Abstract
Previous studies using knockout mice document a key role for the integrin CD103 in promoting organ allograft rejection and graft-vs-host disease (GVHD). However, a determination of whether blockade of the CD103 pathway represents a viable therapeutic strategy for intervention in these processes has proven problematic due to the lack of reagents that efficiently deplete CD103+ cells from wild type hosts. To circumvent this problem, we conjugated the non-depleting anti-CD103 monoclonal antibody, M290, to the toxin, saporin, to produce an immunotoxin (M290-SAP) that efficiently depletes CD103+ cells in vivo. Herein, we show that M290-SAP dramatically reduces the frequency and absolute numbers of CD103-expressing leukocytes in the blood, spleen, mesenteric lymph nodes and intestinal epithelium of treated mice. We further demonstrate that M290-SAP promotes indefinite islet allograft survival in a fully MHC mismatched mouse model. The prolonged islet allograft survival resulting from M290-SAP treatment was associated with multiple effects in the host immune system including not only depletion of CD103-expressing leukocytes, but also an increase in CD4+CD25+FoxP3+ T regulatory cells and a predominance of effector-memory CD8 T cells. Regardless of the underlying mechanisms, these data document that depletion of CD103 expressing cells represents a viable strategy for therapeutic intervention in allograft rejection.
INTRODUCTION
Despite the now routine nature of clinical organ transplantation, even well-matched transplants are recognized and inevitably destroyed by the adaptive immune system [1]. Therapeutic strategies to counter this process continue to rely on drugs that globally suppress the adaptive immune system, leaving the patient vulnerable to tumors and opportunistic infections. Novel targets for therapeutic intervention in these processes are therefore needed. One potential target for therapeutic intervention in organ allograft rejection – and the focus of this communication- is the integrin heterodimer CD103/β7, herein referred to as CD103.
CD103 was initially identified by its expression in the vertebrate gut mucosa [2], where it is expressed at high levels by >95% of CD8+ T cells in the intestinal epithelium. Subsequent studies by Karecla et al. [3] and Cepek et al. [4] documented that the epithelial cell-specific ligand, E-cadherin, is the pertinent ligand of CD103. Of relevance to clinical renal transplantation, CD103 is expressed by a subset of CD8 effector cells that infiltrate rejecting renal allografts [5, 6]. The CD103 ligand, E-cadherin, is broadly expressed by epithelial layers and is known to be highly expressed by the functional elements of commonly transplanted organs [7–11], consistent with a key role for CD103+CD8+ effectors in promoting graft dysfunction. That CD103 plays a causal role in destruction of graft epithelial compartments was established using a mouse pancreatic islet allograft model where it was shown that CD8+ T cells from CD103-deficient mice were strikingly defective in the capacity to reject islet allografts [12]. Subsequent studies documented that CD103 plays an analogous role in destruction of the graft renal tubules during renal allograft rejection [13] and in the destruction of the host intestinal epithelium during graft-vs-host disease [14].
From the perspective of therapeutic intervention in organ allograft rejection, it is important to note that CD103 is promiscuously expressed by leukocyte subsets including several that might reasonably be expected to impact immune responses. In addition to CD8 effector populations[5], CD103 also is expressed by subsets of naïve CD8+ T cells [15] and CD8+ recent thymic emigrants[16]. Furthermore, CD103 is expressed by critical non-CD8 cells including subsets of dendritic cells [17] and CD4+CD25+FoxP3+ T regs[18], raising concern that CD103 blockade might cause global immunosuppressive effects and/or exacerbate transplant immune responses. CD103 is reportedly expressed by epidermal dendritic T cells and mast cells [19], though the extent to which these cell populations contribute to transplant immunity is unknown.
The goal of the present study was to assess the therapeutic potential of CD103 blockade using a well defined murine transplant model. The existing monoclonal antibodies (mAbs) to mouse CD103 - M290 [17] and 2E7 [15] are blocking antibodies that fail to significantly deplete CD103-expressing cells in vivo (GAH, unpublished data). Previous studies have shown that such blocking mAbs are ineffective in prolonging survival of pancreatic islet allografts in wild type hosts even in strain combinations in which rejection is known to be CD103 dependent[12]. To circumvent this barrier, we conjugated the non-depleting anti-CD103 antibody, M290, to the toxin, saporin to produce an anti-CD103 immunotoxin (M290-SAP) that efficiently depletes CD103-expressing cells in vivo. We herein report the development and characterization of this novel reagent. We initially demonstrate the striking efficacy of M290-SAP in depleting CD103-expressing cells in vivo. We further demonstrate that a single injection of M290-SAP promotes long-term acceptance of pancreatic islet allografts. These data provide proof-of-principle that depletion of CD103-expressing cells presents a viable approach for therapeutic intervention in organ allograft rejection.
MATERIALS AND METHODS
Animals
Female 8−10-week-old C57BL/6 (H-2b) recipients and BALB/c (H-2d) donor mice were purchased from Charles River/ NCI and maintained under pathogen-free housing conditions with free access to food and water at the Ohio State University, Columbus, OH. All mouse studies were done in compliance with National Institutes of Health (NIH) and Institutional Animal Care and Use Committee (IACUC) policies and guidelines at the Ohio State University.
CD103-immunotoxin
M290 (rIgG2a) to mouse CD103 [17] was purified from hybridoma culture supernatants and conjugated to saporin by Advanced Targeting Systems (San Diego, CA) to produce M290-SAP.
Islet isolation and transplantation
Islets for transplantation were prepared as described by Gotoh et al [20]. Diabetes was induced in recipient C57BL/6 mice with a single intraperitoneal (i.p.) injection of streptozotocin (200 mg/kg) and confirmed by measuring persistent hyperglycemia (blood glucose >350 mg/dl). Approximately 500 islets were transplanted under the left kidney capsule of the diabetic C57BL/6 recipient. After recovery from anesthesia, and blood glucose levels were monitored 2–3 times per week. Blood glucose levels of less than 200 mg/dl, by day 3 after transplantation, defined primary graft function whereas blood glucose levels of >200 mg/dl allograft dysfunction and loss. Recipient C57BL/6 mice received 2.0 mg/kg M290-SAP ip or the same dose of unconjugated M290 and/or IgG conjugated to saporin (IgG-SAP). on day 3 post-transplantation.
Lymphocyte isolation
Small intestinal epithelial lymphocytes (iIEL) were isolated as previously described [21]. Blood collection from the orbital sinus of mice was anticoagulated with EDTA. Cells were isolated from spleens, lymph node and thymus by mincing the tissue with forceps and passing the resulting homogenate through a cell strainer (40-µm pore size). Splenocytes were further processed by density centrifugation with Lympholyte-M (CEDARLANE Laboratories Ltd, Burlington, NC) to remove red blood cells. TruCOUNT tube (BD bioscience) was used to calculate absolute number of the specific subpopulation.
CFSE-labeled lymphocyte proliferation assay in vivo
20 million splenocytes (prepared by tissue mincing, followed by hypotonic lysis of red blood cells) from syngeneic mice were delivered intravenously by tail vein injection into M290-SAP treated C57BL/6 mice. The cells were labeled with CFSE (carboxyfluorescein succinimidyl ester) as described by Gudmundsdottir et al [22]. 14 days later, the mice were killed, spleen was collected and mononuclear cells were analyzed by flow to monitor CD4 and CD8 population proliferation.
Flow cytometry
Flow cytometry was performed using a FACSCalibur (BD bioscience). Data were analyzed using FCS express (De Novo software, Los Angeles, CA), FlowJo (TreeStar, Inc., Ashland, OR), and WinMDI (Scripps Institute, San Diego, CA) software. The percentage of positive cells for a given marker was based on cutoff points chosen to exclude >99% of the negative control population. For in vitro use, fluorochrome-conjugated mAbs to mouse CD103 (M290 and 2E7), CD4 (GK1.5), CD8a (53-6.7), CD3e (145-2C11), CD25 (3C7), CD11c (HL3), CD44 (IM7), CD62L (MEL-14), and the respective species- and isotype-matched negative control mAbs were purchased from BD PharMingen (San Diego, CA). Fluorochrome-conjugated mAb to mouse FoxP3 (FJK-16s) and isotype-matched negative control mAb were purchased from eBiosicence (San Diego, CA).
Statistical analyses
Analyses were performed using Prism 5 program (GraphPad Software). Graft survivals were compared using the Log-rank test. Other analyses performed included Student’s t-test and one-way ANOVA Dunnett’s multiple comparisons.
RESULTS
M290-SAP depletes CD103-expressing cells in vivo
The initial goal of this study was to develop a reagent that efficiently depletes CD103+CD8+ cells in vivo. To accomplish this, we conjugated the non-depleting anti-CD103 mAb, M290 (rat IgG2a), to the toxin, saporin, to produce the immunotoxin M290-saporin (M290-SAP). To assess the in vivo efficacy of M290-SAP, naïve C57BL/6 mice were treated with titrated doses ranging from 0.1 mg/kg to 2.0 mg/kg. These studies revealed that doses <2 mg/kg resulted in only partial depletion of CD103+CD8+ cells in normal mice (data not shown). We therefore used the dose of 2.0 mg/kg administered i.p. as the standard dose for further experiments. Pharmacokinetic studies indicated that M290-SAP delivered in this manner is cleared from the circulation by day 3 post injection (data not shown).
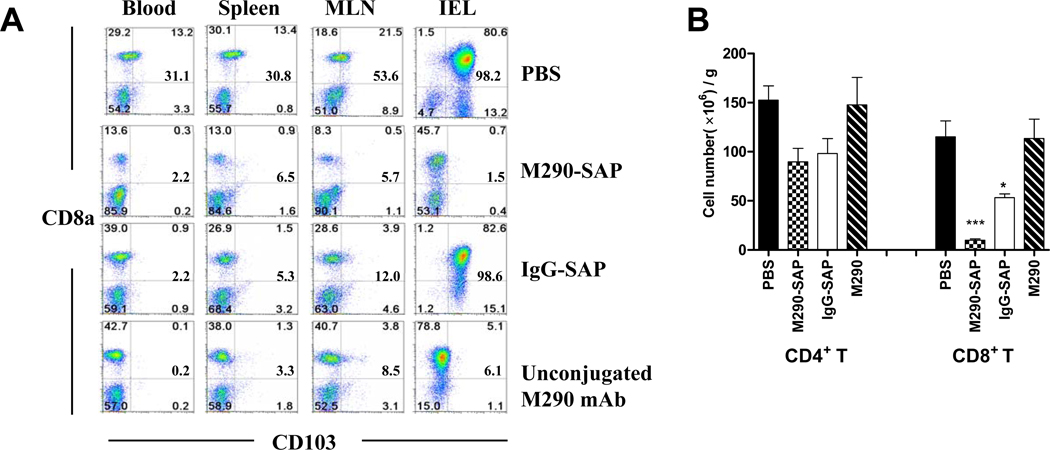
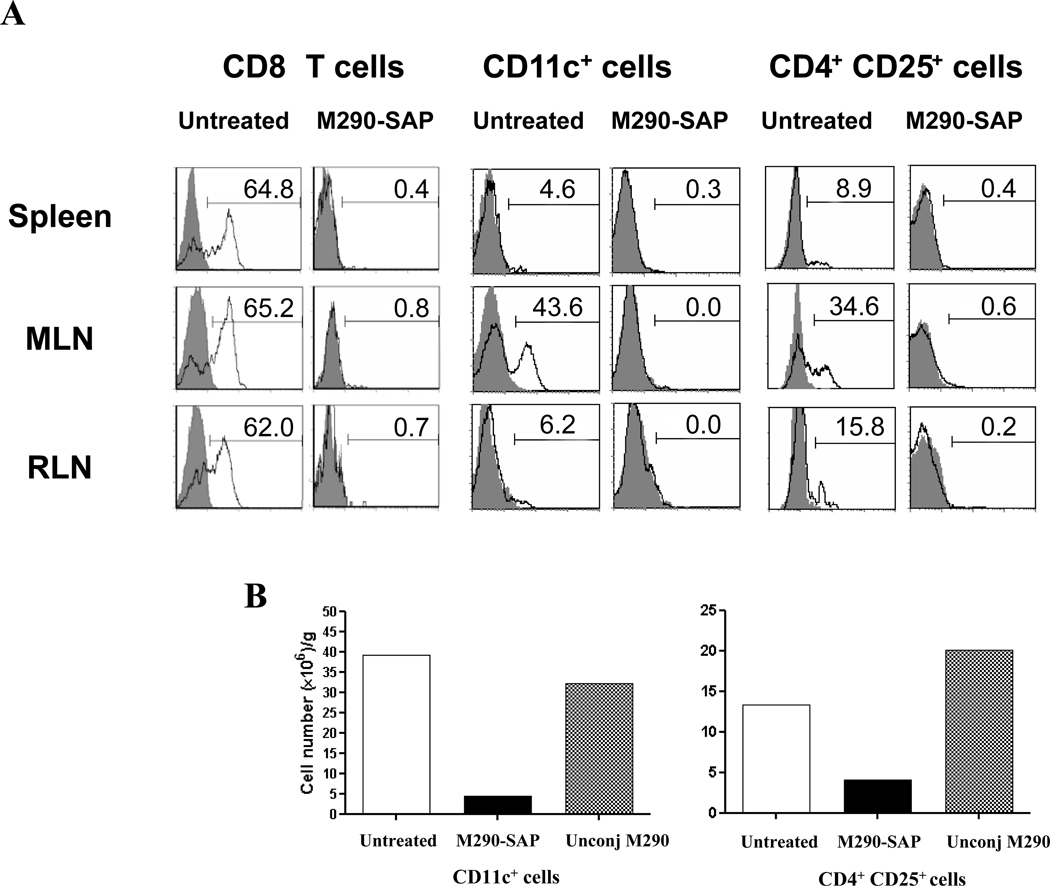
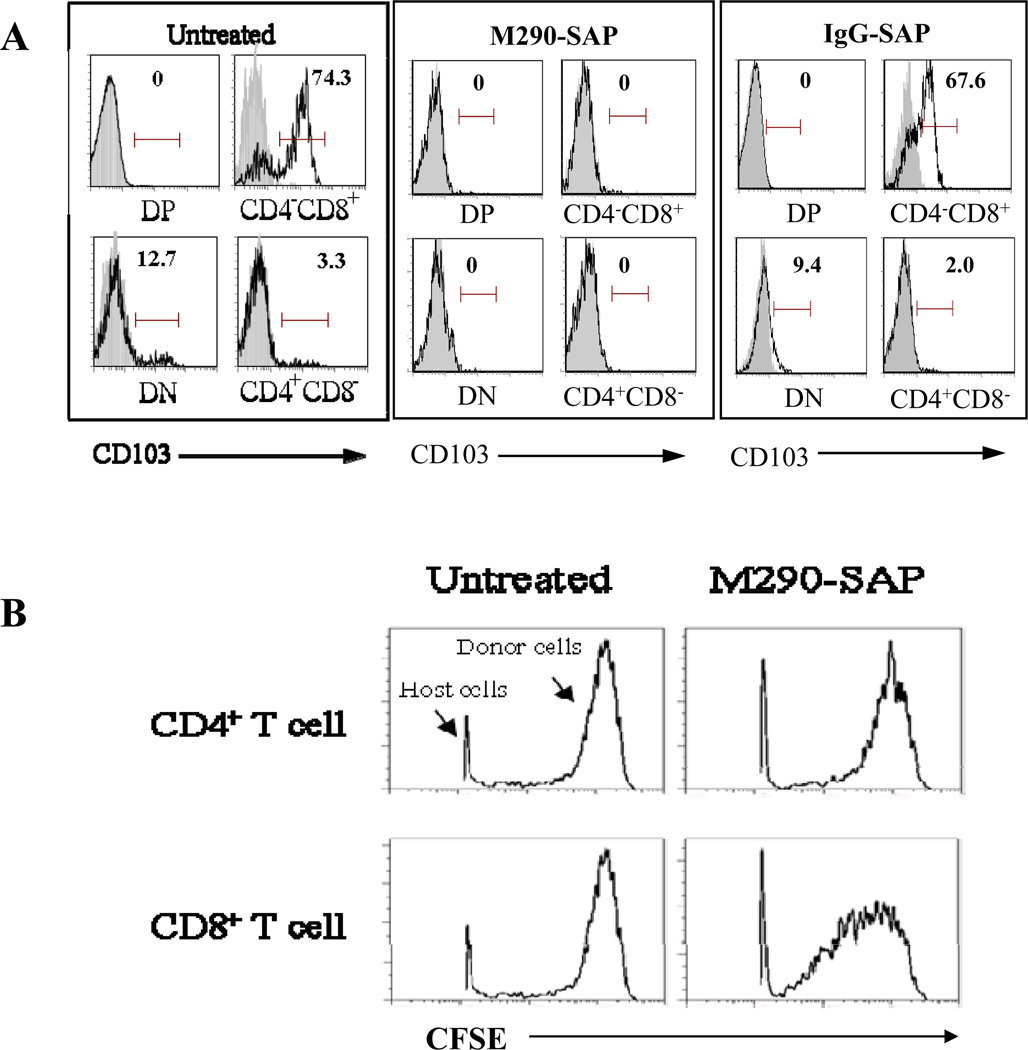
As shown in Fig. 1A, treatment of mice with a single i.p. injection of M290-SAP resulted in a dramatic decrease in the frequency of CD103+CD8+ cells in all compartments examined including peripheral blood, spleen, mesenteric lymph nodes (MLN) and intestinal epithelial lymphocytes (iIEL). Fig. 1B shows that this reflected an absolute reduction of total CD8 cells in the MLN, indicating that the loss of CD103 expression by CD8 cells reflects bona fide depletion of CD103+CD8+ cells by M290-SAP rather than masking or modulation of the CD103 determinant. In contrast, unconjugated M290 masked CD103 expression by peripheral CD8 T cells as evidenced by the loss of CD103 expression (Fig. 1A) in the absence of true depletion (Fig. 1B). Note that isotype control saporin conjugate (IgG-SAP) showed a partial depletion of both CD8+ and CD4+ cells (Fig 1B), consistent with the known non-specific effects of saporin conjugates [23]. Fig. 2 shows that, M290-SAP dramatically reduced the frequencies (Fig. 2A) and absolute numbers (Fig. 2B) of CD11c+ cells (dendritic cells) and CD4+CD25+ cells (T regs) in diverse compartments, consistent with the reported expression of CD103 by these subsets[17, 18].
Figure 1. M290-SAP efficiently depletes CD103+ cells in vivo.
Normal C57BL/6 mice were untreated or injected i.p. with the indicated reagents at 2mg/kg i.p. Six days later, cells from the indicated compartments were isolated and subjected to multi-color FACS analyses using mAbs to CD3, CD4, CD8, CD11c, and CD103. A. Percentages of residual CD103+CD8+ cells in different compartments. Numbers in each quadrant represent percentages of cells within the CD3+ T lymphocyte gate. Data are representative of at least three independent experiments. Bold numbers at middle right of each quadrant are the frequencies of CD103-expressing cells as a percentage of total CD8 T cells. B. Absolute numbers of CD4 and CD8 T cells in MLN from A. Data shown are mean ± SEM from at least four independent experiments. Statistically significant differences from the PBS-treated mice were measured by one-way ANOVA with posttesting by Dunnett’s Multiple Comparison Test. ***denotes p-values<0.001, * denotes p-values<0.05.
Figure 2. M290-SAP depletes CD103-expressing leukocytes.
A. CD103 expression (M290) by gated CD8+ T cells (left), CD11c+ dendritic cells (middle) and CD4+CD25+ natural regulatory T cells (right) in the indicated compartments. Gray peaks show staining with an isotype-matched negative control mAb. Data are representative of at least five independent experiments. B. Absolute numbers of CD11c+ dendritic cells (left) and CD4+CD25+ natural regulatory T cells (right) in MLN from A were enumerated using TruCOUNT tubes (BD bioscience). Data shown are values obtained for a single mouse either untreated or treated with M290-SAP or unconjuated M290 (unconj M290).
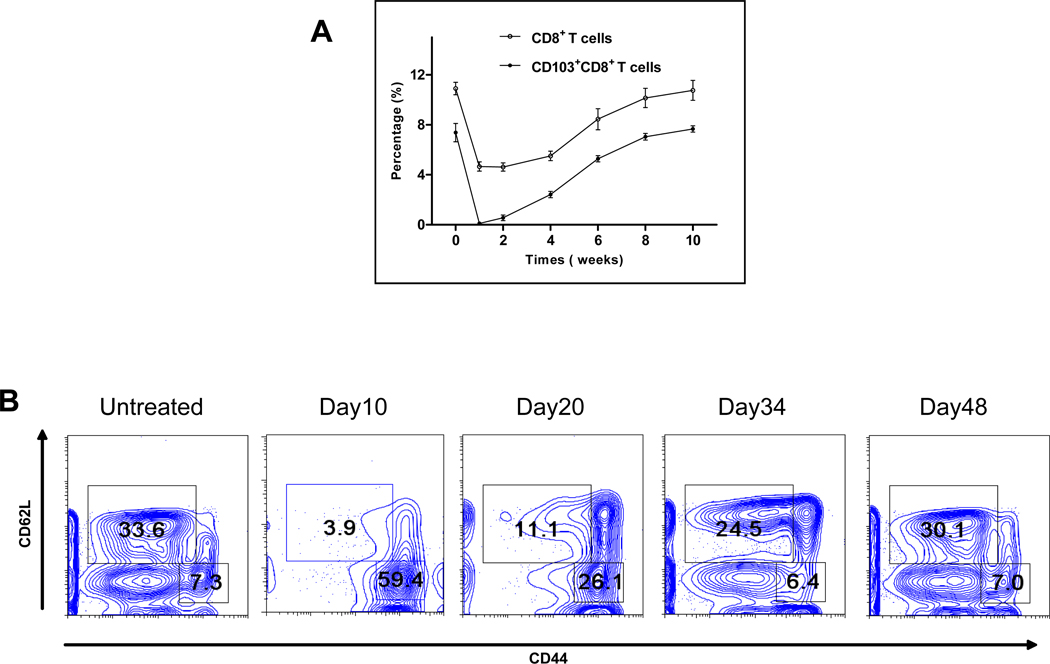
As shown in Fig. 3A, M290-SAP induced a complete loss of CD103+CD8+ cells in the peripheral blood within one week of treatment, with the percentage of CD103+CD8+ T cells gradually recovering to pretreatment levels by week 8. The loss of CD8 T cells in the peripheral blood (Fig. 3A) was substantially less than in the MLN (Fig. 1B) commensurate with the higher frequencies of CD103+CD8+ cells in the latter compartment (Fig. 1A). As shown in Fig. 3B, M290-SAP dramatically depleted peripheral CD8 T cells of CD44lo phenotype while sparing CD8 T cells of CD44hi phenotype with reconstitution substantially complete by day 48 post-treatment (Fig. 3B). In experiments not shown, M290-SAP treatment had little to no impact on peripheral CD4 T cells of either CD44hi or CD44lo phenotype.
Figure 3. Depletion and recovery of peripheral CD8 T cells.
8 to 10-week-old C57BL/6 mice were treated with M290-SAP at 2mg/kg i.p.. Peripheral blood was collected at the designated time points and subjected to three-color FACS analyses using mAbs to CD3, CD103, and CD8. A. Depletion and recovery of peripheral CD8 T cells. Data shown are mean percentages ± SEM of CD8+ (empty circle) and CD103+CD8+ (filled circle) T cells among total lymphocytes (n = 5). B. Depletion and recovery of naïve and memory CD8 T cells. Data shown are two-dimensional plots of CD62L vs. CD44 expression for gated CD8+ lymphocytes.
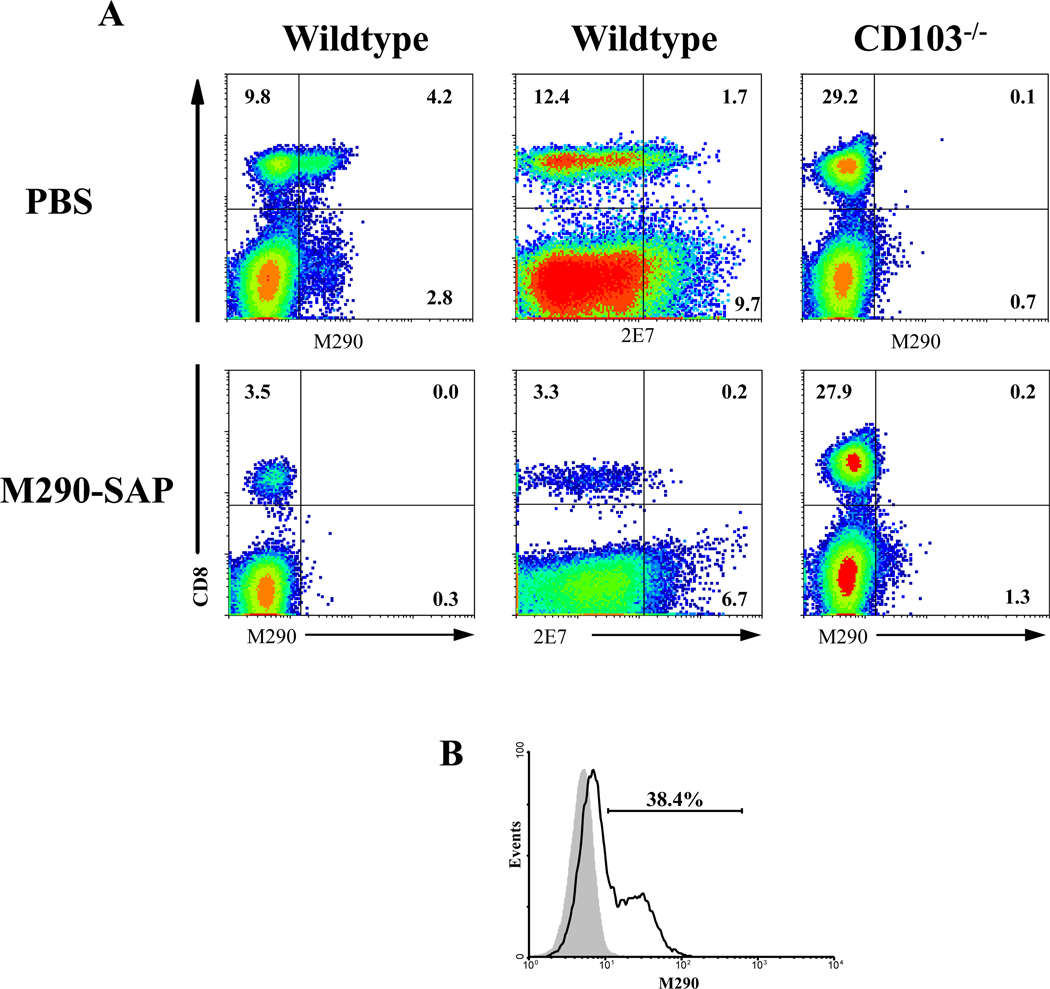
M290-SAP dramatically depleted CD8 T cells from wildtype mice but not CD103−/− mice (Fig. 4B for MLN; SC and IEL not shown), thus confirming the specificity of M290-SAP for CD103-expressing cells. Results obtained with a second anti-CD103 mAb, 2E7, directed to an independent epitope on the CD103 molecule[15], were identical, thus confirming that the loss of peripheral CD8 T cells following M290-SAP treatment represents bona fide depletion rather than masking of the CD103 determinant (Fig. 4A). Fig. 4B shows that the vast majority of CD8 T cells express CD103 at levels above that of the isotype control, thereby providing an explanation for the unexpectedly efficient depletion of CD8 T cells by M290-SAP.
Figure 4. Loss of CD103-expressing cells represents depletion rather than masking of the CD103 determinant.
A. 8 to 10-week-old C57BL/6 mice on the wildtype (left and middle columns) or CD103−/− (right) backgrounds [41] were treated with either PBS (upper row) or M290-SAP at 2mg/kg i.p (lower row).. Peripheral blood was collected at day 6 and subjected to three-color FACS analyses using the anti-CD103 mAbs M290 (left and right) or 2E7 (middle) in combination with mAb to CD8. Data shown are two-dimensional plots of staining of MLN lymphocytes from the indicated hosts with anti-CD103 mAbs M290 (left and right) or 2E7 (middle) in combination with mAb to CD8. B. Data shown are a histogram of CD103 expression by gated CD8+ lymphocytes in the MLN of wildtype C57BL/6 hosts as by determined by M290 (rIgG2a) staining). The gray peak shows staining with an isotype-matched negative control mAb. (rIgG2a).
M290-SAP disrupts peripheral CD8 T cell homeostasis
CD103 is known to be expressed by T cell subsets in the thymus [24], raising the possibility that these cells are susceptible to depletion by M290-SAP. Notably, the CD4−CD8+ T cell subset which represents the immediate precursors of mature naïve CD8 T cells, referred to as recent thymic emigrants [16], has been reported to uniformly express high levels of CD103. As shown in Fig. 5A (left panel), CD103 is preferentially expressed by CD4−CD8+ single positive (SP) thymocytes with the CD4−CD8−, CD4+CD8−, and CD4+CD8+ subsets expressing little to no CD103. As shown in Fig. 5A (middle panel), M290-SAP treatment of naïve mice led to a loss of CD103 expression by the CD4−CD8+ thymocyte subset whereas IgG-SAP had no detectable impact on such expression (Fig. 5A, right panel). These data are consistent with a selective depletion of CD8 T cell precursors in the thymus by M290-SAP, though masking was not excluded in this case.
Figure 5. Disruption of thymopoeisis and CD8 T cell homeostasis by M290-SAP.
A. Normal C57BL/6 mice were treated with M290-SAP or IgG-SAP at 2mg/kg i.p. Six days later, thymocytes were collected and subjected to FACS analyses. Data shown are CD103 expression by the indicated thymic subsets. Gray peaks show staining with an isotypematched negative control mAb. Numbers shown on histograms represent percent positive cells within the indicated region. Data are representative of at least three independent experiments. B. Normal C57BL/6 mice were untreated or treated with M290-SAP at 2 mg/kg i.p. Six days later, 20 million CFSE-labeled syngeneic T cells were adoptively transferred into treated mice or untreated controls by tail vein injection. Splenocytes were obtained and analyzed for CFSE expression by the transferred subsets at 14 days following transfer. Data shown are histograms of CFSE expression by gated CD4+ or CD8+ lymphocytes. Note that the majority of host cells were gated out of the analysis. The fraction of total CFSE labeled cells was 0.3% for gated CD8+ lymphocytes and 1.8% for gated CD4+ lymphocytes at day 14 post-transfer.
The loss and subsequent recovery of naïve CD8 T cells (Fig. 3) despite apparent disruption of thymopoeisis by M290-SAP (Fig. 5A), raised the possibility that M290-SAP may promote homeostatic expansion of peripheral CD8 T cells. To test this hypothesis, we examined the capacity of M290-SAP to induce homeostatic expansion of peripheral CD8 T cells. As shown in Fig. 5B, normal CD8 T cells transferred into syngeneic M290-SAP treated recipients underwent strong proliferation in a syngeneic host, consistent with homeostatic expansion. In contrast, CD4 T cells transferred into the same environment failed to proliferate (Fig. 5B), indicating that the effect is highly specific and thus unlikely due to the nonspecific toxicity of saporin conjugates. No proliferation was observed when cells were transferred into control non-depleted syngeneic recipients (Fig. 5B), indicating that M290-SAP mediated T cell depletion is required for the proliferative effect. These data suggest that M290-SAP selectively induces homeostatic expansion in the host CD8 T cell compartment.
M290-SAP promotes long term survival of pancreatic islet allografts
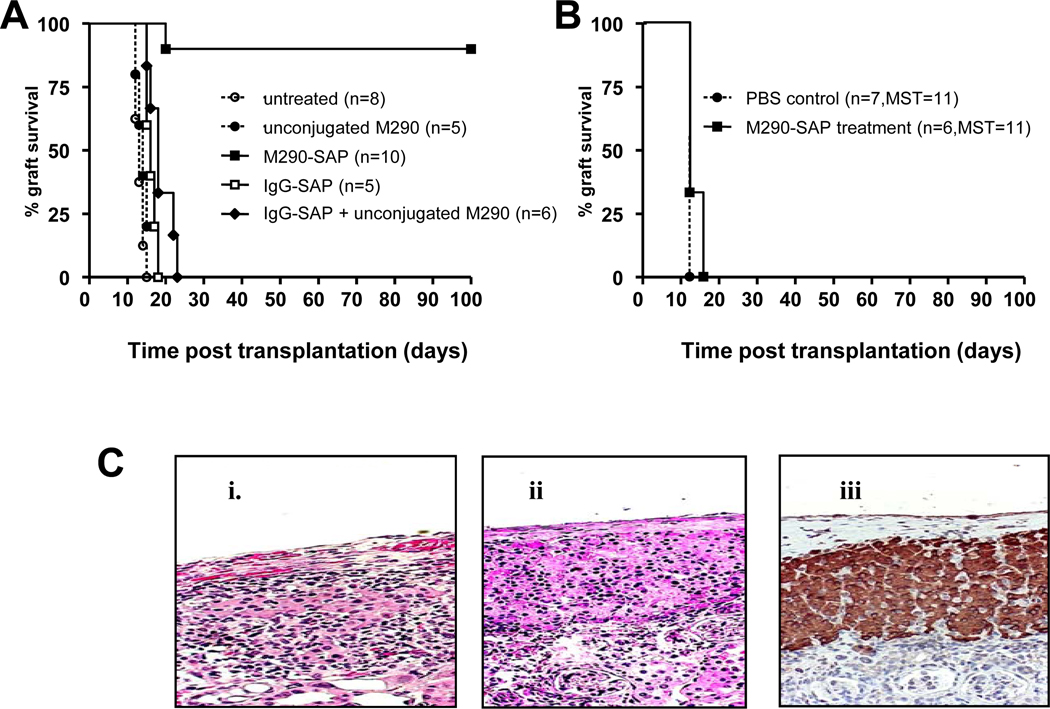
Based on the observation that CD103 knockout mice are defective in the capacity to reject islet allografts [12], we postulated that CD103 depletion would promote islet allograft survival. As shown in Fig. 6A, a single i.p. injection of M290-SAP at 2 mg/kg promoted long-term survival of islet allografts (>100 days) in 90% of recipients (9/10). In contrast, allografts transplanted into untreated recipients were promptly rejected (mean ± SEM = 13±2 days)(Fig. 6A). Note that neither unconjugated M290 antibody nor IgG-SAP had a significant impact on graft survival, indicating that depletion of CD103-expressing cells by M290-SAP rather than possible non-specific effects on the host are responsible for the prolonged allograft survival. The combination of IgG-SAP plus unconjugated M290 failed to prolong allograft survival, indicating that prolongation of graft survival requires the intact M290-SAP conjugate (Fig 6A). Long-term functioning grafts (>100 days) in M290-SAP treated mice showed little, if any, leukocyte infiltration and islets remained pristine as evidenced by normal architecture and strong insulin staining (Fig. 6C).
Figure 6. Graft prolonging effects of M290-SAP.
8 to 10-week old C57BL/6 recipients were transplanted with islet (A, C) or skin allografts (B) then treated with a single dose of M290-SAP at 2 mg/kg i.p. or the indicated controls on day 3 post-transplantation. A. Survival of islet allografts treated with M290-SAP or the indicated controls. B. Survival of skin allografts in recipients treated as indicated above. C. Graft histology. Islet graft-bearing kidneys were removed by survival nephrectomy, fixed, and stained by H&E or immunohistochemistry. i. Islet allografts transplanted into untreated recipients harvested at the time of rejection (blood glucose >200 mg/dL). ii. Long surviving (>100 d) islet allografts removed from M290-SAP treated recipients. iii. Insulin immunostaining of long surviving islet allografts from long recipients (>100 d) treated with M290-SAP.
Fig. 6B shows that M290-SAP does not prolong survival of skin allografts, suggesting that its graft prolonging effects are tissue-specific. Similarly, M290-treated recipients bearing long-term islet allografts rejected both donor (BALB/c) and third party (FVB/N) skin allografts with normal kinetics (data not shown), indicating that the hosts remain fully immunocompetent despite the selective inability to reject islet allografts.
Characteristics of M290-SAP treated hosts bearing long term allografts
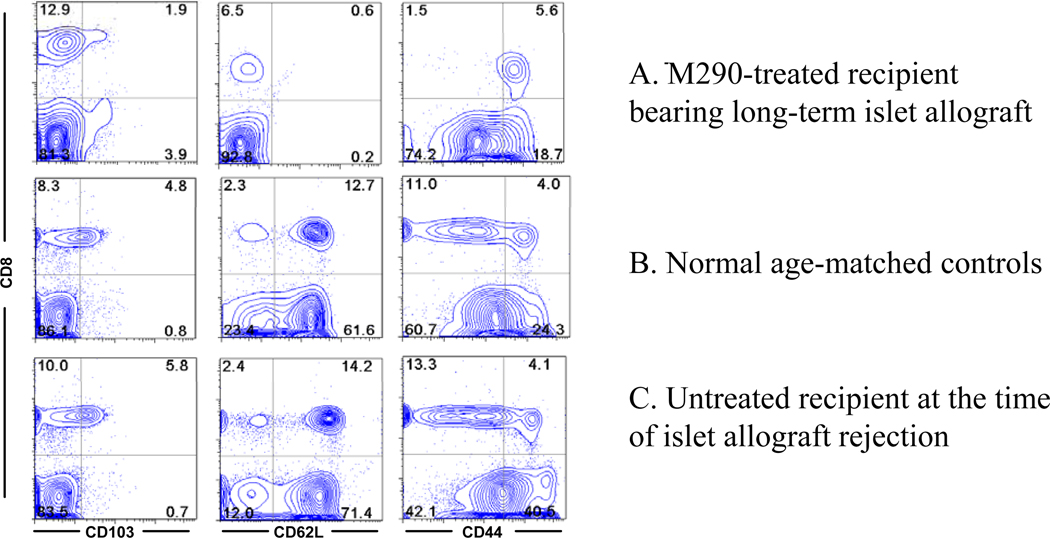
Surprisingly, peripheral CD8 T cells in M290-SAP treated mice exhibited a uniform CD44hi CD62Llo phenotype (Fig. 7A). In contrast, peripheral CD8 T cells in normal age matched controls (Fig. 7B) or untreated hosts transplanted with islet allografts (Fig. 7C) were predominantly CD62Lhi with heterogeneous CD44 expression. Thus, these data unexpectedly revealed that most if not all CD8 T cells in M290-SAP treated mice are of effector-memory phenotype. As discussed below, we postulate that this unusual phenotype results from the unique capacity of M290-SAP to induce homeostatic expansion of CD8 T cells in the absence of new thymic output.
Figure 7. Characteristics of M290-SAP treated hosts.
Blood collected from M290-SAP treated recipient bearing a long-term islet allograft (A), a normal age matched control (B), and islet allograft recipient undergoing unmodified rejection (C), was subjected to multicolor FACS analyses using mAbs to CD8, CD103, CD44, and CD62L. . Data shown are two-dimensional plots of the indicated markers by gated lymphocytes. Numbers in quadrants are percentages of total lymphocytes. Data are representative of at least three independent experiments.
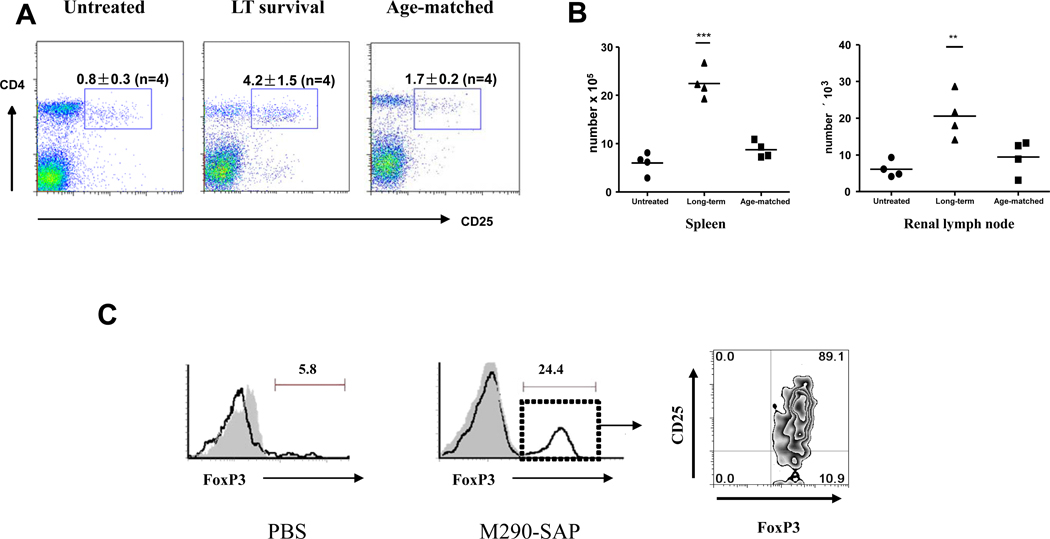
As mentioned above, M290-SAP treatment completely eliminated the CD103-expressing subset of T regs (Fig. 2) but did not lead to accelerated rejection of islet allografts (Fig. 6A), indicating that CD103-expressing T regs are not significantly involved in regulating islet allograft rejection. However, as shown in Fig. 8, spleens from M290-SAP recipients bearing long-term islet allografts contained significantly higher percentages (Fig. 8A) and absolute numbers (Fig. 8B) of CD4+CD25+ cells as compared to those in untreated allograft recipients or age matched normal control mice. As shown in Fig. 8C, these cells co-expressed FoxP3, and were present in long-term surviving grafts at levels increased compared to untreated grafts undergoing rejection, though it remains to be determined if these cells are causally related to prolonged graft survival in M290-SAP treated hosts.
Figure 8. Increased numbers of CD4+CD25+ T regulatory cells in M290-SAP treated recipients bearing long-term islet allografts.
A. Phenotypic analysis of splenocytes from mock injected recipients undergoing unmodified rejection at POD 15 (left), M290-SAP treated recipients bearing long-term islet allografts harvested at POD 100 (middle), or age matched (age 150 days) normal C57BL/6 controls, were subjected to multi-color FACS analyses using mAbs to CD4, CD25. Data shown are two-dimensional plots of the indicated markers by gated lymphocytes. Numbers in quadrants are percentages of total lymphocytes. B. Absolute numbers of CD4+CD25+ cells in the spleen (left) and renal lymph node (right) for the three groups as described in A The horizontal line denotes the mean of the points within each group. Statistically significant differences were measured by one-way ANOVA with posttesting by Dunnett’s Multiple Comparison Test. ***denotes p<0.001, ** denotes p<0.01. C. Phenotypic analysis of lymphocytes infiltrating islet allografts in mock injected controls at POD 15 (left panel) or an M290-SAP treated recipient bearing long-term islet allograft harvested at POD 100 (right panels). Data shown in far right panel is a two-dimensional plot of CD25 vs. FoxP3 expression by gated CD4+ cells in the long-term surviving islet allograft.
DISCUSSION
The salient findings of the present study are that the anti-CD103 immunotoxin, M290-SAP, efficiently depletes CD103-expressing cells in vivo and promotes long-term survival of MHC mismatched pancreatic islet allografts. Previous studies using knockout mice have documented a key role for the integrin CD103 in promoting islet allograft rejection [12]. However, a direct evaluation of the capacity of CD103-expressing cells in the rejection process has proven problematic due to the lack of reagents that significantly deplete CD103+ cells in vivo. The existing mAbs to mouse CD103 (M290 or 2E7) neither deplete CD103-expressing cells in vivo nor prolong islet allograft survival even in a strain combinations in which islet allograft rejection is known to be CD103-dependent [12]. To circumvent this problem, M290-SAP was produced by conjugating the non-depleting CD103 mAb, M290 (rat IgG2a) to saporin, a potent inhibitor of protein synthesis [24]. The strongly increased cytotoxicity of M290-SAP as compared to unconjugated M290 likely reflects mechanisms similar to those previously established for other antibody-saporin conjugates [25]. That is, following binding of the intact immunotoxin to the cell surface of CD103-expressing cells, saporin likely translocates into the cytoplasm where it inhibits cellular protein synthesis and thereby induces apoptosis of the target cell population [25], though this remains a matter of speculation.
Our data indicate that M290-SAP depletes diverse CD103-expressing leukocytes in vivo including peripheral CD8 T cells (Figs. 1–4), CD4+CD25+ T regs (Fig. 2), and CD11c+ dendritic cells (Fig. 2). In contrast, unconjugated M290 and IgG-SAP at the same at the same dose both were ineffectual in eliminating such cells (Fig. 1). We further confirm that loss of CD103-expressing cells following M290-SAP treatment represents true depletion rather than masking or modulation of CD103 determinant in the case of peripheral CD8 T cells, CD11c+ dendritic cells, and CD4+CD25+ T regs, and that depletion of CD8 T cells does not occur in hosts genetically deficient in CD103 expression (Fig. 4A). Whether the loss of CD103-expressing thymocytes (Fig. 5A) represents true depletion remains to be established. M290-SAP preferentially depleted CD8+CD44lo cells (Fig. 3B) as compared to CD4 T cells of either CD44hi or CD44lo phenotype (data not shown) consistent with specific depletion, though we can not exclude the possibility that the latter cells are uniquely resistant to depletion by M290-SAP. Importantly, we demonstrate that the vast majority of CD8 T cells in WT hosts express significant levels of CD103 (Fig. 4B), thereby providing an explanation for the unexpectedly efficient depletion of CD8 T cells by M290-SAP. Similar arguments potentially explain the profound depletion of CD11c+ and CD4+CD25+ cells as shown in Fig. 2B, though given the ambiguity regarding which cells truly express CD103, it is virtually impossible to completely exclude the possibility that depletion of cell populations following M290-SAP treatment extends to CD103− cells.
A key finding of the present study is that a single injection of M290-SAP dramatically prolongs islet allograft survival. However, the underlying mechanisms of action are unclear. We found that peripheral CD8 T cells in M290-SAP treated recipients bearing long-term islet allografts exhibit a unique memory phenotype (Fig. 7), a characteristic of both bona fide memory T cells and T cells undergoing homeostatic expansion [26]. This finding differs from that of Pearl et al. [27] who reported that effector-memory CD4 T cells are the dominant cell type in clinical renal transplant recipients following inductive T cell depletional therapy, suggesting that M290-SAP has a unique mechanism of action. Given that M290-SAP likely disrupts thymopoeisis (Fig. 5A), we postulate that - in the absence of new thymic output - the presence of an allograft promotes differentiation of the remaining naïve CD8 T cells to memory cells thereby accounting for accumulation of memory CD8 T cells in M290-SAP treated mice bearing long-term allografts. These data raise the possibility that the graft prolonging effects of M290-SAP reflect its capacity to disrupt peripheral CD8 T cell homeostasis, though it is possible that effector-memory T cells are uniquely resistant to M290-SAP.
The present study reveals that M290-SAP has substantial non-specific effects on the host immune system, likely due to the well-documented toxicity of saporin conjugates [23]. For example, M290-SAP and isotype control saporin conjugate (IgG-SAP) both significantly reduce levels of peripheral T cells (Fig. 1B). In experiments not shown, M290-SAP was uniformly lethal to normal BALB/c mice at doses of 1 mg/kg or greater, and was lethal to C57BL/6 mice in context of organ transplantation or graft-vs-host disease. Histological analyses (data not shown) suggest that such toxicity reflects the well documented hepatotoxicity of saporin conjugates [23], though the precise mechanisms of lethality remain unclear. Although our data indicate that IgG-SAP by itself or in combination with unconjugated M290 has no effect on islet allograft rejection, we can not exclude the possibility that the graft prolonging effects of M290-SAP partially reflect its non-specific effects. While such non-specific toxicity is problematic from the perspective of clinical application, it remains possible that some of these effects may be beneficial when they occur in the context of specific immunosuppression.
CD103 is known to be expressed by dendritic cells (DC), a highly potent APC population critical to effective transplant responses[28–31], and we herein demonstrate that M290-SAP substantially depletes CD103+CD11c+ cells (Fig. 2). However, several lines of evidence argue against the possibility that the graft prolonging effects of M290-SAP reflect its capacity to deplete DC. For one, the present data indicate that CD103 is expressed by only a small (<10%) proportion of CD11c+ cells in the spleen or renal lymph node (RLN, Fig. 2A). The latter data are particularly insightful given that the RLN is the draining lymphoid compartment for islet allografts transplanted under the renal subcapsule, thereby arguing against a significant overall depletion of host DC in this compartment by M290-SAP. Moreover, FACS analyses revealed that dendritic cells of donor origin present in islet allografts express negligible levels of CD103, and, moreover, that pretreatment of donors with M290-SAP to remove CD103-expressing dendritic cells from the graft does not prolong allograft survival (GAH, unpublished data). We further demonstrate that M290-SAP treated recipients bearing long term islet allografts retain the capacity to reject skin allografts, thus documenting that M290-SAP treated recipients are capable of mounting normal immune responses and thus are not globally immunosuppressed. Given that skin allograft rejection depends on host DC [32], these data further argue against a significant depletion of DC by M290-SAP.
There is now broad acceptance that T regs play essential roles in maintaining allograft tolerance [33], and CD103 has been reported to be a marker of this cell population [34], raising the possibility that CD103 blockade might somehow interfere with s key mechanism of graft tolerance and thereby accelerate allograft rejection. However, the present data are clearly incompatible with this concept. First of all, CD103 expression by T regs ranges from less than 10% in the spleen to ~35% in the MLN (Fig 2A); indicating that the vast majority of T regs fail to express CD103 so would not be susceptible to depletion by M290-SAP. Our data clearly document that depletion of the CD103-expressing T reg subset with M290-SAP does not accelerate rejection but rather is associated with indefinite allograft survival. On the other hand, M290-SAP treated recipients bearing long-term surviving islet allografts contained increased levels of T reg phenotype cells in the graft and peripheral lymphoid compartments. However, it is not clear whether these cells are a cause, or conversely an effect, of long-term allograft survival. The present data do not exclude the possibility that M290-SAP may deplete or otherwise prevent effector responses to islet allografts thereby obscuring the impact of CD103-expressing T regs, though this remains a matter of speculation.
Our data indicate that the graft prolonging effects of M290-SAP are tissue specific. M290-SAP dramatically prolongs islet allograft survival but has no significant impact on skin allograft rejection in the same strain combination (BALB/c-to-B6). It is possible that the apparent tissue specificity of M290-SAP reflects the greater immunogenicity of skin allografts, and our data do not exclude this possibility. However, an equally plausible explanation is that the tissue-specificity of M290-SAP reflects the vulnerability of skin allografts to CD4-mediated rejection [35, 36] whereas islets are dependent on CD8-mediated rejection [37–40]. These data raise the possibility that M290-SAP induces donor specific tolerance in the CD8 T cell compartment while leaving the CD4 T cell compartment intact and thus capable of responding to donor alloantigens. Experiments to test this key hypothesis are currently underway.
The holy grail of transplant immunology is the development of strategies that facilitate long-term acceptance of clinical transplants. Regardless, the present findings provide key insight into the therapeutic potential of reagents that efficiently deplete CD103-expressing cells, and thereby establish baseline information on which to design subsequent studies to achieve this important clinical objective. We are currently exploring the use of less toxic means to deplete CD103-expressing cells in vivo and prolong allograft survival. The data described in this communication provide a requisite foundation for the pursuit of these studies.
In summary, the present data indicate that depletion of CD103-expressing cells provides a novel method to prevent destruction of transplanted tissues. Regardless of the underlying mechanisms, these data prove the requisite background information to design more refined and less toxic strategies to achieve this important clinical objective. The present data argue that depletion of CD103 expressing cells represents a viable strategy for therapeutic intervention in allograft rejection. It will now be important to elucidate the underlying mechanisms of action and develop more clinically applicable strategies for depletion of CD103-expressing cells.
Acknowledgments
Funding Sources: These studies were supported by grants to G.A.H from the National Institutes of Health (AI036532) and the Roche Organ Transplantation Research Foundation (501313640).
REFERENCES
- 1.Gjertson DW. Determinants of long-term survival of adult kidney transplants: a 1999 UNOS update. Clin Transpl. 1999:341–352. [PubMed] [Google Scholar]
- 2.Cerf-Bensussan N, et al. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 3.Karecla PI, et al. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin alpha M290 beta 7 (alpha E beta 7) Eur J Immunol. 1995;25(3):852–856. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- 4.Cepek KL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372(6502):190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 5.Hadley GA, et al. The epithelial cell-specific integrin, CD103 (alpha E integrin), defines a novel subset of alloreactive CD8+ CTL. J Immunol. 1997;159(8):3748–3756. [PubMed] [Google Scholar]
- 6.Robertson H, et al. Tubulitis after renal transplantation: demonstration of an association between CD103+ T cells, transforming growth factor beta1 expression and rejection grade. Transplantation. 2001;71(2):306–313. doi: 10.1097/00007890-200101270-00024. [DOI] [PubMed] [Google Scholar]
- 7.Dogan A, Wang ZD, Spencer J. E-cadherin expression in intestinal epithelium. J Clin Pathol. 1995;48(2):143–146. doi: 10.1136/jcp.48.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirulli V, et al. Expression of neural cell adhesion molecule (N-CAM) in rat islets and its role in islet cell type segregation. J Cell Sci. 1994;107(Pt 6):1429–1436. doi: 10.1242/jcs.107.6.1429. [DOI] [PubMed] [Google Scholar]
- 9.Nouwen EJ, et al. Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int. 1993;44(1):147–158. doi: 10.1038/ki.1993.225. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri A, Watanabe R, Tomita Y. E-cadherin expression in renal cell cancer and its significance in metastasis and survival. Br J Cancer. 1995;71(2):376–379. doi: 10.1038/bjc.1995.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terada T, et al. Expression of epithelial-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development: a possible role in bile duct morphogenesis. J Hepatol. 1998;28(2):263–269. doi: 10.1016/0168-8278(88)80013-8. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, et al. CD103 expression is required for destruction of pancreatic islet allografts by CD8(+) T cells. J Exp Med. 2002;196(7):877–886. doi: 10.1084/jem.20020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan R, et al. Critical role for CD103+CD8+ effectors in promoting tubular injury following allogeneic renal transplantation. J Immunol. 2005;175(5):2868–2879. doi: 10.4049/jimmunol.175.5.2868. [DOI] [PubMed] [Google Scholar]
- 14.El-Asady R, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201(10):1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefrancois L, et al. Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur J Immunol. 1994;24(3):635–640. doi: 10.1002/eji.1830240322. [DOI] [PubMed] [Google Scholar]
- 16.Staton TL, et al. Murine CD8+ recent thymic emigrants are alphaE integrin-positive and CC chemokine ligand 25 responsive. J Immunol. 2004;172(12):7282–7288. doi: 10.4049/jimmunol.172.12.7282. [DOI] [PubMed] [Google Scholar]
- 17.Kilshaw PJ. Expression of the mucosal T cell integrin alpha M290 beta 7 by a major subpopulation of dendritic cells in mice. Eur J Immunol. 1993;23(12):3365–3368. doi: 10.1002/eji.1830231246. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann J, et al. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Natl Acad Sci U S A. 2002;99(20):13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith TJ, et al. Murine M290 integrin expression modulated by mast cell activation. Immunity. 1994;1(5):393–403. doi: 10.1016/1074-7613(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 20.Gotoh M, et al. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40(4):437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Guy-Grand D, Griscelli C, Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gudmundsdottir H, Turka LA. A closer look at homeostatic proliferation of CD4+ T cells: costimulatory requirements and role in memory formation. J Immunol. 2001;167(7):3699–3707. doi: 10.4049/jimmunol.167.7.3699. [DOI] [PubMed] [Google Scholar]
- 23.Stirpe F, et al. Hepatotoxicity of immunotoxins made with saporin, a ribosome-inactivating protein from Saponaria officinalis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53(5):259–271. doi: 10.1007/BF02890252. [DOI] [PubMed] [Google Scholar]
- 24.Stirpe F, et al. Ribosome-inactivating proteins from plants: present status and future prospects. Biotechnology (N Y) 1992;10(4):405–412. doi: 10.1038/nbt0492-405. [DOI] [PubMed] [Google Scholar]
- 25.Bagga S, Seth D, Batra JK. The cytotoxic activity of ribosome-inactivating protein saporin-6 is attributed to its rRNA N-glycosidase and internucleosomal DNA fragmentation activities. J Biol Chem. 2003;278(7):4813–4820. doi: 10.1074/jbc.M207389200. [DOI] [PubMed] [Google Scholar]
- 26.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174(11):6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 27.Pearl JP, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 28.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 29.Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation--friend or foe? Immunity. 2001;14(4):357–368. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- 30.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 31.van den Broek M. Dendritic cells break bonds to tolerize. Immunity. 2007;27(4):544–546. doi: 10.1016/j.immuni.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Galkowska H, et al. Skin allografts--lymph veiled (dendritic) cells are responsible for initiation of rejection in canine skin/SCID mouse chimera model. Ann Transplant. 1999;4(3–4):5–10. [PubMed] [Google Scholar]
- 33.Waldmann H, Cobbold S. Regulating the immune response to transplants. a role for CD4+ regulatory cells? Immunity. 2001;14(4):399–406. doi: 10.1016/s1074-7613(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 34.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 35.Hall BM. Cells mediating allograft rejection. Transplantation. 1991;51(6):1141–1151. doi: 10.1097/00007890-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Steinmuller D. Which T cells mediate allograft rejection? Transplantation. 1985;40(3):229–233. doi: 10.1097/00007890-198509000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Desai NM, et al. Islet allograft, islet xenograft, and skin allograft survival in CD8+ T lymphocyte-deficient mice. Transplantation. 1993;55(4):718–722. doi: 10.1097/00007890-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Desai NM, et al. Pancreatic islet allograft and xenograft survival in CD8+ T-lymphocyte-deficient recipients. Transplant Proc. 1993;25(1 Pt 2):961–962. [PubMed] [Google Scholar]
- 39.Osorio RW, et al. Major histocompatibility complex class I deficiency prolongs islet allograft survival. Diabetes. 1993;42(10):1520–1527. doi: 10.2337/diab.42.10.1520. [DOI] [PubMed] [Google Scholar]
- 40.Osorio RW, Ascher NL, Stock PG. Prolongation of in vivo mouse islet allograft survival by modulation of MHC class I antigen. Transplantation. 1994;57(6):783–788. doi: 10.1097/00007890-199403270-00001. [DOI] [PubMed] [Google Scholar]
- 41.Schon MP, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162(11):6641–6649. [PubMed] [Google Scholar]