Abstract
The contributions of prolactin (PRL) to breast cancer are becoming increasingly recognized. To better understand the role for PRL in this disease, its interactions with other oncogenic growth factors and hormones must be characterized. Here, we review our current understanding of PRL crosstalk with other mammary oncogenic factors, including estrogen, epidermal growth factor (EGF) family members, and insulin-like growth factor-I (IGF-I). The ability of PRL to potentiate the actions of these targets of highly successful endocrine and molecular therapies suggests that PRL and/or its receptor (PRLR) may be an attractive therapeutic target(s). We discuss the potential benefit of PRL/PRLR-targeted therapy in combination with established therapies and implications for de novo and acquired resistance to treatment.
Keywords: Prolactin, Breast cancer, Signaling crosstalk, Targeted therapeutics
1. Introduction
The concept that prolactin (PRL) significantly contributes to the pathogenesis and progression of breast cancer was brought to the forefront in the 1970s, although it remained controversial for many years (Clevenger et al., 2003). Through numerous experimental, clinical, and epidemiological studies, a striking body of evidence implicating a role for PRL in mammary carcinogenesis has gained increasing acceptance (Arendt and Schuler, 2008a; Trott et al., 2008; Tworoger and Hankinson, 2008; Wagner and Rui, 2008). However, PRL is just one component of a highly integrated signaling network. To further define the role of PRL in disease, interactions with other critical regulators of mammary growth and differentiation must be characterized. This review will focus on recent data elucidating PRL crosstalk, its contributions to tumor pathogenesis and progression, and therapeutic implications.
2. PRL and breast cancer
Because of its crucial role in mammary proliferation and differentiation, it was speculated that PRL was involved in carcinogenesis, but conflicting outcomes of hypophysectomy or bromocriptine treatment to remove pituitary PRL fed a prolonged debate (Vonderhaar, 1998; Goffin et al., 1999). More recently, large prospective epidemiological studies have correlated circulating PRL levels with risk for estrogen receptor-alpha (ERα)-positive breast cancer in both pre-and post-menopausal women (Tworoger and Hankinson, 2008). These findings are strengthened by the discovery that PRL is produced locally within the mammary epithelium and adjacent adipose tissue, further increasing PRL exposure (Ginsburg and Vonderhaar, 1995; Clevenger and Plank, 1997; Zinger et al., 2003). These reports incited new interest in PRL as a potential modulator of mammary carcinogenesis. Recently, it was shown that PRL expression in tumors is higher than in normal or hyperplastic epithelium (McHale et al., 2008). Moreover, PRL receptor (PRLR) is expressed in 70–95% of primary breast tumors and at a higher level within tumors compared to normal adjacent tissue (Ormandy et al., 1997a; Reynolds et al., 1997; Touraine et al., 1998; Gill et al., 2001; Meng et al., 2004). In mouse models, targeted mammary overexpression of PRL or activated PRLR is oncogenic (Arendt and Schuler, 2008a), and conversely, inhibition of PRLR with a ligand antagonist (Tomblyn et al., 2005) or gene ablation (Oakes et al., 2007) delays tumor development. Together, these data point to an important role for PRL in breast cancer, and suggest that PRL signaling may be a target for therapeutic exploitation.
3. PRL-initiated signals
PRL exerts its biological effects via binding to PRLR and initiation of signals to downstream mediators, including Janus kinases (JAKs), STATs, mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3′-kinase (PI3K), AKT, src family kinases, the protein kinase C family, Rho GTPases, and RUSH (Clevenger et al., 2003; Chilton and Hewetson, 2005). These pathways can contribute to key features of neoplastic progression, including proliferation, survival, and invasion (Vivanco and Sawyers, 2002; Sebolt-Leopold and Herrera, 2004;Wagner and Rui, 2008), as well as resistance to current breast cancer therapies (Schiff et al., 2004; McCubrey et al., 2006).
3.1. JAK/STAT
JAK2/STAT5 is the most characterized PRL-initiated signaling cascade. This pathway appears to mediate most PRL actions in lobuloalveolar development and lactation, as demonstrated by mouse models with genetic ablations of these signaling components (Horseman et al., 1997; Ormandy et al., 1997b; Liu et al., 1997; Teglund et al., 1998; Shillingford et al., 2002). However, the role of this pathway in mammary oncogenesis is more complex. STAT5-overexpressing mice develop mammary tumors (Iavnilovitch et al., 2004), and loss of STAT5 significantly delays tumor onset in multiple mouse models of breast cancer (Humphreys and Hennighausen, 1999; Ren et al., 2002). Nonetheless, STAT5 activation in primary tumors is associated with a favorable prognosis (Cotarla et al., 2004; Nevalainen et al., 2004), and STAT5 suppresses invasion in vitro (Sultan et al., 2005; Nouhi et al., 2006; Gutzman et al., 2007). This correlates well with findings that STAT5 expression is lost in invasive carcinomas but retained in well-differentiated histo-types (Bratthauer et al., 2006; Strauss et al., 2006). Thus, a dual role for this pathway has been proposed: one that supports tumor-initiation, but fosters differentiation and suppresses invasion in advanced disease (Wagner and Rui, 2008). Adding to this complexity, in one study, nuclear STAT5 in primary tumors predicted a positive response to endocrine therapy (Yamashita et al., 2006), yet in vitro models implicate several STAT family members, including STAT5, in antiestrogen resistance (Silva and Shupnik, 2007).
3.2. MAP kinases
PRL activates MAPKs, including ERK1/2, ERK5, p38 and JNK1/2 (Schwertfeger et al., 2000; Gutzman et al., 2004a). ERK1/2 are activated in response to multiple growth factors and cytokines, and elicit a myriad of effects associated with malignancy. Several transcription factors are activated downstream of ERK1/2, including AP-1, which regulates genes involved in proliferation, survival, and metastasis, such as cyclin D1 and MMPs (Shaulian and Karin, 2002; Eferl and Wagner, 2003). ERK1/2 are hyperexpressed and hyperactivated in human mammary neoplasias (Santen et al., 2002; Sivaraman et al., 1997), and unlike STAT5, are associated with poor patient prognosis in some (Gee et al., 2001), but not all (Milde-Langosch et al., 2005; Bergqvist et al., 2006) studies. Moreover, ERK1/2 activation has been reported to predict poor responses to endocrine therapy and contribute to resistance to antiestrogens and EGFR/HER-2-targeted therapies (Vilora-Petit and Kerbel, 2004; Riggins et al., 2007).
3.3. PI3K/AKT
PRL also activates the PI3K/AKT cascade, which plays prominent roles in proliferation and survival. Multiple effector proteins associated with tumorigenesis function downstream of this pathway, including GSK-3β, cyclin D1, mTOR, BAD, procaspase-9, and Fork-head transcription factors (Vivanco and Sawyers, 2002; Hennessy et al., 2005). Furthermore, this pathway contributes to tumor invasion and the epithelial-mesenchymal transition (Shaw et al., 1997; Grille et al., 2003; Zhou et al., 2004). Recent studies have shown that AKT activity increases with tumor progression, correlating with increased relapse and decreased survival for patients with metastatic disease (Kirkegaard et al., 2005; Liu et al., 2007). Finally, like MAP kinases, AKT has been implicated in resistance to antiestrogens and EGFR/HER-2-targeted therapies (Vilora-Petit and Kerbel, 2004; Riggins et al., 2007).
4. PRL crosstalk in breast cancer
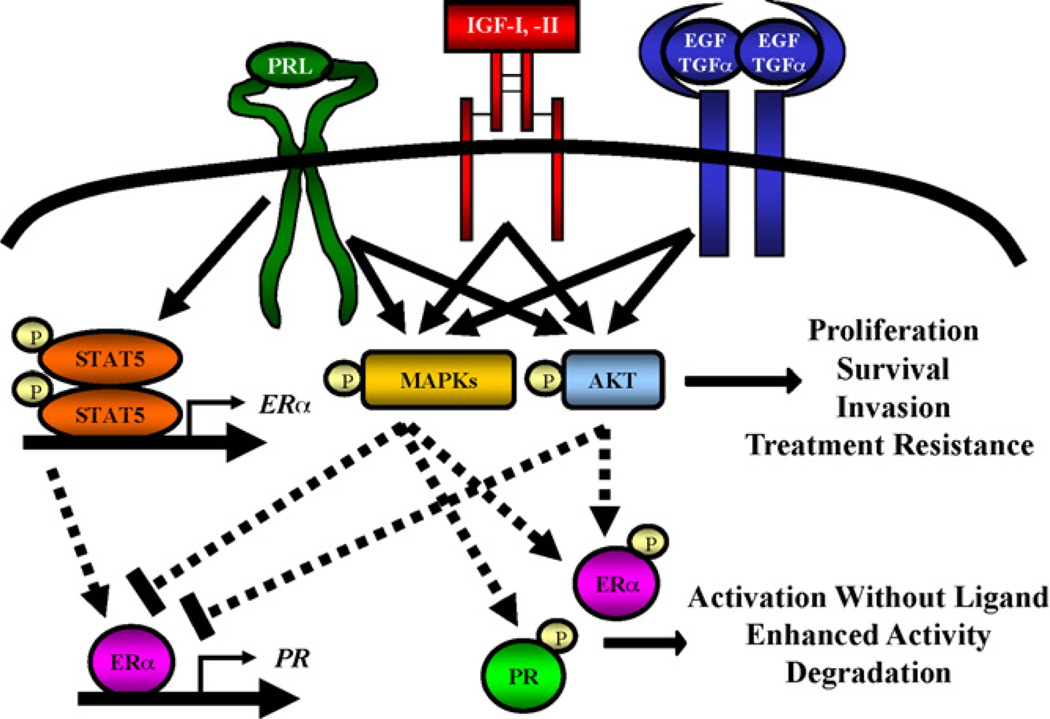
Mammary development requires coordinated interactions of multiple growth factors and hormones. Many of these normal developmental processes, such as proliferation and morphogenesis, often become deregulated during tumorigenesis. Thus, it is critical to understand growth factor and hormone interactions in the context of developing breast pathology. The pathways described above are shared by many of these factors and represent potential sites for crosstalk, as well as putative sites for therapeutic intervention. Interestingly, pathways other than JAK2/STAT5 appear to be the most robust sites for PRL-growth factor interaction. The association of these alternative pathways with neoplastic progression and treatment resistance as discussed above suggests profound prognostic and therapeutic implications for PRL actions in this disease. Accumulating evidence points to synergistic interactions between PRL and estrogen, members of the EGF family, and IGFs (Fig. 1).
Fig. 1.
PRL crosstalk with estrogen, progesterone, EGF/TGF-α, and IGF-I. PRL can synergize with IGF-I and EGF family ligands to activate MAPKs and AKT. These pathways contribute to expression of genes involved in proliferation, survival, and invasion. Furthermore, activation of these pathways is associated with resistance to endocrine, molecular, and chemo- and radio-therapies. ERK1/2 and AKT can also phosphorylate ERα and PR, leading to enhanced transcriptional activity and degradation. PRL signaling through the JAK2/STAT5 pathway increases expression of ERα, potentially enhancing estrogen sensitivity and expression of classical estrogen target genes, including PR. See text for further discussion.
4.1. PRL and estrogen
Crosstalk between PRL and estrogen can occur at multiple levels and is bidirectional. PRL increases ERα and ERβ transcription via STAT5 (Frasor and Gibori, 2003), and increases mammary ERα expression in mouse models (Edery et al., 1985; Muldoon, 1987). By this mechanism, PRL can enhance estrogen sensitivity, with implications for tumor differentiation and treatment sensitivity (Webb et al., 1992; Sharma et al., 2006). In MCF-7 cells engineered to inducibly overexpress PRL, endogenous PRL increasesERα expression, and also estrogen responsiveness as determined using several markers including proliferation, ERE-luciferase activity, and increased expression of target genes such as cyclin D1, progesterone receptor (PR), and Bcl-2 (Gutzman et al., 2004b).
PRL can also enhance non-classical and unliganded estrogen signaling. In MCF-7 cells, PRL and estrogen augment AP-1 activity through increased phosphorylation of p38, ERK1/2, and c-Fos (Gutzman et al., 2005). Additionally, PRL induces phosphorylation of ERα at S118 and S167, likely through MAPK and PI3K/AKT pathways (Glaros et al., 2006;Arendt and Schuler, 2008b). Phosphorylation of multiple serine residues can enhance ERα transcriptional activity even in the absence of estrogen, which may be further augmented by phosphorylation of coactivators by these same kinases (Likhite et al., 2006; Weigel and Moore, 2007; Gonzalez et al., 2009).
Conversely, estrogen also can alter PRL-induced signaling. Estrogen upregulates transcription of PRL in breast cancer cells (Dong et al., 2006; Duan et al., 2008), as well as pituitary lactotrophs (Ben-Jonathan et al., 2008), enhancing the mammary PRL autocrine/paracrine loop. Estrogen can potentiate PRL-activated Stat5 activity in some mammary cells, including some breast cancer cell lines (Bjornstrom et al., 2001; Wang and Cheng, 2004). However, the outcome of this crosstalk was inhibitory in some studies (Faulds et al., 2004; Wang and Cheng, 2004), underscoring the importance of cell context.
4.2. PRL and EGF/TGF-α
Multiple lines of evidence point to cooperation between PRL and EGF family members in breast cancer. PRL and EGF synergistically induce signals to ERK1/2 and AKT, but not STAT5, and PRL also can activate HER2 (Yamauchi et al., 2000; Arendt et al., 2006, 2009; Frank, 2008). Interestingly, PRL also reduces ligand-induced EGFR downregulation via threonine phosphorylation (Frank, 2008). Functionally, PRL potentiates EGF-induced cell motility in multiple breast cancer cell lines (Maus et al., 1999).
In genetically modified NRL-PRL/TGF-α mice, local mammary PRL and TGF-α in combination significantly enhance proliferation in morphologically normal structures, increase development of preneoplastic lesions, and dramatically reduce tumor latency in nonparous females (Arendt et al., 2006). These effects are accompanied by enhanced phosphorylation of mammary ERK1/2 and AKT, confirming that these kinases are points of signaling crosstalk (Arendt et al., 2006, 2009). Interestingly, PR expression is dramatically reduced in the bitransgenic mice. The literature suggests several mechanisms whereby these kinases may reduce PR transcription (Petz et al., 2004; Cui et al., 2003), or increase degradation of PR protein (Lange et al., 2007). Moreover, the combination of PRL with TGF-α can also cause mammary tumors in male mice, in contrast to the lack of tumors in single transgenic males (Arendt and Schuler, 2008b).
However, not all interactions between PRL and EGF family members are positive. Some studies of murine cell lines in vitro demonstrate inhibitory interactions between PRL and EGF (Fenton and Sheffield, 1993; Horsch et al., 2001). The relationship of these observations to the other models is unclear.
4.3. PRL and IGFs
PRL requires local production of IGF-II to mediate upregulation of cyclin D1 and subsequent mammary development in mouse models (Brisken et al., 2002; Hovey et al., 2003). However, in MCF-7 breast cancer cells this requirement is bypassed, although PRL retains its ability to increase IGF-II synthesis (Carver and Schuler, 2008). This demonstrates that the effects of PRL can differ in normal and tumor cells.
In addition to IGF-II, PRL also increases local IGF-I expression, creating a favorable environment for crosstalk between PRL and IGF-IR ligands in breast cancer cells. Like crosstalk downstream of PRLR and EGFR, PRL and IGF-I cooperatively activate ERK1/2 and AKT, but not STAT5 (Carver and Schuler, 2008). Together, PRL and IGF-I augment critical processes associated with neoplastic progression, including proliferation, survival, and invasion, more than the sums of effects elicited by PRL or IGF-I alone. One mechanism underlying this synergy is the ability of PRL to enhance IGF-I-induced phosphorylation of Tyr1135/1136 within the kinase domain of IGF-IR. This augmentation is a result of decreased IGF-IR dephosphorylation, caused by reduced association of IGF-IR with the tyrosine phosphatase, SHP-2, and diminished IGF-IR internalization (Carver et al., submitted).
5. Therapeutic implications
The evidence described above reveals PRL as an important modulator of the actions of estrogen, EGF family members, and IGF-I in breast cancer. These factors are the targets of widely employed and highly successful endocrine and molecular therapies, and continue to be the focus of pharmaceutical development. However, acquired resistance to existing therapies after the initial patient response has proven a major obstacle in clinical oncology, driving exploration of new targets. With the abundance of evidence implicating PRL in the pathogenesis of breast cancer, PRL and/or PRLR are promising targets. The relatively restricted distribution of PRLR and limited phenotype of the PRLR−/− mice apart from the mammary gland (Goffin et al., 2002) suggest that therapeutics directed toward PRL/PRLR will be well tolerated, increasing its appeal.
5.1. Inhibition of PRL signals
Due to the recent acceptance of a role for PRL in breast cancer, there is a relative dearth of PRL/PRLR-specific therapeutics that also address local PRL production. Inhibitors of PRL-associated kinases such as JAK2 and src family members have been developed, but these kinases are shared by many receptors, and are critical for physiological activities of other cytokines (Thomas and Brugge, 1997; Kisseleva et al., 2002). The production of antibodies raised against PRLR has proven difficult; only one study (Sissom et al., 1988) has shown anti-tumor efficacy in vivo, and these findings have yet to be reproduced (Clevenger et al., 2008). PRL antagonists evolved over several generations (Walker, 2006; Tallet et al., 2008) appear to be the most promising intervention to date, but these are in early stages of development, and issues surrounding binding affinity and stability must be resolved before they can enter the clinic.
Small molecule inhibitors represent an untapped class of potential PRL-specific therapeutics. The majority of identified small molecule inhibitors are directed toward known protein pockets, such as ATP binding sites in tyrosine kinase receptors, which have no counterpart in PRLR. However, these molecules can also interact with so-called “hot spots”, which may destabilize protein-protein interactions, such as PRLR-kinase or adaptor protein interactions.
Our laboratory has begun to investigate this possibility via high-throughput screening efforts. We have screened diverse “drug-like” libraries and discovered promising compounds that are currently being characterized (Carver et al., in preparation). Completion of these studies will enable future structural, functional, and pharmacological optimization. Importantly, these compounds will complement the development of PRL antagonists and will add to the meager repertoire of tools available to examine the contributions of PRL signals to oncogenic processes in the breast.
5.2. Combinatorial therapy
With the increasing number of treatment options for breast cancer patients, therapeutic regimens are becoming more individualized. Pre-clinical data strongly support the rationale for combinatorial therapy (Johnston et al., 2007). However, while some clinical trials combining antiestrogens, EGFR/HER2 inhibitors, anti-angiogenics, and/or chemotherapies have yielded encouraging results (Romond et al., 2005; Polychronis et al., 2005; Tabernero, 2007), others have shown no benefit compared to monotherapy (Leary et al., 2007). In some cases, one subpopulation responds well, while another exhibits no effect (Marcom et al., 2007). These results suggest that careful selection is required to identify the subset of patients who will receive the most benefit. Expression of the target protein itself (e.g. HER2) may not be sufficient, and additional predictive markers are needed.
Combining current treatment options with therapeutics targeting PRL/PRLR may be quite beneficial. The PRLR antagonist, G129R-PRL, enhances the efficacy of trastuzumab in xenograft models (Scotti et al., 2008), and potentiates the cytotoxic effects of doxorubicin and paclitaxel in vitro (Howell et al., 2008). Moreover, a study of women with metastatic breast cancer and concomitant hyperprolactinemia showed that reduction of pituitary PRL with cabergoline, in combination with docetaxel, resulted in a higher rate of tumor regression compared to docetaxel alone (Frontini et al., 2004). This is significant evidence that PRL/PRLR could be a prime target in the large number of patients with tumors expressing high levels of PRLR, particularly in patients with tumors demonstrating activated growth factor-stimulated pathways and/or ERα expression.
5.3. Resistance to treatment
Despite many successful responses to endocrine and molecular therapies, a significant patient subpopulation either does not respond initially (de novo resistance) or becomes refractory to such treatment (acquired resistance), often resulting in tumor relapse (Normano et al., 2005). Many implicated factors or signals are sites of crosstalk with PRL. For example, resistance to endocrine therapies is associated with increased MAPK and PI3K/AKT signaling, overexpression of HER2, and/or increased activation of IGF-IR (Nicholson et al., 2007; Massarweh et al., 2008). Resistance to EGFR- or HER2-targeted therapies is associated with increased PI3K/AKT signaling and/or IGF-IR activation (Bianco et al., 2005; Suzuki and Toi, 2007). Increased activation of IGF-IR and the PI3K/AKT cascade are also common mechanisms of chemo- and radio-resistance (Casa et al., 2008).
Combinatorial therapy has thus become an attractive strategy to overcome the resistant phenotype or to delay the onset of acquired resistance in some pre-clinical (du Manoir et al., 2006) and clinical studies (Dowsett et al., 2005). Concomitant inhibition of PRL signals may prove beneficial. High serum PRL levels before treatment are associated with endocrine therapy failure, recurrent disease, and worse overall survival (Dowsett et al., 1983; Bhatavdekar et al., 1994; Barni et al., 1998; Tworoger and Hankinson, 2008). The ability of PRL to potently augment activation of growth factor receptors and downstream signals associated with poor prognoses argues for inhibiting PRL action. Intriguingly, NRL-PRL/TGF-α bitransgenic mice have significantly elevated ERα expression in preneoplastic lesions, yet are non-responsive to estrogen, resembling an endocrine resistant state (Arendt et al., 2009). Finally, PRL protects breast cancer cells from irradiation-induced cell death (Chakravarti et al., 2005). These data support targeting PRL/PRLR in combination with other therapies to reduce or delay acquired treatment resistance.
6. Perspectives and conclusions
An increasingly abundant body of evidence supports a significant role for PRL in breast cancer. As described herein, PRL strongly interacts with oncogenic growth factors and hormones, contributing to tumor development and progression. Understanding the mechanism(s) underlying this crosstalk will further delineate the actions of PRL in breast carcinogenesis. Interestingly, studies to date suggest that PRL/EGF and PRL/IGF-I cooperation is selective, since the JAK2/STAT5 pathway is not affected. This is important to note, as activation of the latter pathway may be favorable in human tumors. However, PRL-growth factor crosstalk greatly increases the activation of the MAPK and PI3K/AKTpathways, which are associated with a poor prognosis and therapeutic resistance. Thus, combining therapeutics targeting PRL/PRLR with endocrine or EGFR/HER2-and IGF-IR-targeted therapies may more effectively inhibit tumor growth and/or prevent or delay acquired treatment resistance. Mixed results of several therapeutic combinations currently in clinical trials have underscored the importance of patient selection. Identification of markers that predict response will be extremely valuable as more targeted therapies are developed and treatment regimens become even more individualized. However, in order to exploit the role PRL plays in this disease, we must first develop PRL/PRLR-targeted therapies beyond the pre-clinical stage.
Acknowledgements
This work was supported by NIH RO1 CA78312 and DK62783 (L.A.S.), K01 RR21858 (L.M.A.), and T32 GM08349 (K.C.C.).
References
- Arendt LM, Rose-Hellekant TA, Sandgren EP, Schuler LA. Prolactin potentiates transforming growth factor-alpha induction of mammary neoplasia in transgenic mice. Am. J. Pathol. 2006;168:1365–1374. doi: 10.2353/ajpath.2006.050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt LM, Schuler LA. Transgenic models to study actions of prolactin in mammary neoplasia. J. Mammary Gland Biol. Neoplasia. 2008a;13:29–40. doi: 10.1007/s10911-008-9073-9. [DOI] [PubMed] [Google Scholar]
- Arendt LM, Schuler LA. Prolactin drives ERα-dependent ductal expansion and synergizes with transforming growth factor-alpha to induce mammary tumors in males. Am. J. Pathol. 2008b;172:194–202. doi: 10.2353/ajpath.2008.070597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt LM, Grafwallner-Huseth TL, Schuler LA. Prolactin-growth factor crosstalk reduces mammary estrogen responsiveness despite elevated ERα expression. Am. J. Pathol. 2009;174:1065–1074. doi: 10.2353/ajpath.2009.080719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barni S, Lissoni P, Meregalli S, Ardizzoia A, Mengo S, Musco F, Merlini D, Tancini G. Clinical efficacy of the aromatase inhibitor anastrozole in relation to prolactin secretion in heavily pretreated metastatic breast cancer. Tumori. 1998;84:45–47. doi: 10.1177/030089169808400109. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr. Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist J, Elmberger G, Ohd J, Linderholm B, Bjohle J, Helborg H, Nordgren H, Borg AL, Skoog L, Bergh J. Activated ERK1/2 and phosphorylated oestrogen receptor-alpha are associated with improved breast cancer survival in women treated with tamoxifen. Eur. J. Cancer. 2006;42:1104–1112. doi: 10.1016/j.ejca.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Bhatavdekar JM, Patel DD, Karelia NH, Shah NG, Ghosh N, Vora HH, Suthar TP, Balar DB, Doctor SS. Can plasma prolactin predict tamoxifen resistance in patients with advanced breast cancer? Eur. J. Surg. Oncol. 1994;20:118–121. [PubMed] [Google Scholar]
- Bianco R, Toriani T, Tortora G, Ciardiello F. Intrinsic and acquired resistance to EGFR inhibitors in human cancer therapy. Endocr. Relat. Cancer. 2005;12 Suppl. 1:S159–S171. doi: 10.1677/erc.1.00999. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Kilic E, Norman M, Parker MG, Sjoberg M. Crosstalk between STAT5b and estrogen receptor-alpha and -beta in mammary epithelial cells. J. Mol. Endocrinol. 2001;27:93–106. doi: 10.1677/jme.0.0270093. [DOI] [PubMed] [Google Scholar]
- Bratthauer GL, Strauss BL, Tavassoli FA. STAT5a expression in various lesions of the breast. Virchows Arch. 2006;448:165–171. doi: 10.1007/s00428-005-0056-6. [DOI] [PubMed] [Google Scholar]
- Brisken C, Ayyanna A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev. Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Carver KC, Schuler LA. Prolactin does not require insulin-like growth factor intermediates but synergizes with insulin-like growth factor I in human breast cancer cells. Mol. Cancer Res. 2008;6:634–643. doi: 10.1158/1541-7786.MCR-07-2069. [DOI] [PubMed] [Google Scholar]
- Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front. Biosci. 2008;13:3273–3287. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- Chakravarti P, Henry MK, Quelle FW. Prolactin and heregulin override DNA damage-induced growth arrest and promote phosphatidylinositol-3 kinase-dependent proliferation in breast cancer cells. Int. J. Oncol. 2005;26:509–514. [PubMed] [Google Scholar]
- Chilton BS, Hewetson A. Prolactin and growth hormone signaling. Curr. Top. Dev. Biol. 2005;68:1–23. doi: 10.1016/S0070-2153(05)68001-5. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Plank TL. Prolactin as an autocrine/paracrine factor in breast tissue. J. Mammary Gland Biol. Neoplasia. 1997;2:59–68. doi: 10.1023/a:1026325630359. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr. Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger CV, Zheng J, Jablonski EM, Galbaugh TL, Fang F. From bench to bedside: future potential for the translation of prolactin inhibitors as breast cancer therapeutics. J. Mammary Gland Biol. Neoplasia. 2008;13:147–156. doi: 10.1007/s10911-008-9074-8. [DOI] [PubMed] [Google Scholar]
- Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA. STAT5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int. J. Cancer. 2004;108:665–671. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, Lee AV. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol. Endocrinol. 2003;17:575–588. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- Dong J, Tsai-Morris CH, Dufau ML. A novel estradiol/ERα-dependent transcriptional mechanism controls expression of the human prolactin receptor. J. Biol. Chem. 2006;281:18825–18836. doi: 10.1074/jbc.M512826200. [DOI] [PubMed] [Google Scholar]
- Dowsett M, McGarrick GE, Harris AL, Coombes RC, Smith IE, Jeffcoate SL. Prognostic significance of serum prolactin levels in advanced breast cancer. Br. J. Cancer. 1983;47:763–769. doi: 10.1038/bjc.1983.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Nicholson RI, Pietras RJ. Biological characteristics of the pure antiestrogen fulvestrant: overcoming endocrine resistance. Breast Cancer Res. Treat. 2005;93 Suppl. 1:S11–S18. doi: 10.1007/s10549-005-9037-3. [DOI] [PubMed] [Google Scholar]
- Duan R, Ginsburg E, Vonderhaar BK. Estrogen stimulates transcription from the human prolactin distal promoter through AP-1 and estrogen responsive elements in T47D human breast cancer cells. Mol. Cell. Endocrinol. 2008;281:9–18. doi: 10.1016/j.mce.2007.10.004. [DOI] [PubMed] [Google Scholar]
- du Manoir JM, Francia G, Man S, Mossoba M, Medin JA, Viloria-Petit A, Hicklin DJ, Emmenegger U, Kerbel RS. Strategies for delaying or treating in vivo acquired resistance to trastuzumab in human breast cancer xenografts. Clin. Cancer Res. 2006;12:904–916. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- Edery M, Imagawa W, Larson L, Nandi S. Regulation of estrogen and progesterone receptor levels in mouse mammary epithelial cells grown in serum-free collagen gel cultures. Endocrinology. 1985;116:105–112. doi: 10.1210/endo-116-1-105. [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- Faulds MH, Olsen H, Helguero LA, Gustafsson JA, Hadosen LA. Estrogen receptor functional activity changes during differentiation of mammary epithelial cells. Mol. Endocrinol. 2004;18:412–421. doi: 10.1210/me.2003-0290. [DOI] [PubMed] [Google Scholar]
- Fenton SE, Sheffield LG. Prolactin inhibits EGF-stimulated signaling events in mouse mammary epithelial cells by altering EGF receptor function. Mol. Biol. Cell. 1993;4:773–780. doi: 10.1091/mbc.4.8.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SJ. Mechanistic aspects of crosstalk between GH and PRL and ErbB receptor family signaling. J. Mammary Gland Biol. Neoplasia. 2008;13:119–129. doi: 10.1007/s10911-008-9065-9. [DOI] [PubMed] [Google Scholar]
- Frasor J, Gibori G. Prolactin regulation of estrogen receptor expression. Trends Endocrinol. Metab. 2003;3:118–123. doi: 10.1016/s1043-2760(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Frontini L, Lissoni P, Vaghi M, Perego MS, Pescia S, Ardizzoia A, Gardani G. Enhancement of the efficacy of weekly low-dose taxotere by the long acting anti-prolactinemic drug cbergoline in pretreated metastatic breast cancer. Anticancer Res. 2004;24:4223–4226. [PubMed] [Google Scholar]
- Gee JMW, Robertson JFR, Ellis IO, Nicholson RI. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int. J. Cancer. 2001;95:247–254. doi: 10.1002/1097-0215(20010720)95:4<247::aid-ijc1042>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gill S, Peston D, Vonderhaar BK, Shousha S. Expression of prolactin receptors in normal, benign, and malignant breast tissue: an immunohistological study. J. Clin. Pathol. 2001;54:956–960. doi: 10.1136/jcp.54.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg E, Vonderhaar BK. Prolactin synthesis and secretion by human breast cancer cells. Cancer Res. 1995;55:2591–2595. [PubMed] [Google Scholar]
- Glaros S, Atanaskova N, Zhao C, Skafar DF, Reddy KB. Activation function-1 domain of estrogen receptor regulates the agonistic and antagonistic actions of tamoxifen. Mol. Endocrinol. 2006;20:996–1008. doi: 10.1210/me.2005-0285. [DOI] [PubMed] [Google Scholar]
- Goffin V, Touraine P, Pichard C, Bernichtein S, Kelly PA. Should prolactin be reconsidered as a therapeutic target in human breast cancer? Mol. Cell. Endocrinol. 1999;151:79–87. doi: 10.1016/s0303-7207(99)00023-4. [DOI] [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu. Rev. Physiol. 2002;64:47–67. doi: 10.1146/annurev.physiol.64.081501.131049. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Zambrano A, Lazaro-Trueba I, Lopez E, Gonzalez JJ, Martin-Perez J, Aranda A. Activation of the unliganded estrogen receptor by prolactin in breast cancer cells. Oncogene. 2009;28:1298–1308. doi: 10.1038/onc.2008.473. [DOI] [PubMed] [Google Scholar]
- Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase AKT induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- Gutzman JH, Rugowski DE, Schroeder MD, Watters JJ, Schuler LA. Multiple kinase cascades mediate prolactin signals to activating protein-1 in breast cancer cells. Mol. Endocrinol. 2004a;18:3064–3075. doi: 10.1210/me.2004-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzman JH, Miller KK, Schuler LA. Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor alpha and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2004b;88:69–77. doi: 10.1016/j.jsbmb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Gutzman JH, Nikolai SE, Rugowski DE, Watters JJ, Schuler LA. Prolactin and estrogen enhance the activity of activating protein 1 in breast cancer cells: role of extracellularly regulated kinase 1/2-mediated signals to c-fos. Mol. Endocrinol. 2005;19:1765–1778. doi: 10.1210/me.2004-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzman JH, Rugowski DE, Nikolai SE, Schuler LA. Stat5 activation inhibits prolactin-induced AP-1 activity: distinct prolactin-initiated signals in tumorigenesis dependent on cell context. Oncogene. 2007;26:6341–6348. doi: 10.1038/sj.onc.1210454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu YL, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Horsch K, Schaller MD, Hynes NE. The protein tyrosine phosphatase-PEST is implicatedin the negative regulation of epidermal growth factor on PRL signaling in mammary epithelial cells. Mol. Endocrinol. 2001;15:2182–2196. doi: 10.1210/mend.15.12.0743. [DOI] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:2935–6926. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey RC, Harris J, Hadsell DL, Lee AV, Ormandy CJ, Vonderhaar BK. Local insuin-like growth factor-II mediates prolactin-induced mammary gland development. Mol. Endocrinol. 2003;17:460–471. doi: 10.1210/me.2002-0214. [DOI] [PubMed] [Google Scholar]
- Howell SJ, Anderson E, Hunter T, Farnie G, Clarke RB. Prolactin receptor antagonism reduces the clonogenic capacity of breast cancer cells and potentiates doxorubicin and paclitaxel cytotoxicity. Breast Cancer Res. 2008;10:R68. doi: 10.1186/bcr2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys RC, Hennighausen L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. 1999;10:685–694. [PubMed] [Google Scholar]
- Iavnilovitch E, Cardiff RD, Groner B, Barash I. Deregulation of STAT5 expression and activation causes mammary tumors in transgenic mice. Int. J. Cancer. 2004;112:607–619. doi: 10.1002/ijc.20484. [DOI] [PubMed] [Google Scholar]
- Johnston SRD, Martin LA, Leary A, Head J, Dowsett M. Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J. Steroid Biochem. Mol. Biol. 2007;106:180–186. doi: 10.1016/j.jsbmb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JM. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J. Pathol. 2005;207:139–146. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- Lange CA, Gioeli D, Hammes SR, Marker PC. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu. Rev. Physiol. 2007;69:171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- Leary AF, Sirohi B, Johnston SR. Clinical trials update: endocrine and biological therapy combinations in the treatment of breast cancer. Breast Cancer Res. 2007;9:112–121. doi: 10.1186/bcr1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, DNA, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol. Endocrinol. 2006;20:3120–3132. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner K-U, Garrett L, Wynshaw-Boris A, Hennighausen L. STAT5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Liu W, Bagaitkar J, Watabe K. Roles of AKT signal in breast cancer. Front. Biosci. 2007;12:4011–4019. doi: 10.2741/2367. [DOI] [PubMed] [Google Scholar]
- Marcom PK, Isaacs C, Harris L, Wong ZW, Kommarreddy A, Novielli N, Mann G, Tao Y, Ellis MJ. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancer. Breast Cancer Res. Treat. 2007;102:43–49. doi: 10.1007/s10549-006-9307-8. [DOI] [PubMed] [Google Scholar]
- Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoattractant for human breast cancers. Endocrinology. 1999;140:5447–5550. doi: 10.1210/endo.140.11.7245. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Roles of RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- McHale K, Tomaszewski JE, Puthiyaveetil R, Livolsi VA, Clevenger CV. Altered expression of prolactin receptor-associated signaling proteins in human breast carcinoma. Mod. Pathol. 2008;21:565–571. doi: 10.1038/modpathol.2008.7. [DOI] [PubMed] [Google Scholar]
- Meng J, Tsai-Morris CH, Dufau ML. Human prolactin receptor variants in breast cancer: low ratio of short forms to long-form human prolactin receptor associated with mammary carcinoma. Cancer Res. 2004;64:5677–5682. doi: 10.1158/0008-5472.CAN-04-1019. [DOI] [PubMed] [Google Scholar]
- Milde-Langosch K, Bamberger AM, Rieck G, Grund D, Hemminger G, Muller V, Loning T. Expression and prognostic relevance of activated extracellular-regulated kinases (ERK1/2) in breast cancer. Br. J. Cancer. 2005:2206–2215. doi: 10.1038/sj.bjc.6602655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon TG. Prolactin mediation of estrogen-induced changes in mammary tissue estrogen and progesterone receptors. Endocrinology. 1987;121:141–149. doi: 10.1210/endo-121-1-141. [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, Sauter G, Rui H. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J. Clin. Oncol. 2004;22:2053–2060. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Hutcheson IR, Jones HE, Hiscox SE, Giles M, Taylor KM, Gee JM. Growth factor signaling in endocrine and anti-growth factor resistant breast cancer. Rev. Endocr. Metab. Disord. 2007;8:241–253. doi: 10.1007/s11154-007-9033-5. [DOI] [PubMed] [Google Scholar]
- Normano N, Di Maio M, De Maio E, De Luca A, de Matteis A, Giordano A, Perrone F. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr. Relat. Cancer. 2005;12:721–747. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- Nouhi Z, Chughati N, Hartley S, Cocolakis E, Lebrun JJ, Ali S. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res. 2006;66:1824–1832. doi: 10.1158/0008-5472.CAN-05-2292. [DOI] [PubMed] [Google Scholar]
- Oakes SR, Robertson FG, Kench JG, Gardiner-Garden M, Wand MP, Green JE, Ormandy CJ. Loss of mammary epithelial prolactin receptor delays tumor formation by reducing cell proliferation in low-grade preinvasive lesions. Oncogene. 2007;26:543–553. doi: 10.1038/sj.onc.1209838. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Hall RE, Manning DL, Robertson JFR, Blarney RW, Kelly PA, Nicholson RI, Sutherland RL. Coexpression and cross-regulation of the prolactin receptor and sex steroid hormone receptors in breast cancer. J. Clin. Endocrinol. Metab. 1997a;82:3692–3699. doi: 10.1210/jcem.82.11.4361. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive effects in the mouse. Genes Dev. 1997b;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Petz LN, Ziegler YS, Schultz JR, Nardulli AM. Fos and jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site. Mol. Endocrinol. 2004;18:521–532. doi: 10.1210/me.2003-0105. [DOI] [PubMed] [Google Scholar]
- Polychronis A, Sinnett HD, Hadjiminas D, Singhal H, Monsi JL, Shivapatham D, Shousha S, Jiang J, Peston D, Barrett N, Vigushin D, Morrision K, Beresford E, Ali S, Slade MJ, Coombes RC. Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen receptor-positive and epidermal growth factor receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomized trial. Lancet Oncol. 2005;6:383–391. doi: 10.1016/S1470-2045(05)70176-5. [DOI] [PubMed] [Google Scholar]
- Ren S, Cai HR, Li M, Furth PA. Loss of STAT5a delays mammary cancer progression in a mouse model. Oncogene. 2002;21:4335–4339. doi: 10.1038/sj.onc.1205484. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV. Expression of prolactin and its receptor in human breast carcinoma. Endocrinology. 1997;138:5555–5560. doi: 10.1210/endo.138.12.5605. [DOI] [PubMed] [Google Scholar]
- Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer Lett. 2007;256:1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tanh-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy in operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activate protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin. Cancer Res. 2004;10:331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- Schwertfeger KL, Hunter S, Heasley LE, Levresse V, Leon RP, DeGregori J, Anderson SM. Prolactin stimulates activation of c-jun N-terminal kinase (JNK) Mol. Endocrinol. 2000;13:1592–1602. doi: 10.1210/mend.14.10.0536. [DOI] [PubMed] [Google Scholar]
- Scotti ML, Langenheim JF, Tomblyn S, Springs AE, Chen WY. Additive effects of a prolactin receptor antagonist, G129R, and herceptin on inhibition of HER2-overexpressing breast cancer cells. Breast Cancer Res. Treat. 2008;111:241–250. doi: 10.1007/s10549-007-9789-z. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Sharma D, Saxena NK, Davidson NE, Vertino PM. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res. 2006;66:6370–6378. doi: 10.1158/0008-5472.CAN-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Robinson GW, Grimm SL, Rosen JM, Neubauer H, Pfeffer K, Hennighausen L. JAK2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 2002;16:563–570. doi: 10.1210/mend.16.3.0805. [DOI] [PubMed] [Google Scholar]
- Silva CM, Shupnik MA. Integration of steroid and growth factor pathways in breast cancer: focus on signal transducers and activators of transcription and their potential role in resistance. Mol. Endocrinol. 2007;21:1499–1512. doi: 10.1210/me.2007-0109. [DOI] [PubMed] [Google Scholar]
- Sissom JF, Eigenbrodt ML, Porter JC. Anti-growth action on mouse mammary and prostate glands of a monoclonal antibody to prolactin receptor. Am. J. Pathol. 1988;133:589–595. [PMC free article] [PubMed] [Google Scholar]
- Sivaraman VS, Wang HY, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J. Clin. Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss BL, Bratthauer GL, Tavassoli FA. STAT5a expression in the breast is maintained in secretory carcinoma, in contrast to other histologic types. Hum. Pathol. 2006;37:586–592. doi: 10.1016/j.humpath.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. STAT5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene. 2005;24:746–760. doi: 10.1038/sj.onc.1208203. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Toi M. Improving the efficacy of trastuzumab in breast cancer. Cancer Sci. 2007;98:767–771. doi: 10.1111/j.1349-7006.2007.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol. Cancer Res. 2007;5:203–220. doi: 10.1158/1541-7786.MCR-06-0404. [DOI] [PubMed] [Google Scholar]
- Tallet E, Rouet V, Jomain JB, Kelly PA, Bernichtein S, Goffin V. Rational design of competitive prolactin/growth hormone receptor antagonists. J. Mammary Gland Biol. Neoplasia. 2008;13:105–117. doi: 10.1007/s10911-008-9066-8. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. STAT5a and STAT5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tomblyn S, Langenheim JF, Jacquemart IC, Holle E, Chen WY. The role of human prolactin and its antagonist,G129R, in mammary gland development and DMBA-initiated tumorigenesis in transgenic mice. Int. J. Oncol. 2005;27:1381–1389. [PubMed] [Google Scholar]
- Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicolas A, Trivin C, Postel-Vinay MC, Kuttenn F, Kelly PA. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J. Clin. Endocrinol. Metab. 1998;83:667–674. doi: 10.1210/jcem.83.2.4564. [DOI] [PubMed] [Google Scholar]
- Trott JF, Vonderhaar BK, Hovey RC. Historical perspectives of prolactin and growth hormone as mammogens, lactogens and galactagogues—agog for the future! J. Mammary Gland Biol. Neoplasia. 2008;13:3–11. doi: 10.1007/s10911-008-9064-x. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Hankinson SE. Prolactin and breast cancer etiology: an epidemiological perspective. J. Mammary Gland Biol. Neoplasia. 2008;13:41–53. doi: 10.1007/s10911-008-9063-y. [DOI] [PubMed] [Google Scholar]
- Vilora-Petit AM, Kerbel RS. Acquired resistance to EGFR inhibitors: mechanisms and prevention strategies. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:914–926. doi: 10.1016/j.ijrobp.2003.09.091. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK. Prolactin: the forgotten hormone of breast cancer. Pharmacol. Ther. 1998;79:169–178. doi: 10.1016/s0163-7258(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Wagner K-U, Rui H. JAK2/STAT5 signaling in mammogenesis, breast cancer initiation and progression. J. Mammary Gland Biol. Neoplasia. 2008;13:93–103. doi: 10.1007/s10911-008-9062-z. [DOI] [PubMed] [Google Scholar]
- Walker AM. Therapeutic potential of S179D prolactin—from prostate cancer to angioproliferative disorders: the first selective prolactin receptor modulator. Expert Opin. Investig. Drugs. 2006;15:1257–1267. doi: 10.1517/13543784.15.10.1257. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng CH. Estrogen receptor alpha and STAT5 crosstalk: interaction through c-terminal portions of the proteins decreases STAT5a phosphorylation, nuclear translocation, and DNA binding. FEBS Lett. 2004;572:238–244. doi: 10.1016/j.febslet.2004.06.098. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Greene GL, Baxter JD, Kushner PJ. The limits of the cellular capacity to mediate an estrogen response. Mol. Endocrinol. 1992;6:157–167. doi: 10.1210/mend.6.2.1569962. [DOI] [PubMed] [Google Scholar]
- Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol. Endocrinol. 2007;21:2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Nishio M, Ando Y, Zhang Z, Hamaguchi M, Mita K, Kobayashi S, Fujii Y, Iwase H. STAT5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocr. Relat. Cancer. 2006;13:885–893. doi: 10.1677/erc.1.01095. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Yamauchi N, Ueki K, Sugiyama T, Waki H, Miki H, Tobe K, Matsuda S, Tsushima T, Yamamoto T, Fujita T, Taketani Y, Fukayama M, Kimura S, Yazaki Y, Nagai R, Kadowaki T. Constitutive tyrosine phosphorylation of ErbB-2 via JAK2 by autocrine secretion of prolactin in human breast cancer. J. Biol. Chem. 2000;275:33937–33944. doi: 10.1074/jbc.M000743200. [DOI] [PubMed] [Google Scholar]
- Zhou BHP, Deng J, Xia WY, Xu JH, Li YM, Gunduz M, Hung MC. Dual regulation of snail by GSK-3 beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- Zinger M, McFarland M, Ben-Jonathan N. Prolactin expression and secretion by human breast glandular and adipose tissue explants. J. Clin. Endocrinol. Metab. 2003;88:689–696. doi: 10.1210/jc.2002-021255. [DOI] [PubMed] [Google Scholar]