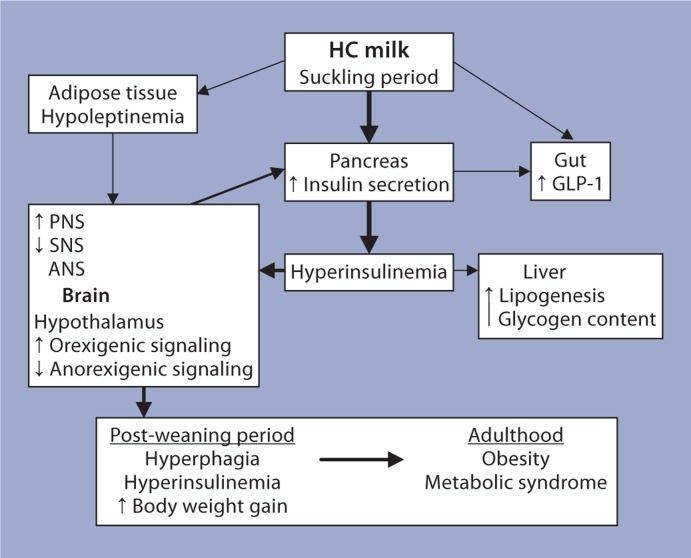
An immediate response to the HC milk formula was the onset of hyperinsulinemia which persisted throughout the period of the HC dietary modification. The responses evident in target tissues during the period of the HC dietary modification can be seen in figure 3. Several adaptive responses in pancreatic islets supported the hyperinsulinemia in the HC rats during the period of the HC milk formula consumption [36,37,38]. At the cellular level, a greater number of small-sized islets and an increase in the number of islets per unit area were observed in the HC pancreas. At the biochemical level, a marked leftward shift in the insulin secretory response to glucose and alterations in glucose metabolism in HC islets were observed. Upregulation of parasympathetic signaling and downregulation of sympathetic signaling in islets from HC rat pups further enhanced their ability to secrete increased amounts of insulin. Increases in the mRNA levels of muscarinic-type 3 receptor, phospholipase C and protein kinase Cα, and decreases in the mRNA levels of α2a-adrenergic receptor (α2aAR) provided further support to an altered autonomic nervous system regulation of insulin secretion in HC rats. At the molecular level, increased insulin biosynthesis and increased gene expression of preproinsulin and of the transcription factors that regulate its expression were observed in HC islets. Further, gene array analyses indicated alterations in the mRNA levels of several clusters of genes involved in multiple functions in islets. Taken together, these observations indicate that several regulatory mechanisms supporting insulin secretion were altered in HC rat pups to meet the increased demand for insulin during this period [36,37,38].
Fig. 3.
A representation of the responses to the HC milk formula and the possible cross-talk between the target organs, which support the development of the obesity/metabolic syndrome in the post-weaning period. The thickness of the arrows indicates the relative contribution to metabolic programming. The shorter arrows in the boxes indicate the direction of the changes. ANS/PNS/SNS = Autonomic/parasympathetic/sympathetic nervous system.
Although the body weights of the HC rats up to the time of weaning were not significantly different from those of age-matched control rats, increased mRNA levels of NPY, agouti-related protein and decreases in the mRNA levels of proopiomelanocortin, cocaine- and amphetamine-regulated transcript, corticotrophin-releasing factor and melanocortin-4-receptor in the hypothalamus of HC rat pups indicated programming effects predisposing for hyperphagia [39].
Long-Term Consequences of the HC Dietary Modification Observed in the Post-Weaning Period
Interestingly, hyperinsulinemia persisted in the post-weaning period in the HC rats. Functional alterations in terms of the secretory capacity of islets in adult HC rats were similar to those observed in HC rats during the period of the HC dietary modification. Additionally, the pattern of autonomic nervous system regulation of insulin secretion in adult HC rats was also similar to that observed in the HC pups. Vagotomy in adult HC rats resulted in reductions in plasma insulin levels providing additional support to the altered autonomic nervous system regulation of insulin secretion in adult HC rats [36,37,38]. HC rats weaned onto a standard laboratory rodent chow diet on postnatal day 24 demonstrated hyperphagia and increased body weight gains in the post-weaning period compared to controls (fig. 3). In the adult hypothalamus, alterations in the gene expression of orexigenic and anorexigenic neuropeptides were similar to those observed in the HC rat pups [36,37,38]. Taken together, these observations suggest that an increased proportion of carbohydrate in the diet during infancy, via ‘malprogramming’ of the pancreatic islets and the hypothalamic energy regulatory mechanism, can predispose the organism for adult-onset obesity and related metabolic disorders.
Generational Effect in the HC Rat Model – A Lasting Consequence of the HC Dietary Modification in the Immediate Postnatal Period
A significant observation derived from our studies on the HC rat model was that female rats that were artificially raised on the HC milk formula in their immediate postnatal life spontaneously transferred HC phenotype (chronic hyperinsulinemia and adult-onset obesity) to their offspring [40]. The HC intrauterine environment was characterized by hyperinsulinemia, hyperleptinemia, increased plasma levels of several proinflammatory markers and significantly increased body weight compared to control female pregnant rats. Embryo transfer experiments (mother-fed embryos to HC mother and vice versa) indicated that mere fetal development in the adverse intrauterine environment of the HC female rat was sufficient for fetal programming of the offspring [41]. Cross-breeding of HC female rats with normal and HC male rats and cross-fostering of HC offspring by control dams also supported the above observation [Vadlamudi and Patel, unpubl. data]. HC term fetuses were hyperinsulinemic and HC fetal islets secreted increasing amounts of insulin in the presence of glucose and amino acids [42]. Surprisingly, adaptations supporting hyperphagia were evident in the fetal HC hypothalamus [41]. Although during the suckling period plasma insulin levels in HC pups were similar to those of age-matched control pups (due most likely to a suppressive effect of high-fat/low-carbohydrate content of rat milk), immediately after weaning HC offspring developed hyperinsulinemia which persisted into adulthood. Hyperinsulinemia in the HC offspring in the post-weaning period was supported by alterations in the insulin secretory capacity of islets similar to those observed for HC rats that were subjected to the HC dietary modification [43]. HC offspring demonstrated increased body weight gain in the post-weaning period and were markedly obese in adulthood. The consumption of the HC milk formula by newborn female rat pups resulted in a cyclic effect of transfer of the maternal characteristics to its offspring.
Lactational Environment in the Mother
Diabetic Mother
That the lactational environment in the diabetic dam could program hypothalamic neuronal circuits for later development of obesity in the offspring was shown by Fahrenkrog et al. [44]. Malprogramming of the hypothalamic neuropeptidergic system – such as an increased immunopositivity in the ARC for NPY and agouti-related protein and decreased immunopositivity for proopiomelanocortin and melanocyte-stimulating hormone, as well as an increased total number of neurons in the paraventricular nuclei – was evident in normal rat pups suckled by diabetic dams. It was suggested that these changes could drive the development of hyperphagia, obesity and diabetogenic disturbances in these rats [44]. Increased levels of glucose and insulin have been noted in human breast milk from diabetic mothers; this increase was attributed to the diffusion from maternal circulation into breast milk [45,46]. Milk synthesis and ejection are reduced in untreated diabetic rat dams and this condition could lead to undernourishment for the offspring [47]. Furthermore, diabetes during lactation could alter maternal behavior towards the offspring and could further affect neurodevelopment in the offspring. These factors, either singly or in combination, could contribute to the development of the altered phenotype in normal offspring nursed by a diabetic mother.
Obese Mother
Oben et al. [48] demonstrated that cross-fostering of offspring of lean rat dams by obese dams resulted in an exaggerated dysmetabolic, insulin-resistant and non-alcoholic fatty liver disease phenotype compared to offspring of obese or lean dams nursed by their natural mothers. This observation suggests that an adverse ex utero environment (being suckled by an obese dam in this study) alone produced an exaggerated diseased phenotype in the offspring. Based on this observation, these authors suggested that in utero exposure to obesity appeared to protect the offspring to some extent against the programming effects of lactation by an obese dam. Furthermore, it was speculated that the increased levels of leptin present in the breast milk of obese dams and the increased levels of insulin present in the offspring of obese dams could singly or in combination contribute to the observed phenomenon [48].
Reifsnyder et al. [49] noted that when the offspring of the cross between the New Zealand obese mouse and the nonobese mouse were reared by lactating obese or nonobese females, the body weights of pups fostered by obese dams were significantly heavier than those of the pups fostered by lean lactating dams on postnatal day 18. These observations suggest that a genetic predisposition to increased body weight gain could be enhanced by the maternal environment during lactation. The authors hypothesized that the increased lipid and leptin contents of breast milk from obese dams could be responsible for this observation.
In yet another study on the role of lactation by an obese dam, Gorski et al. [50] showed that genetically obesity-resistant rat pups cross-fostered to genetically obese dams showed increases in diet-induced adiposity, insulin resistance and hypothalamic changes in the energy homeostasis mechanisms. There was a selective increase in insulin levels and fatty acid composition in the breast milk of the obese dam. These observations demonstrate that postnatal factors can overcome genetic predisposition for the determination of the development of obesity in later life.
Malnourished Mother
Malnourishment in the lactating mother has also been shown to cause aberrations in the offspring. For example, protein malnourishment in the mother during lactation resulted in reproductive defects in the offspring due to disturbances in the follicular development and alterations in the endometrial angiogenesis [51]. Protein deprivation only during the early lactation period produced alterations in the innate immune response [52] as well as in the insulin sensitivity in peripheral tissues in adulthood [53].
Alterations in Milk Energy Density (Combined Effect of Quality and Quantity)
Studies on rhesus macaques indicated that the quality of mother's milk can alter brain development and result in aberrations in behavioral dispositions in later life. Hinde and Capitanio [54] showed that infant monkeys nursed by mothers whose milk had a greater available milk energy had higher activity levels and greater confidence in a stressful setting compared to the infants nursed by dams whose milk had a lower available milk energy.
Mechanisms Supporting Postnatal Programming
Although the precise mechanisms that support metabolic programming effects due to altered nutritional experiences in the immediate postnatal period are not well understood, altered hormonal levels have been implicated in this process. Since insulin and leptin function as trophic factors during early periods of life, a causal relationship has been extrapolated between abnormal levels of these hormones and altered development of target organs. For example, hyperinsulinemia in the immediate postnatal period in rats has been associated with adult-onset obesity [55,56]. It has been suggested that the hyperinsulinemia and hyperleptinemia evident in SL rats underlie the observed malprogramming of the hypothalamic energy circuitry, resulting in hyperphagia and increased body weight gains in the post-weaning period of these rats [57] (fig. 2). In the ob/ob mouse, the lack of leptin has been shown to alter normal development of neuronal projections within specific nuclei in the hypothalamus, leading to the development of obesity in these mice [58]. The administration of leptin to neonatal ob/ob mice rescued the development of these neuronal projections, and reduced food intake was observed in the immediate post-weaning period [59]. Administration of leptin to neonatal rats prevented the development of obesity in rats on a high-fat diet in the post-weaning period [60]. These observations underscore the harmful enduring effects of an abnormal hormonal profile during early periods of life on regulatory mechanisms, with the consequence of a predisposition to a diseased state in later life.
Recently, epigenetic regulation of gene expression has been shown to contribute to fetal programming effects. For example, Park et al. [61] showed that epigenetic silencing of the Pdx-1 gene in pancreatic islets contributed to the development of type 2 diabetes in intrauterine growth-retarded rats. Histone code modifications were shown to underlie the repression of the Glut-4 gene in the muscle of intrauterine growth-retarded offspring [62]. There are not many reports on the epigenetic regulation of gene expression for postnatal metabolic programming effects. Plagemann et al. [63] have shown that the promoter region of proopiomelanocortin was hypermethylated within two Sp-1-related binding sequences in the hypothalamus of SL rats. Furthermore, the same group demonstrated that overnourishment in the suckling period produced hypermethylation of the insulin receptor promoter region in the hypothalamus of SL rats [64].
For the HC rats, the nutritional challenge is the increased carbohydrate content of the milk formula. Unlike the SL or LL rats, the HC rat is not subject to alterations in calorie availability. Hyperinsulinemia is evident in the HC pups within 24 h of being fed the HC milk formula. The immediate onset of hyperinsulinemia indicates that it is a necessary response for survival of these pups on the HC milk formula. Figure 3 is the hypothetical representation of the possible effects of the HC milk formula on target organs and the cross-talk between them, which could contribute to adult-onset obesity in these rats. During the period of the HC dietary modification, the HC rat pups were hyperinsulinemic as well as hypoleptinemic. Such an altered hormonal environment in the HC rat pup may underlie the observed malprogramming of the appetite-regulatory mechanism in the hypothalamus of these pups. The persistence of these early hypothalamic adaptations in the post-weaning period results in hyperphagia and increased body weight gains in the post-weaning period of these rats. An increased parasympathetic tone and a decreased sympathetic tone have been observed to contribute to the increased insulin-secretory capacity of HC islets. Since specific neuropeptides can directly function at the level of islets, the altered levels of hypothalamic neuropeptides may additionally regulate insulin secretion in HC rats. Taken together, these observations suggest that the brain and the pancreas reciprocally modify regulatory mechanisms in each other in the HC rat (fig. 3). The reduced sympathetic tone in the HC rats in addition to its effects on the islets could also regulate nonshivering thermogenesis and via this mechanism support obesity in this model. In the context of reversal of the development of obesity in the HC rat, we observed that pair-feeding of HC female rats from the time of weaning normalized plasma insulin levels and body weight in adulthood of these rats [42]. This observation further underscores the role of hyperinsulinemia in this model.
So far, our studies have focused mainly on the adaptations in the islets and the hypothalamus. Our results indicate that these organs respond immediately to the HC milk formula and that these early metabolic effects persist to contribute to the development of the obese phenotype in the HC adult rats. Limited studies in other organs such as the gut (increased plasma glucagon-like-peptide 1 levels) and the liver (increased glycogen content and lipogenic capacity) indicate that regulatory mechanisms could be affected in other organs. More detailed studies are needed in this area in the HC rat. Epigenetic mechanisms may be involved in the permanence of the immediate effects to support chronic hyperinsulinemia and predisposition to hyperphagia, but these mechanisms are yet to be unraveled in this model.
Infant Feeding Practices and Possibility of Lactational Programming Effects in Humans
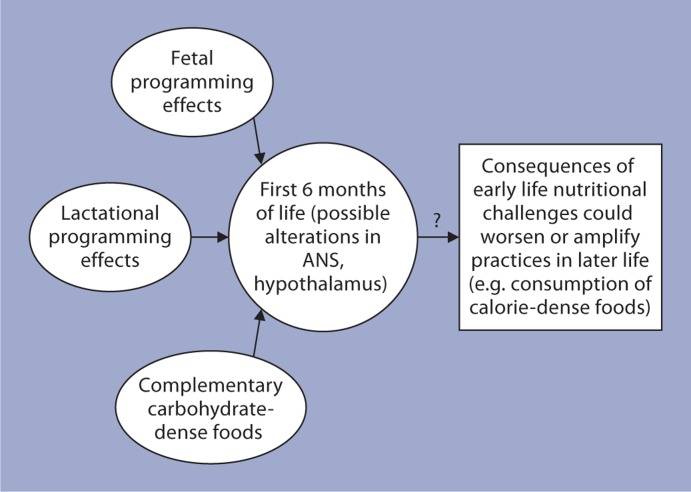
From the discussion above, it appears that nutritional alterations during the suckling period alone can significantly increase the risk for the development of metabolic disorders in adulthood (fig. 4). This observation has a direct impact on infant feeding practices with specific reference to reduced rates of breastfeeding and early introduction of complementary foods. The American Dietetic Association has suggested that infants should be exclusively breastfed for the first 6 months of life and that complimentary foods should be introduced only after 6 months of age [65]. Breast milk has the ideal combination of nutrients, hormones and other factors essential for the proper development of babies. The beneficial effects of breastfeeding for the infant are well documented. For example, it has been shown that breastfeeding was associated with a reduction in the risk for obesity, and type 1 and 2 diabetes, etc. [66]. On the other hand, increased height and weight gains and adult-onset obesity have been reported in infants who were only formula fed in their infancy [67,68]. Shorter periods of breastfeeding and early introduction of complementary foods have been shown to result in altered growth patterns in infants [69]. A recent report on the analysis of data from five cohorts in low- and middle-income countries after adjusting for confounding factors showed that later introduction (after 6 months) of complementary foods was associated with lower body weight in adulthood [70].
Fig. 4.
Based on animal studies and observations in humans, malprogramming and nutritional alterations during early postnatal life could significantly increase the risk for development of metabolic disorders in adulthood. ANS = Autonomic nervous system.
Although breastfeeding for infants has been advocated by the responsible authorities, dietary practices for infants have undergone major changes. In the first half of the 20th century, formula feeding for infants began to gain popularity with a concomitant decrease in breastfeeding rates and early introduction of complementary foods [68]. The impetus for the promotion of formula feeding has been attributed to commercial interests and changes in public health policies [71].
In addition to reduced breastfeeding rates and increased rate of formula feeding, there appears to be a disturbing trend with regard to early introduction of complementary foods. It can be seen from table 1 that complementary foods for babies are mostly carbohydrate dense (mainly simple sugars) and include fruits, fruit juices and vegetables. Table 2 indicates the reciprocal changes in carbohydrate-derived calories and fat-derived calories in the total available calories for infants when milk (human or formula) is supplemented with complementary foods. At the level of 30% supplementation, there is a marked increase in carbohydrate-derived calories and a marked decrease in fat-derived calories. Since babies receive such foods via the bottle and spoon, there is a possibility of over-feeding babies due to these modes of feeding. This could further increase the carbohydrate load for the babies. As observed in the HC rat model, substantial increases in the availability of carbohydrate-derived calories during infancy could predispose these infants to overweight and obesity in their later life.
Table 1.
Total calorie content and calories derived per serving from carbohydrate, protein and fat for some of the popular commercially available first foods for babies
| Food | Calories |
|||
|---|---|---|---|---|
| total | carbohydrate | protein | fat | |
| Banana | 70 | 66 (52) | <4 | 0 |
| Sweet potato | 45 | 44 (24) | <4 | 0 |
| Pears | 40 | 40 (28) | <4 | 0 |
| Apple sauce | 40 | 36 (32) | <4 | 0 |
| Carrots | 25 | 22 (12) | <4 | 0 |
| Apple juice | 60 | 56 (52) | <4 | 0 |
| Banana/strawberry | 70 | 68 (64) | <4 | 0 |
| Apple/grapes | 60 | 60 (56) | <4 | 0 |
| White grapes | 80 | 80 (80) | <4 | 0 |
On average, the total calories per serving in baby first foods (fruits, fruit juices and vegetables) are 54.4 (ranging from 25–80 calories for 9 different foods) and calories derived from carbohydrates are 52 (ranging from 20 to 80 calories for 9 different foods). On average, the caloric contribution from simple sugars in these foods is 81.6% of total calories and 85.5% of total carbohydrate-derived calories. Fat-derived calories are negligible and protein-derived calories from baby first foods are about 14.5% of the total calories in these supplemental foods. The calorie content for fat and protein are only approximate estimates. They may vary depending on the nature of the infant food used. These calculations are based on data derived from commercially available infant foods (major brands). Numbers in parentheses represent the calories derived from simple sugars.
Table 2.
Estimation of the increased intake of carbohydrate-derived calories by infants due to early supplementation (prior to 6 months of age) of milk (human or formula) with baby first foods without increase in daily total calorie intake
| Macronutrients | Macronutrient-derived calories in human milk or infant formula at 100% of daily total calorie intake % | Alterations in macronutrient-derived calories due to supplementation (exchange) with baby first foods at the indicated level of daily total calorie intake1 |
||
|---|---|---|---|---|
| milk (90%) +baby first foods (10%) | milk (80%) +baby first foods (20%) | milk (70%) +baby first foods (30%) | ||
| Carbohydrate | 41 | 45.5 | 49.9 | 54.35 |
| Fat | 52 | 46.8 | 41.6 | 36.4 |
| Protein | 7 | 7.75 | 8.5 | 9.25 |
The caloric contribution of the macronutrients in human milk is derived from lenness [75]. The caloric contribution of the macronutrients in commercially available infant milk formula (carbohydrate: 42.5%; fat: 47.8%, and protein: 9.6%; average values calculated from 6 commercially available brands) closely resembles that of human milk.
Our calculations, as shown above, are based on the assumption that the daily caloric intake remains constant when supplemental first foods are introduced for infants replacing equivalent calories from human milk or infant milk formula.
Although it has been shown that consumption of sugar-enriched drinks increased the risk for overweight/ obesity and other diseases [72], the WIC (Women, Infants and Children) Infant Feeding Practices Study found high levels of consumption of sweet drinks (e.g. sugar water or fruit-flavored drinks) by infants. In the first month of life, 14% of infants received these drinks; by 4 months, about 30% were given these drinks. This practice was predominant amongst Hispanic infants [73,74]. Further, it was noted that a large number of infants received fruit juice earlier than the recommended age of 6 months – about 50% by the age of 4 months [73]. Based on the observations on the HC rat model, it is possible that early introduction of sugar-dense complementary foods for babies could be a contributing factor in the etiology of obesity in children and adults.
Accumulated evidence shows that the consequences of an early life nutritional challenge could be exacerbated by dietary practices in adult life. As an example, it has been noted that SL mice overnourished during the suckling period demonstrated increased body weight gains on a high-fat diet in the post-weaning period compared to SL mice weaned onto laboratory chow [32]. It is possible that the metabolic adaptations occurring in infants due to early introduction of sweetened foods (before the recommended age of 6 months) could be amplified by consumption of calorie-dense foods in their later life. Such a situation could result in the development of obesity at an earlier age and to a greater extent.
Conclusions
In the context of the worldwide prevailing obesity epidemic, it is to be recognized that feeding practices during the first 6 months of life may play a critical role in the determination of the future health of these infants. Results from animal models as described above indicate malprogramming of vital regulatory mechanisms as a response to an altered nutritional experience in the immediate postnatal life. It appears that such responses lay the foundation for the subsequent development of metabolic disorders including obesity and related diseases. The immediate postnatal period is therefore a vulnerable period for permanent programming of appetite and growth dynamics and hence requires targeting by healthcare providers for implementation of proper guidelines for mothers with regard to feeding practices for their newborn babies.
Disclosure Statement
M.S.P. serves as a consultant to Sanofi-Aventis. Research performed in his laboratory and summarized in this article has been supported by NIH grants. The writing of this article was supported by the Nestlé Nutrition Institute.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Inge TH, Garcia V, Daniels S, Langford L, Kirk S, Roehrig H, Amin R, Zeller M, Higa K. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg. 2004;39:442–447. doi: 10.1016/j.jpedsurg.2003.11.025. discussion 446–447. [DOI] [PubMed] [Google Scholar]
- 3.Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99:565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–371. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Kanaka-Gantenbein C. Fetal origins of adult diabetes. Ann NY Acad Sci. 2010;1205:99–105. doi: 10.1111/j.1749-6632.2010.05683.x. [DOI] [PubMed] [Google Scholar]
- 8.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- 9.Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25:669–677. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- 10.Remmers F, Delemarre-van de Waal HA. Developmental programming of energy balance and its hypothalamic regulation. Endocr Rev. 2011;32:272–311. doi: 10.1210/er.2009-0028. [DOI] [PubMed] [Google Scholar]
- 11.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427:333–347. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 12.Xita N, Tsatsoulis A. Fetal origins of the metabolic syndrome. Ann NY Acad Sci. 2010;1205:148–155. doi: 10.1111/j.1749-6632.2010.05658.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaung HL. Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev Dyn. 1994;200:163–175. doi: 10.1002/aja.1002000208. [DOI] [PubMed] [Google Scholar]
- 14.Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 15.Codo W, Carlini EA. Postnatal undernutrition in rats: attempts to develop alternative methods to food deprive pups without maternal behavioral alteration. Dev Psychobiol. 1979;12:475–484. doi: 10.1002/dev.420120507. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Soldado I, Munilla MA, Herrera E. Long-term consequences of undernutrition during suckling on glucose tolerance and lipoprotein profile in female and male rats. Br J Nutr. 2006;96:1030–1037. doi: 10.1017/bjn20061949. [DOI] [PubMed] [Google Scholar]
- 17.Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dorner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146–155. doi: 10.1016/s0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- 18.Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- 19.Lopez M, Seoane LM, Tovar S, Garcia MC, Nogueiras R, Dieguez C, Senaris RM. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia. 2005;48:140–148. doi: 10.1007/s00125-004-1596-z. [DOI] [PubMed] [Google Scholar]
- 20.Davidowa H, Plagemann A. Inhibition by insulin of hypothalamic VMN neurons in rats overweight due to postnatal overfeeding. Neuroreport. 2001;12:3201–3204. doi: 10.1097/00001756-200110290-00012. [DOI] [PubMed] [Google Scholar]
- 21.Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- 22.Zippel U, Heidel E, Plagemann A, Davidowa H. Action of CCK and 5-HT on lateral hypothalamic neurons depends on early postnatal nutrition. Nutr Neurosci. 2001;4:143–152. doi: 10.1080/1028415x.2001.11747358. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14(suppl 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 24.Davidowa H, Plagemann A. Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport. 2000;11:2795–2798. doi: 10.1097/00001756-200008210-00037. [DOI] [PubMed] [Google Scholar]
- 25.Davidowa H, Plagemann A. Insulin resistance of hypothalamic arcuate neurons in neonatally overfed rats. Neuroreport. 2007;18:521–524. doi: 10.1097/WNR.0b013e32805dfb93. [DOI] [PubMed] [Google Scholar]
- 26.Davidowa H, Li Y, Plagemann A. Differential response to NPY of PVH and dopamine-responsive VMH neurons in overweight rats. Neuroreport. 2002;13:1523–1527. doi: 10.1097/00001756-200208270-00007. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Plagemann A, Davidowa H. Increased inhibition by agouti-related peptide of ventromedial hypothalamic neurons in rats overweight due to early postnatal overfeeding. Neurosci Lett. 2002;330:33–36. doi: 10.1016/s0304-3940(02)00722-x. [DOI] [PubMed] [Google Scholar]
- 28.Davidowa H, Li Y, Plagemann A. Hypothalamic ventromedial and arcuate neurons of normal and postnatally overnourished rats differ in their responses to melanin-concentrating hormone. Regul Pept. 2002;108:103–111. doi: 10.1016/s0167-0115(02)00153-2. [DOI] [PubMed] [Google Scholar]
- 29.Davidowa H, Li Y, Plagemann A. Altered responses to orexigenic (AGRP, MCH) and anorexigenic (alpha-MSH, CART) neuropeptides of paraventricular hypothalamic neurons in early postnatally overfed rats. Eur J Neurosci. 2003;18:613–621. doi: 10.1046/j.1460-9568.2003.02789.x. [DOI] [PubMed] [Google Scholar]
- 30.Plagemann A, Rake A, Harder T, Melchior K, Rohde W, Dorner G. Reduction of cholecystokinin-8S-neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Neurosci Lett. 1998;258:13–16. doi: 10.1016/s0304-3940(98)00823-4. [DOI] [PubMed] [Google Scholar]
- 31.Xiao XQ, Williams SM, Grayson BE, Glavas MM, Cowley MA, Smith MS, Grove KL. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology. 2007;148:4150–4159. doi: 10.1210/en.2007-0373. [DOI] [PubMed] [Google Scholar]
- 32.Glavas MM, Kirigiti MA, Xiao XQ, Enriori PJ, Fisher SK, Evans AE, Grayson BE, Cowley MA, Smith MS, Grove KL. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology. 2010;151:1598–1610. doi: 10.1210/en.2009-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues AL, De Souza EP, Da Silva SV, Rodrigues DS, Nascimento AB, Barja-Fidalgo C, De Freitas MS. Low expression of insulin signaling molecules impairs glucose uptake in adipocytes after early overnutrition. J Endocrinol. 2007;195:485–494. doi: 10.1677/JOE-07-0046. [DOI] [PubMed] [Google Scholar]
- 34.Waterland RA, Garza C. Early postnatal nutrition determines adult pancreatic glucose-responsive insulin secretion and islet gene expression in rats. J Nutr. 2002;132:357–364. doi: 10.1093/jn/132.3.357. [DOI] [PubMed] [Google Scholar]
- 35.Hall WG. Weaning and growth of artificially reared rats. Science. 1975;190:1313–1315. doi: 10.1126/science.1198116. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan M, Patel MS. Metabolic programming in the immediate postnatal period. Trends Endocrinol Metab. 2008;19:146–152. doi: 10.1016/j.tem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Patel MS, Srinivasan M. Metabolic programming due to alterations in nutrition in the immediate postnatal period. J Nutr. 2010;140:658–661. doi: 10.3945/jn.109.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel MS, Srinivasan M, Laychock SG. Metabolic programming: role of nutrition in the immediate postnatal life. J Inherit Metab Dis. 2009;32:218–228. doi: 10.1007/s10545-008-1033-4. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan M, Mitrani P, Sadhanandan G, Dodds C, Shbeir-ElDika S, Thamotharan S, Ghanim H, Dandona P, Devaskar SU, Patel MS. A high-carbohydrate diet in the immediate postnatal life of rats induces adaptations predisposing to adult-onset obesity. J Endocrinol. 2008;197:565–574. doi: 10.1677/JOE-08-0021. [DOI] [PubMed] [Google Scholar]
- 40.Vadlamudi S, Kalhan SC, Patel MS. Persistence of metabolic consequences in the progeny of rats fed a HC formula in their early postnatal life. Am J Physiol. 1995;269:E731–E738. doi: 10.1152/ajpendo.1995.269.4.E731. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan M, Dodds C, Ghanim H, Gao T, Ross PJ, Browne RW, Dandona P, Patel MS. Maternal obesity and fetal programming: effects of a high-carbohydrate nutritional modification in the immediate postnatal life of female rats. Am J Physiol Endocrinol Metab. 2008;295:E895–E903. doi: 10.1152/ajpendo.90460.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasan M, Aalinkeel R, Song F, Mitrani P, Pandya JD, Strutt B, Hill DJ, Patel MS. Maternal hyperinsulinemia predisposes rat fetuses for hyperinsulinemia, and adult-onset obesity and maternal mild food restriction reverses this phenotype. Am J Physiol Endocrinol Metab. 2006;290:E129–E134. doi: 10.1152/ajpendo.00248.2005. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan M, Aalinkeel R, Song F, Patel MS. Programming of islet functions in the progeny of hyperinsulinemic/obese rats. Diabetes. 2003;52:984–990. doi: 10.2337/diabetes.52.4.984. [DOI] [PubMed] [Google Scholar]
- 44.Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, Plagemann A. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. 2004;134:648–654. doi: 10.1093/jn/134.3.648. [DOI] [PubMed] [Google Scholar]
- 45.Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr. 1991;54:69–80. doi: 10.1093/ajcn/54.1.69. [DOI] [PubMed] [Google Scholar]
- 46.Neville MC, Allen JC, Archer PC, Casey CE, Seacat J, Keller RP, Lutes V, Rasbach J, Neifert M. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. 1991;54:81–92. doi: 10.1093/ajcn/54.1.81. [DOI] [PubMed] [Google Scholar]
- 47.Lau C, Sullivan MK, Hazelwood RL. Effects of diabetes mellitus on lactation in the rat. Proc Soc Exp Biol Med. 1993;204:81–89. doi: 10.3181/00379727-204-43638. [DOI] [PubMed] [Google Scholar]
- 48.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE, Sigala B, Novelli M, Poston L, Taylor PD. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–920. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 49.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2006;291:R768–R778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- 51.Bittencourt Brasil F, Silva Faria T, Barcellos Sampaio FJ, da Fonte Ramos C. The effect of maternal malnutrition during lactation on the endometrial ERα expression, collagen type and blood vessels in rats offspring at puberty. Anat Rec (Hoboken) 2010;293:162–170. doi: 10.1002/ar.21028. [DOI] [PubMed] [Google Scholar]
- 52.Silva SV, Garcia-Souza EP, Moura AS, Barja-Fidalgo C. Maternal protein restriction during early lactation induces changes on neutrophil activation and TNF-alpha production of adult offspring. Inflammation. 2010;33:65–75. doi: 10.1007/s10753-009-9159-6. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Souza EP, da Silva SV, Felix GB, Rodrigues AL, de Freitas MS, Moura AS, Barja-Fidalgo C. Maternal protein restriction during early lactation induces GLUT4 translocation and mTOR/Akt activation in adipocytes of adult rats. Am J Physiol Endocrinol Metab. 2008;295:E626–E636. doi: 10.1152/ajpendo.00439.2007. [DOI] [PubMed] [Google Scholar]
- 54.Hinde K, Capitanio JP. Lactational programming? Mother's milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) Am J Primatol. 2010;72:522–529. doi: 10.1002/ajp.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dorner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm Metab Res. 1994;26:213–221. doi: 10.1055/s-2007-1001668. [DOI] [PubMed] [Google Scholar]
- 56.Plagemann A, Heidrich I, Rohde W, Götz F, Dörner G. Hyperinsulinism during differentiation of the hypothalamus is a diabetogenic and obesity risk factor in rats. Neuroendocrinol Lett. 1992;5:373–378. [Google Scholar]
- 57.Plagemann A. Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav. 2005;86:661–668. doi: 10.1016/j.physbeh.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 58.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7:179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 60.Pico C, Oliver P, Sanchez J, Miralles O, Caimari A, Priego T, Palou A. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int J Obes (Lond) 2007;31:1199–1209. doi: 10.1038/sj.ijo.0803585. [DOI] [PubMed] [Google Scholar]
- 61.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118:2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem. 2008;283:13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M, Ziska T, Schellong K, Rodekamp E, Melchior K, Dudenhausen JW. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587:4963–4976. doi: 10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plagemann A, Roepke K, Harder T, Brunn M, Harder A, Wittrock-Staar M, Ziska T, Schellong K, Rodekamp E, Melchior K, Dudenhausen JW. Epigenetic malprogramming of the insulin receptor promoter due to developmental overfeeding. J Perinat Med. 2010;38:393–400. doi: 10.1515/jpm.2010.051. [DOI] [PubMed] [Google Scholar]
- 65.James DC, Lessen R. Position of the American Dietetic Association: promoting and supporting breastfeeding. J Am Diet Assoc. 2009;109:1926–1942. doi: 10.1016/j.jada.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 66.Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007;153:1–186. [PMC free article] [PubMed] [Google Scholar]
- 67.Kramer MS, Guo T, Platt RW, Vanilovich I, Sevkovskaya Z, Dzikovich I, Michaelsen KF, Dewey K. Feeding effects on growth during infancy. J Pediatr. 2004;145:600–605. doi: 10.1016/j.jpeds.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 68.Smith J. The contribution of infant food marketing to the obesogenic environment in Australia. Breastfeed Rev. 2007;15:23–35. [PubMed] [Google Scholar]
- 69.Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–1588. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 70.Fall CH, Borja JB, Osmond C, Richter L, Bhargava SK, Martorell R, Stein AD, Barros FC, Victoria CG, COHORTS group Infant-feeding patterns and cardiovascular risk factors in young and adulthood: data from five cohorts in low- and middle-income countries. Int J Epidemiol. 2011;40:47–62. doi: 10.1093/ije/dyq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bryder L. From breast to bottle: a history of modern infant feeding. Endeavour. 2009;33:54–59. doi: 10.1016/j.endeavour.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97:667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCann MF, Baydar N, Williams RL. Consumption of soft drinks and other sweet drinks by WIC infants. Am J Public Health. 2008;98:1735. doi: 10.2105/AJPH.2008.142471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCann MF, Baydar N, Williams RL. Breastfeeding attitudes and reported problems in a national sample of WIC participants. J Hum Lact. 2007;23:314–324. doi: 10.1177/0890334407307882. [DOI] [PubMed] [Google Scholar]
- 75.Jenness R. The composition of human milk. Semin Perinatol. 1979;3:225–239. [PubMed] [Google Scholar]