Abstract
Background
Fluorodeoxyglucose positron emission tomography (FDG PET) imaging of atherosclerosis has been used to quantify plaque inflammation and to measure the effect of plaque stabilizing drugs. Here we explore how atherosclerotic plaque inflammation varies across arterial territories and how it relates to arterial calcification. We also test the hypotheses that the degree of local arterial inflammation measured by PET is correlated with the extent of systemic inflammation and presence of risk factors for vascular disease.
Methods and Results
Forty-one subjects underwent vascular PET/CT imaging with FDG. All had either vascular disease or multiple risk factors for it. Forty subjects underwent carotid imaging, twenty-seven underwent aortic, twenty-four iliac and thirteen femoral imaging. Thirty-three subjects had a panel of biomarkers analyzed.
We found strong associations between FDG uptake in neighboring arteries (left vs. right carotid r=0.91, p<0.001, ascending aorta vs. aortic arch r=0.88, p<0.001). Calcification and inflammation rarely overlapped within arteries – carotid artery FDG uptake vs. calcium score r=−0.42, p=0.03). Carotid artery FDG uptake was greater in those with a history of coronary artery disease (target to background ratio (TBR) 1.83 vs. 1.61, p<0.01), and in males vs. females (TBR 1.83 vs. 1.63, p<0.05). Similar findings were also noted in the aorta and iliac arteries. Subjects with the highest levels of FDG uptake also had the greatest concentrations of inflammatory biomarkers: descending aorta TBR vs. matrix metalloproteinase 3 (MMP 3): r=0.53, p=0.01 and carotid TBR vs. MMP 9: r=0.50, p=0.01. Non-significant positive trends were seen between FDG uptake and levels of interleukin 18, fibrinogen and C-reactive protein. Finally, we found that the atheroprotective biomarker adiponectin was negatively correlated with the degree of arterial inflammation in the descending aorta: r=−0.49, p=0.03).
Conclusions
This study shows that FDG PET imaging can increase our knowledge of how atherosclerotic plaque inflammation relates to calcification, serum biomarkers and vascular risk factors. Plaque inflammation and calcification rarely overlap, supporting the theory that calcification represents a late, burnt-out stage of atherosclerosis. Inflammation in one arterial territory is associated with inflammation elsewhere, and the degree of local arterial inflammation is reflected in the blood levels of several circulating biomarkers. We suggest that FDG PET imaging could be used as a surrogate marker of both atherosclerotic disease activity and drug effectiveness. Prospective, event driven studies are now underway to determine the role of this technique in clinical risk prediction.
Keywords: Atherosclerosis, Imaging, Inflammation, Positron Emission Tomography, Fluorodeoxyglucose, Calcification
Introduction
The complications of atherosclerosis are the commonest cause of death worldwide and an increasing burden to healthcare systems. Over the last two decades, however, several effective drugs have improved risk factor control and reduced mortality1. In parallel, non-invasive techniques for imaging atherosclerosis have become more widely available. They can help to illuminate the underlying pathology of the disease and may improve the prediction of future clinical events. Additionally, imaging can identify treatment-related changes in plaque structure and function that can serve as surrogate markers of drug efficacy2.
Clinical events related to atherosclerosis are driven by inflammation within the plaque3. FDG PET imaging has emerged as a non-invasive modality that can quantify this inflammation. The degree of FDG uptake within an atherosclerotic plaque is strongly correlated with its macrophage content4, 5, a key contributor to plaque inflammation. FDG PET can identify symptomatic lesions in the carotid6 and vertebral artery7 territories and can track the effect of anti-inflammatory plaque therapies in both human8,9 and animal10 models of disease.
This study employs FDG PET imaging of atherosclerotic arteries to address several questions relating to the pathology of the disease. We investigated the extent to which the degree of arterial inflammation within the carotid arteries, aorta and peripheral vasculature were correlated. Second, we examined the relationship between arterial inflammation and calcification. Third, we explored whether the presence of cardiovascular risk factors increased arterial inflammation, and finally we assessed the relationship between the level of several circulating inflammatory biomarkers and the degree of local arterial FDG uptake. We used a prospective study design and a vascular-specific PET imaging protocol that is highly reproducible11, 12.
Methods
Patient recruitment
We prospectively recruited, at the Mount Sinai Medical Center, a heterogeneous group of 41 patients with either vascular disease (defined as previous myocardial infarction, stroke or peripheral vascular disease) or at least three cardiovascular risk factors. Patients were identified in the catheter lab and in vascular outpatient clinics. All gave written informed consent and the study protocols were approved by the local institutional review board.
Baseline risk factor data were documented including: age, gender, ethnicity, history of coronary artery disease (defined as angina, previous myocardial infarction or coronary artery disease at angiography), history of cerebrovascular disease (defined as transient ischemic attack (TIA) or stroke), history of cigarette smoking, diagnosed or treated hypertension, ethnicity, history of diabetes, family history of heart disease, body mass index (BMI) and medication use.
PET/CT imaging
Of the 41 patients, 40 underwent carotid imaging, 27 aortic imaging, 24 iliac imaging and 13 femoral imaging. All studies were performed after at least 6 hours of fasting with a GE Lightspeed PET/CT scanner (Milwaukee, Wisconsin) after injection of 370 MBq FDG. Subjects with pre-scan blood glucose of >200mg/dl were excluded. Body scanning (encompassing the aorta±iliac±femoral arteries) was performed first, 90 minutes after FDG injection in 2D mode, with 10 minute acquisitions at each bed position.
Carotid artery imaging was undertaken approximately two hours after FDG injection, immediately after body imaging. The head and neck were placed into a soft head holder and a single bed position PET scan was acquired in 3D mode for 15 minutes. The external auditory meatus was the upper limit of the scan. CT was used for attenuation correction, anatomical co-registration and quantification of arterial calcification. No CT contrast agent was administered.
Image reconstruction
The 2D PET data were reconstructed using the ordered subset expectation maximization algorithm13 with a final voxel size of 4.25mm. The 3D PET data had the same corrections applied, and were reconstructed using a 3D reprojection algorithm14 yielding the same voxel size.
Image analysis
PET/CT image analysis was performed on a dedicated workstation (Xeleris 2.0, GE Medical Systems, Milwaukee, Wisconsin). Using the CT images, the aorta was divided into ascending, arch, descending thoracic and abdominal aorta. Similarly, below the aortic bifurcation, the arteries of the pelvis and thigh were separated into iliac and femoral arteries. Data from the common and external iliac arteries were labelled together as ‘iliac artery’; similarly, the common femoral and superficial femoral arteries were combined under the label of ‘femoral artery’. The transition point between iliac and femoral arteries was the inguinal ligament. Carotid artery PET studies were quantified after identifying the artery on the CT images.
Arterial FDG uptake (as a measure of arterial inflammation) was measured by drawing regions of interest (ROI’s) around the artery on each slice of the transaxial co-registered PET/CT images. The maximum standard uptake value (SUV) of FDG within the ROI was calculated from the maximum pixel activity. The SUV is the decay-corrected tissue concentration of FDG, corrected for injected FDG dose and body weight15. It is a widely accepted method for quantification of FDG PET data16.
SUV values for all artery slices in each territory were averaged over the whole artery. Finally, the SUV’s were normalized to blood FDG activity by dividing by a blood ROI (at least 8 venous ROI measurements) from the inferior vena cava (body studies) or the jugular vein (carotid studies). This resulted in a blood-normalized, whole artery SUV, known as the arterial target-to-background ratio (TBR)4. In this paper, the TBR measure is used throughout as a surrogate measure of arterial inflammation.
Calcification quantification
Calcium scores were calculated for the ascending aorta, aortic arch, descending aorta and both carotid arteries. Arterial calcification was measured from the CT images using the GE AW workstation (Milwaukee, Wisconsin) using Smart Score software. The Agatston method was used with a threshold for calcium ≥ 130 Hounsfield units17.
Biomarker analysis
Thirty-three patients had blood drawn for biomarker analysis. The remaining eight patients had already undergone imaging before the facility to analyze the samples became available. Biomarkers were chosen to cover different aspects of the atherothrombotic process (inflammatory, thrombotic and atheroprotective). The following biomarkers were measured: matrix metalloproteinases (MMP) 1, 3 and 9, interleukins (IL) 6, 10 and 18, tumour necrosis factor α, adiponectin, plasminogen activator inhibitor – 1 (PAI-1), C-reactive protein (CRP) and a full lipid panel.
Venous blood samples were collected on the day of imaging, prior to FDG injection. The samples were centrifuged to obtain plasma and serum aliquots (3000 rpm for 20 minutes at 4C for plasma and 3000 rpm for 10 minutes at 20C for serum). The aliquots were stored at −80C until the completion of the study. All biomarker analyses were carried out at the Molecular and Hemostasis Lab at the Center for Disease Control and Prevention, Atlanta, GA. Fluorokine-MA1P MultiAnalyte Profiling Human Base kits were used with the BioRad BioPlex™ Luminex xMAP (Austin, Tx) platform for biomarker quantification. Plasma samples were diluted in the appropriate calibrator dilutent and incubated with the diluted microparticle analyte-multiplex according to the manufacturer’s protocol. Analyte-specific antibodies were pre-coated onto color-coded microparticles. Microparticles, standards, samples and appropriate controls were added to the wells. After washing, a biotinylated antibody cocktail specific to the analytes of interest was added to each well. Following another wash to remove any unbound biotinylated antibody, streptavidin-phycoerythrin conjugate (Streptavidin-PE) was added to each well. After a final wash, the microparticles were resuspended in buffer and read using the Luminex analyzer.
Statistical methods
Continuous variables are summarized by their mean and standard deviation (SD), whereas dichotomous measures are given as percentages. To investigate the relationship between atherosclerotic risk factors and FDG uptake, the average of the left and right TBR values for carotid, iliac and femoral arteries was used. The same method was used when comparing arterial FDG uptake with circulating biomarker levels, and for the analysis of associations between FDG uptake in different arterial regions. However, left and right arteries were treated independently when links between calcification and TBR within individual arteries were assessed.
The association between arterial FDG uptake in different vascular beds was explored using Pearson’s correlation coefficient after first ascertaining approximate normality. Associations between FDG uptake and circulating biomarkers were evaluated using the Spearman rank correlation test because several biomarkers had non-normal distributions. Simple linear regression analysis was performed to explore the associations between FDG variables (outcome variables) and biomarkers (explanatory variables). This analysis was restricted to only the 33 subjects for whom biomarker and FDG data were available. Student’s t tests were used to compare TBR measurements across risk factor groups.
Statistical significance was set at the 5% level and analysis was performed using SPSS version 16. We did not apply a statistical correction for multiple testing, as we felt that standard corrections such as Bonferroni might be too conservative because of the large number of tests. Instead, we have indicated the exact level of significance at either 5%, 1% or 0.1%, and we offer caution in interpretation of p values at the 5% level.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Baseline characteristics
Patient characteristics and risk factor data from all 41 patients are shown in Table 1. Thirty-five patients had a history of previous vascular disease - coronary artery disease, cerebrovascular disease or peripheral vascular disease whilst six patients had at least three risk factors for atherosclerosis. Typical examples of arterial FDG uptake are shown in Figures 1 and 2.
Table 1.
Patient characteristics (n=41). SD - standard deviation, BMI - body mass index. Age and BMI are shown as mean±SD.
| Characteristic | % of female subjects (n=11) |
% of male subjects (n=30) |
% of subjects overall (n=41) |
|---|---|---|---|
| Female (%) | n/a | n/a | 26.8% |
| Age | 64.7±7.2 | 63.7±8.6 | 64.0±8.2 |
| Current or ex-smoker | 27.2% | 56.7% | 48.8% |
| History of coronary artery disease | 45.5% | 83.3% | 73.2% (n=30) |
| History of other vascular disease | 27.3% | 3.3% | 12.2% (n=5) |
| History of hypertension | 54.5% | 63.3% | 61.0% |
| History of diabetes | 27.3% | 40.0% | 36.6% |
| Statin use | 81.8% | 90.0% | 87.8% |
| BMI | 24.0±5.3 | 27.5±4.6 | 25.6±5.0 |
| Ethnicity | |||
| Hispanic | 9.0% | 20.0% | 17.1% |
| African American | 9.0% | 16.7% | 14.6% |
| Indian | 9.0% | 13.3% | 12.2% |
| Caucasian | 72.7% | 50.0% | 56.1% |
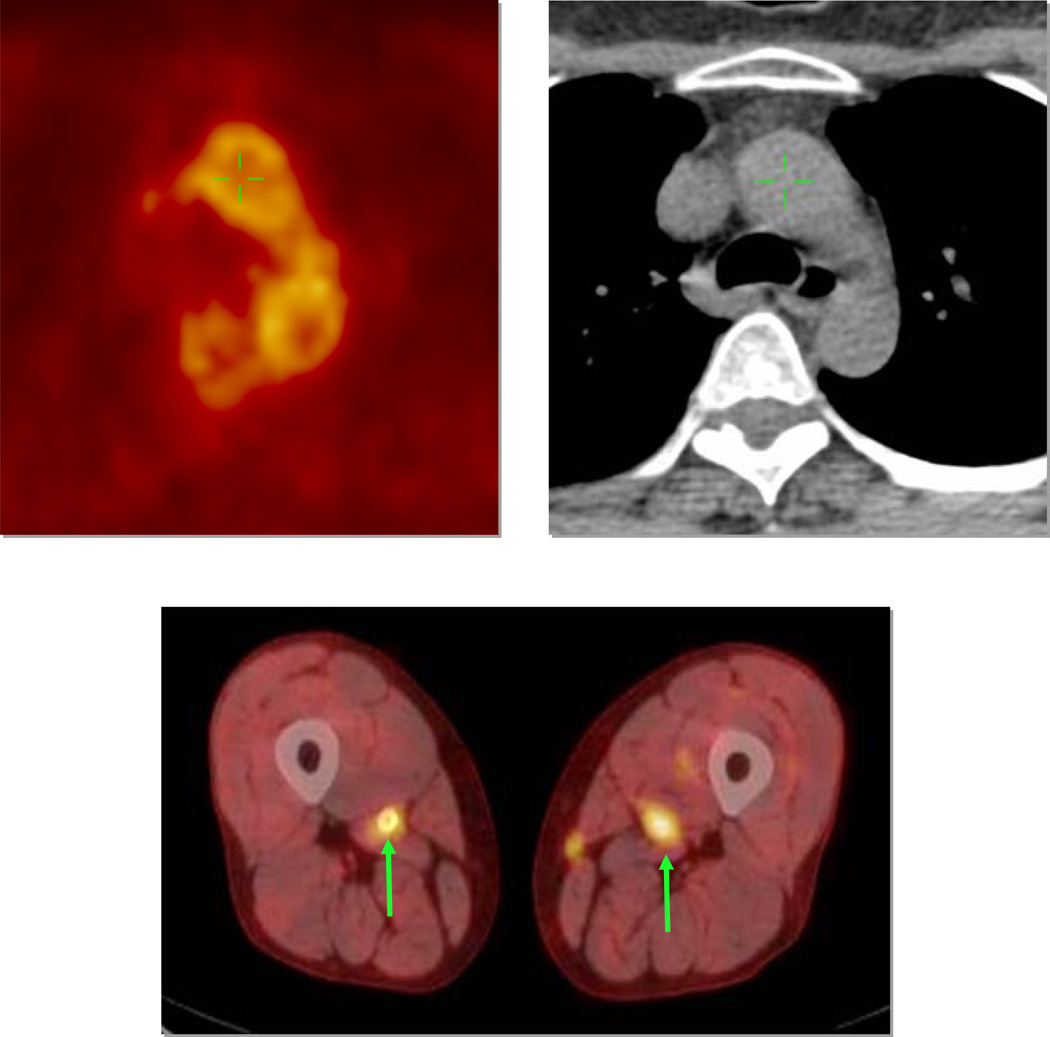
Fig 1.
Examples of FDG uptake. Top panel shows aortic arch imaging by FDG PET (left) and non-contrast CT (right). There is heterogeneous FDG uptake seen within the artery wall. Bottom panel demonstrates bilateral femoral artery FDG uptake on fused PET/CT images (arrows).
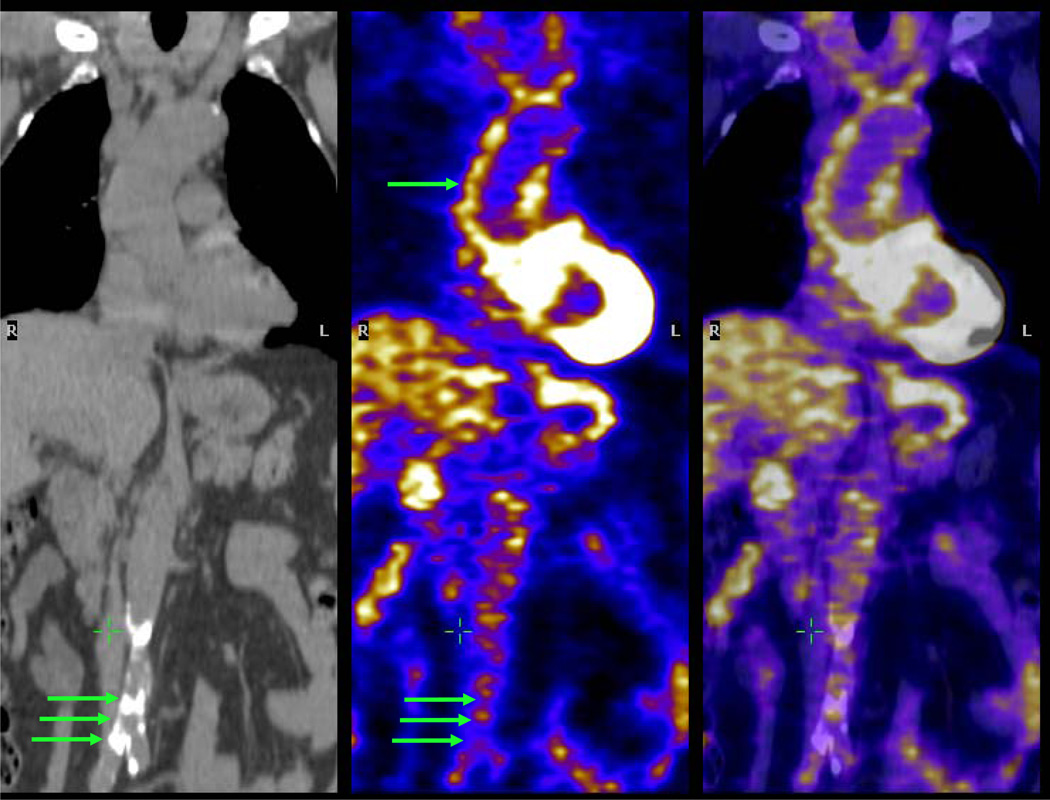
Fig 2.
Aorta imaging with PET/CT. The left hand image is a non-contrast coronal CT image showing calcification of the abdominal aorta (group of three green arrows). Center and right hand images are co-registered PET and fused PET/CT images respectively, demonstrating significant FDG uptake within the ascending aorta (single arrow) but relatively less FDG uptake in the calcified abdominal aorta. The green cross is in the inferior vena cava, where there is low FDG uptake.
Arterial FDG uptake is highly correlated across different arterial territories
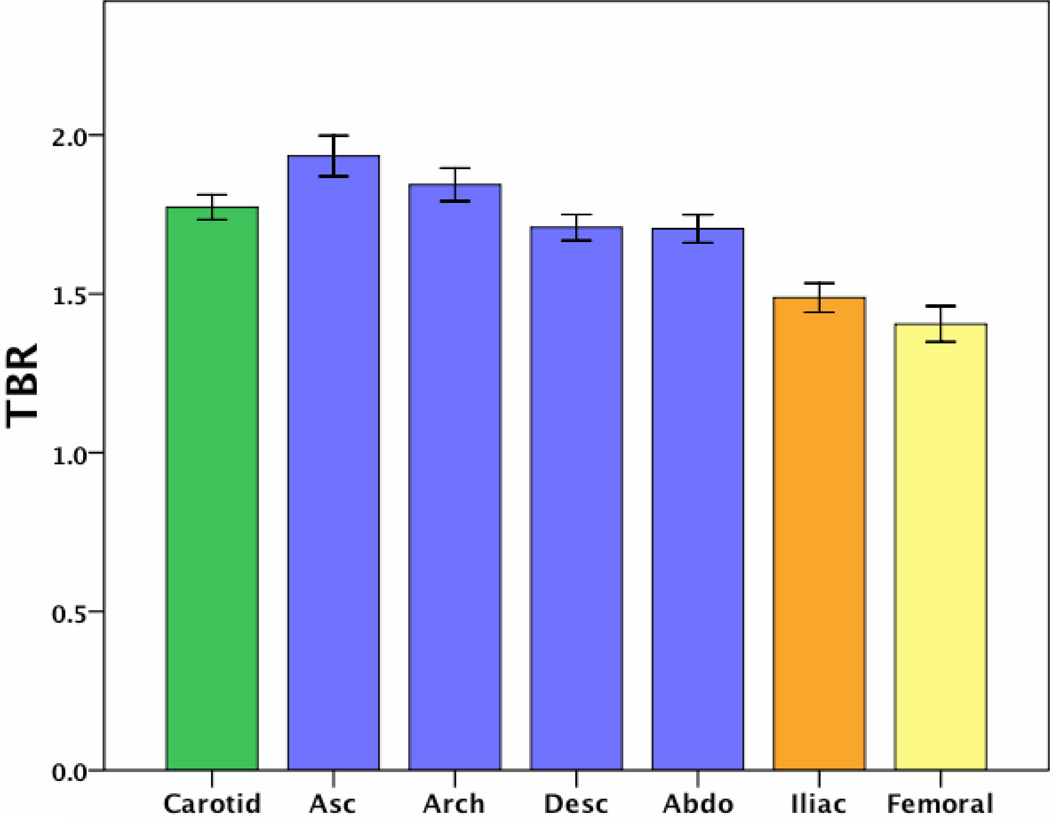
The average TBR value in each of the arterial territories is shown in Figure 3. It is clear that there are regional variations in inflammation, with the carotid and all regions of the aorta being more inflamed than the peripheral arteries. Associations between FDG uptake in each territory are shown in Table 2. We noted associations in the degree of FDG uptake across different arterial territories, more strongly between adjacent territories (neighboring aortic regions, carotid artery and aorta) than between more distant regions (for example carotid versus femoral arteries). When analyzed individually, anatomic artery pairs had particularly strong correlations between their measured degrees of inflammation, for example left and right carotid r=0.91, p<0.001, left and right femoral r=0.96, p<0.001.
Fig 3.
Average TBR value in each arterial territory imaged. Error bars represent standard error of the mean.
Table 2.
Pearson correlation coefficient values for FDG uptake between various arterial regions. Results marked with one asterisk are significant at the 5% level; those with two are significant at the 1% level.
| Ascending | Arch | Descending | Abdominal | Carotid | Iliac | Femoral | |
|---|---|---|---|---|---|---|---|
| Ascending | _ | 0.88** | 0.84** | 0.88** | 0.61** | 0.79** | _ |
| Arch | _ | _ | 0.81** | 0.89** | 0.64** | 0.84** | _ |
| Descending | _ | _ | _ | 0.89** | 0.48* | 0.78** | _ |
| Abdominal | _ | _ | _ | _ | 0.62** | 0.86** | _ |
| Carotid | _ | _ | _ | _ | _ | 0.57** | 0.43 |
| Iliac | _ | _ | _ | _ | _ | _ | 0.71** |
| Femoral | _ | _ | _ | _ | _ | _ | _ |
Arterial FDG uptake and calcification are uncommon in the same artery
In those arteries in which both calcium scoring and FDG imaging was performed, there were negative correlations noted between inflammation and calcification. This was demonstrated in the carotid arteries (left carotid calcium vs. left carotid FDG r=−0.42, p=0.03 and right carotid calcium vs. right carotid FDG r=−0.42, p=0.03). A similar finding was noted in the ascending aorta but this did not reach statistical significance (calcium vs. FDG r=−0.30, p=0.13).
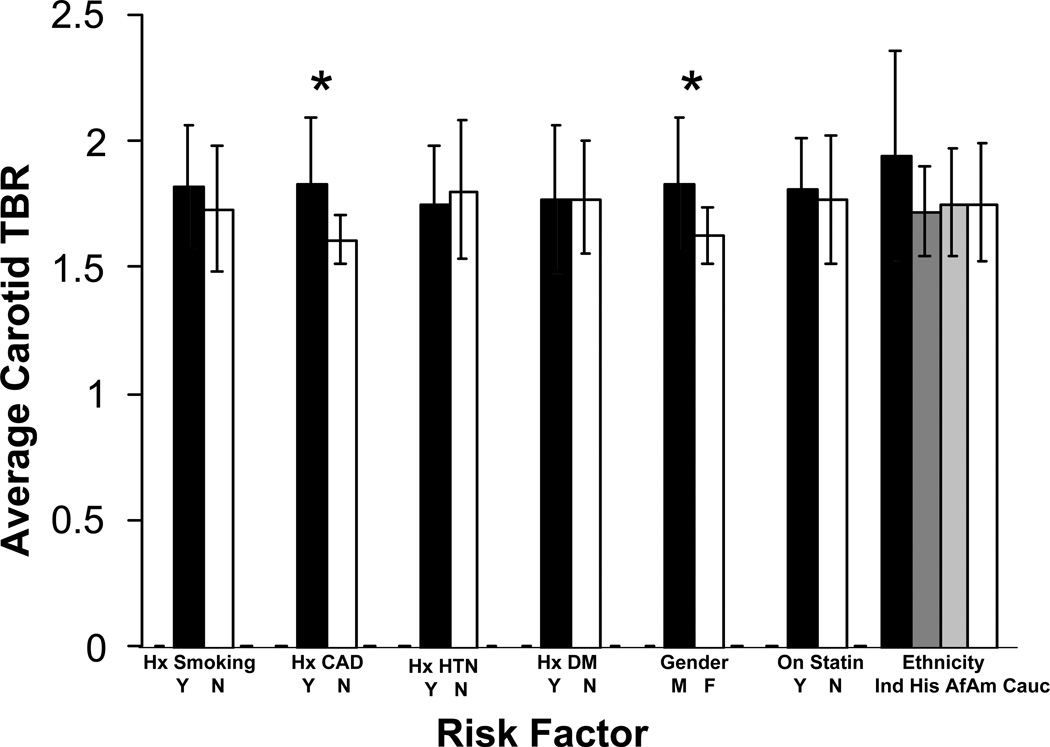
Male sex and the presence of coronary artery disease are associated with arterial FDG uptake
In the 40 subjects that underwent carotid imaging, FDG uptake was significantly greater in males (mean TBR in males 1.83 vs. 1.63 in females, p<0.05). Arterial FDG uptake was also more marked in patients with a prior history of coronary artery disease (CAD) versus no history of CAD (mean TBR 1.83 vs. 1.61, p<0.01) – Figure 4.
Fig 4.
Carotid artery inflammation variations amongst different risk factor groups. Results marked with an asterisk are significant at the 5% level.
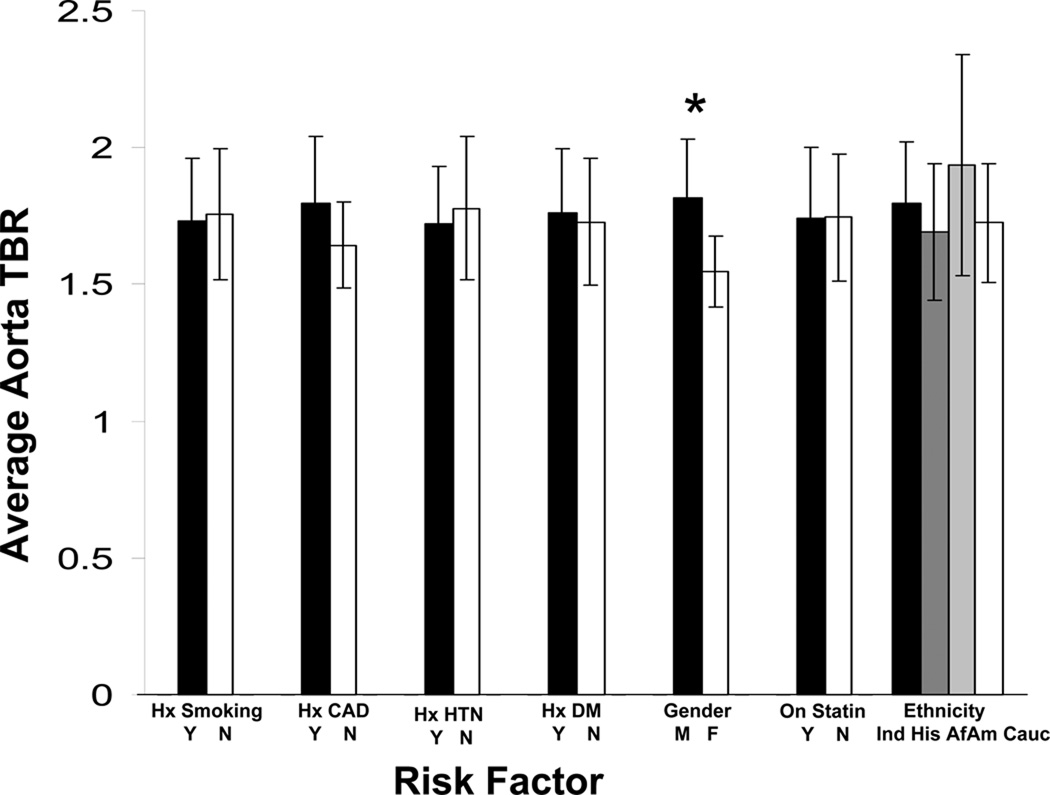
Aortic FDG uptake (n=27) was also associated with male gender (mean TBR 1.81 vs. 1.55, p<0.01) and a trend towards higher FDG uptake in those with a history of CAD (mean TBR 1.80 vs. 1.64, p=0.06, Figure 5).
Fig 5.
Aortic inflammation variations amongst different risk factor groups. Results marked with an asterisk are significant at the 5% level.
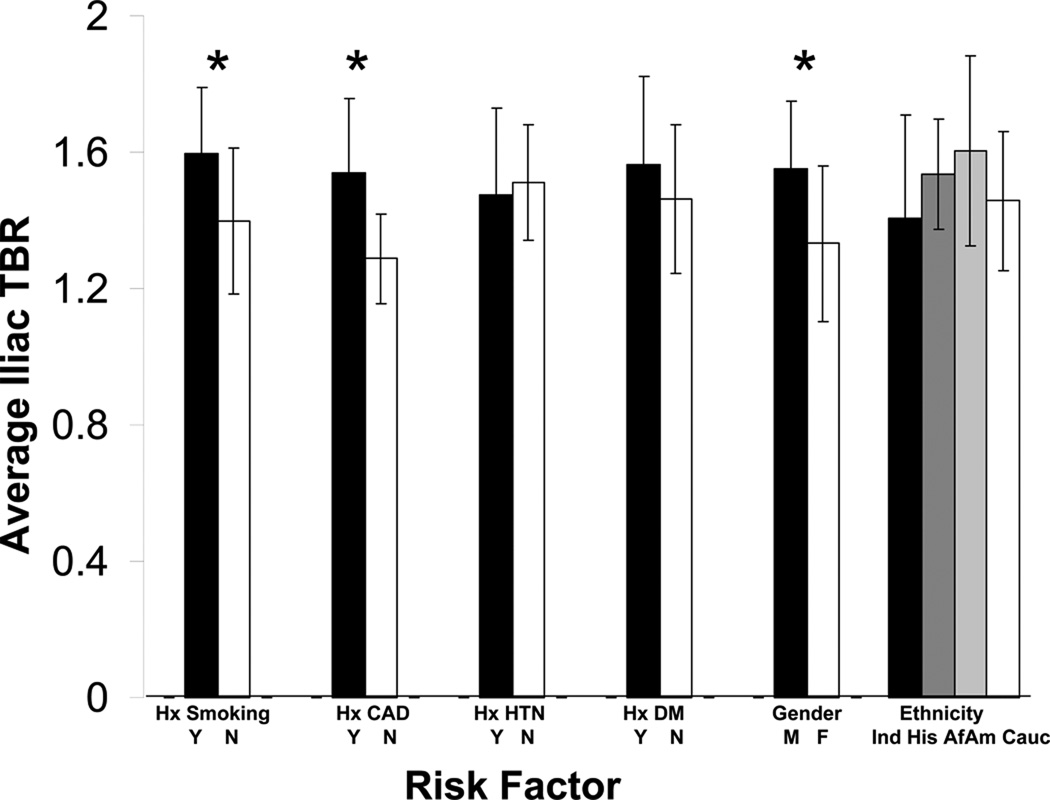
FDG uptake in the iliac arteries (n=24) was similarly associated with male gender (mean TBR 1.55 vs. 1.33, p<0.05) and a prior history of CAD (mean TBR 1.54 vs. 1.29, p<0.01) but also was higher in those with a history of cigarette smoking (mean TBR 1.59 vs. 1.40, p<0.05, Figure 6).
Fig 6.
Iliac artery inflammation variations amongst different risk factor groups. Results marked with an asterisk are significant at the 5% level.
Neither increased age, the presence of diabetes or hypertension, the use of statin medication nor ethnicity had a significant impact on FDG uptake in any arterial region.
Arterial FDG uptake positively correlates with levels of several circulating inflammatory biomarkers
Thirty-three patients from the cohort of forty-one underwent both PET/CT imaging and biomarker analysis. The mean age of the biomarker sub-group (33 patients) was 64 years, with 73% males and 36% diabetic, similar to the overall patient group (41 patients).
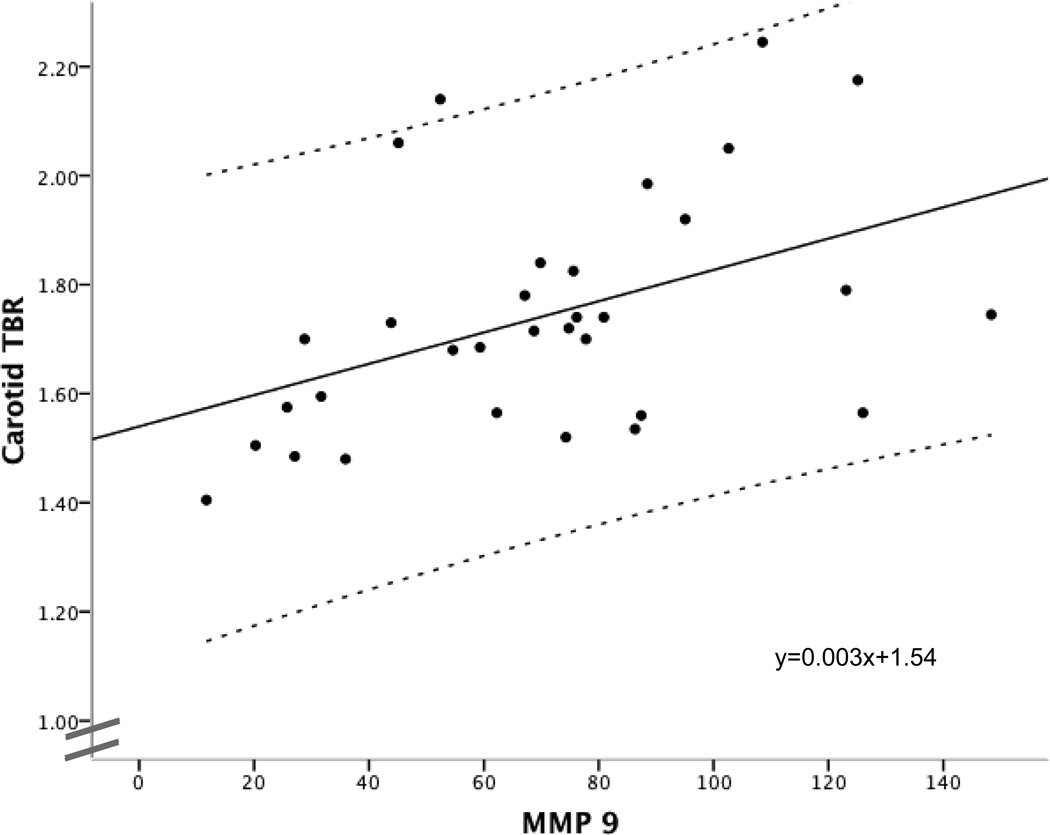
There were significant correlations between MMP 3 levels and inflammation in three regions of the aorta: MMP 3 versus ascending aorta FDG uptake (r=0.49, p=0.02), arch (r=0.44, p=0.05), descending (r=0.53, p=0.01) and borderline significant in the abdominal aortic region (r=0.41, p=0.07). MMP 9 was significantly positively associated with both carotid FDG uptake (r=0.50, p=0.01), and descending and abdominal aortic FDG uptake (descending aorta r=0.44, p=0.05, abdominal aorta r=0.44, p=0.05) – see Figure 7.
Fig 7.
Relationship between the degree of carotid artery inflammation (expressed as TBR) and circulating levels of the inflammatory biomarker MMP 9. Regression line, equation and 95% confidence intervals are provided.
A strong positive trend was seen between levels of interleukin 18 and FDG uptake in the descending and abdominal aorta (descending (r=0.41, p=0.06), abdominal (r=0.40, p=0.07)). In addition, non-significant trends were observed between serum fibrinogen levels and FDG uptake in the descending aorta (r=0.41, p=0.06), and between serum CRP levels and FDG uptake in the same territory (r=0.43, p=0.06).
We saw no relationships between arterial inflammation as assessed by FDG PET/CT and the other biomarkers measured, including serum lipid levels.
Arterial FDG uptake is inversely related to atheroprotective biomarkers
Serum levels of adiponectin were inversely related to inflammation in the descending aorta (r=−0.49, p=0.03), and PAI-1 to inflammation in the carotid arteries (r=−0.39, p=0.03).
Discussion
We prospectively evaluated 41 subjects with either risk factors for, or established atherosclerosis using FDG PET/CT as a surrogate marker of vascular inflammation. Previous studies have established that the degree of arterial uptake of FDG correlates strongly with macrophage infiltration in two different animal models of disease5, 18 and in patients with symptomatic carotid atherosclerosis4, 6. Tahara and colleagues highlighted the ability of FDG PET to track atherosclerotic inflammation reduction resulting from a modest dose of simvastatin8, and similar results were noted in an animal model of atherosclerosis10. Most recently, it has been shown that arterial FDG PET imaging is capable of reporting on the reduction of vascular inflammation resulting from cardiovascular risk factor modification over a one year period9. These intervention studies have set the stage for the use of FDG PET imaging as a potential surrogate marker for evaluating drug efficacy, analogous to its use in cancer therapy trials16.
The current study shows how FDG PET imaging can extend our insight into the disease process of atherosclerosis. Here we have shown that the presence of inflammation in one arterial territory is highly predictive of inflammation in others. This finding suggests a form of systemic arterial activation. There seems to be some degree of regionality however, because inflammation across neighboring territories is more correlated than inflammation across arterial areas more anatomically remote from each other.
Supporting this theory of systemic activation, we also noted that the degree of arterial FDG uptake was associated with blood levels of several systemic inflammatory biomarkers, including those from the matrix metalloproteinase family, and strong trends amongst both the interleukin group and CRP. A previous study demonstrated a link between carotid FDG uptake and level of MMP 1 in a group of patients with carotid disease awaiting surgery19. We found no association in our population with this particular MMP, but did observe moderately strong correlations between FDG uptake and levels of both MMP 3 and MMP 9, which, along with MMP 1, have been implicated in plaque rupture20–22. Over-expression of MMP 9 in ApoE knockout mice leads to rapid plaque destabilization23, highlighting the key role of these macrophage-secreted enzymes. One particular advantage of FDG PET atheroma imaging over measurement of circulating biomarkers is the ability to pinpoint a particular arterial segment as being inflamed, allowing it to be targeted for treatment. Wu et al demonstrated that arteries that were most highly inflamed on FDG PET prior to carotid intervention had the greatest release of harmful MMP 1 during the procedure19.
We noted a modest relationship between arterial FDG uptake and serum CRP levels. Some previous publications have demonstrated positive correlations between FDG uptake and CRP, whilst others have not demonstrated any sort of relationship4, 8, 19, 24. Therefore, although this biomarker can help to refine the risk of future cardiovascular events at a population level25, its role in individual subjects is not yet certain.
The lack of a relationship between arterial inflammation and serum LDL levels, and the inverse relationships between arterial inflammation and the biomarkers adiponectin and PAI-1 are interesting. Adiponectin is known to act as a brake on inflammation in both healthy individuals and those with vascular disease26, 27, so it is intriguing that in those with the highest levels of this hormone, there were the lowest degrees of FDG arterial uptake. Serpins such as PAI-1 have pro- or anti-inflammatory actions, depending on the degree of vascular activation28. Other groups have previously noted no relationship between FDG uptake and LDL level8, 9, 29, and it may be that oxidised LDL levels are more important in determining the amount of artery inflammation than LDL.
In this study inflammation and calcification within arteries rarely overlapped. This has been previously suggested30; but in a retrospective study of cancer patients where the degree of inflammation was not numerically quantified. Our finding supports the view that plaques of different age might coexist in arteries, with episodes of inflammation leading to rupture events and having an end stage of calcification.
The observation that males have greater arterial FDG uptake than females suggests that atherosclerotic plaques in males may be more highly inflamed, perhaps providing a pathological link for their higher rate of cardiovascular events. Higher FDG uptake in the carotid and iliac arteries of patients with a prior history of CAD reinforces the global nature of atherosclerotic disease, whereas in patients with a history of cigarette smoking, FDG uptake was only increased in the iliac arteries. It is well known that iliac artery disease is common amongst smokers31, and this result suggests a site-specific nature of the response to certain risk factors.
The fact that the small number of diabetic subjects in our study did not have higher FDG uptake than non-diabetics may be due to effective medical therapy in our study group, or to the competitive effect of hyperglycemia on FDG uptake. Some diabetic medications, such as glitazones, have been shown to have anti-inflammatory actions32 while metformin has been associated with a decreased risk of future cardiovascular events in obese type 2 diabetic patients33. It is therefore plausible that taking these medications could reduce the degree of arterial inflammation detected by PET imaging. Since we excluded patients with high blood glucose from the study, it is unlikely that significant competition between FDG and glucose would explain the lack of difference. Furthermore, it seems that FDG uptake into inflammatory lesions may be less sensitive to elevated serum glucose levels than tumour cells34.
Our study did not show an association of statin use and FDG uptake, in contrast to other work8. This is most likely due to the small sample size in our non-statin subgroup (as shown in table 2, 88.8% of the subjects were taking statins).
Context and conclusions
Our results are in broad agreement with the study published by Tahara et al in 200729. That study demonstrated higher FDG uptake in the arteries of males compared to females, and an association between FDG uptake and several components of the metabolic syndrome. However, their study was retrospective and limited to patients with cancer, was performed on a standalone PET scanner and did not include many patients with known cardiovascular disease. Our study specifically imaged patients with known vascular disease including a high proportion with CAD. We also used a vascular-tailored imaging protocol, and for the first time obtained data on risk factors, biomarkers, calcification and FDG uptake at multiple sites within the same patient. In conclusion, we have demonstrated that arterial FDG PET imaging can provide new insights into the pathobiology of atherosclerosis. We suggest future studies might test an arterial inflammation score derived from FDG PET and circulating biomarkers as a means of predicting clinical events in high-risk individuals. However, large, prospective event-driven studies of non-invasive inflammation imaging techniques are the best way to determine the place of these modalities in future clinical practice. Such studies are already underway (see http://www.hrpinitiative.com) and are due to report in 2011.
Clinical Impact Summary.
Atherosclerotic plaque inflammation is thought to be central to plaque rupture and, by extension, clinical events such as acute coronary syndrome, stroke and transient ischaemic attack. By identifying such plaques before symptoms become apparent, preventive therapies such as statins might be initiated or intensified. FDG PET/CT is already established for cancer diagnosis, staging and the prediction of tumour response to therapy. Our study applied this non-invasive imaging technology to atherosclerosis of the carotid, femoral, iliac arteries and aorta. We explored the links between plaque inflammation, arterial calcification, circulating biomarkers and atherogenic risk factors. We found that there was strong symmetry in the distribution of arterial inflammation, and that calcification was inversely linked with inflammation. We also confirmed previous work by demonstrating that arterial inflammation was greater in male patients and in those with a diagnosis of coronary artery disease. Clinical applications of this work may include the use of imaging for prediction of plaque rupture, insights into the pathobiology of atherosclerosis and monitoring of anti-atherosclerosis therapies. Underpinning these applications, studies are already underway that will link imaging findings with clinical events. Additionally, FDG PET/CT atherosclerosis imaging is already being used as a surrogate marker of anti-atherosclerotic drug efficacy by several pharmaceutical companies seeking to exploit its uniquely high sensitivity and reproducibility.
Acknowledgments
Funding sources
Dr Rudd was funded by a British Heart Foundation International Fellowship. Dr Myers was funded by a Doris Duke Fellowship. Partial funding was provided by NIH/NHLBI ROI HL71021 (ZAF). The study was supported in part by the National Institute of Health Research Cambridge Biomedical Research Centre
Footnotes
Disclosures
None of the authors has any relevant disclosure to declare.
References
- 1.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay AC, Choudhury RP. Form to function: current and future roles for atherosclerosis imaging in drug development. Nat Rev Drug Discov. 2008;7:517–529. doi: 10.1038/nrd2588. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am.Coll.Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 5.Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnstrom P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging Atherosclerotic Plaque Inflammation With [18F]-Fluorodeoxyglucose Positron Emission Tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 7.Davies JR, Rudd JH, Fryer TD, Graves MJ, Clark JC, Kirkpatrick PJ, Gillard JH, Warburton EA, Weissberg PL. Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke. 2005;36:2642–2647. doi: 10.1161/01.STR.0000190896.67743.b1. [DOI] [PubMed] [Google Scholar]
- 8.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am.Coll.Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49:1277–1282. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa M, Magata Y, Kato T, Hatano K, Ishino S, Mukai T, Shiomi M, Ito K, Saji H. Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med. 2006;47:1845–1850. [PubMed] [Google Scholar]
- 11.Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA. Atherosclerosis Inflammation Imaging with 18F-FDG PET: Carotid, Iliac, and Femoral Uptake Reproducibility, Quantification Methods, and Recommendations. J Nucl Med. 2008;49:871–878. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 12.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Gundlich B, Musmann P, Weber S, Nix O, Semmler W. From 2D PET to 3D PET: issues of data representation and image reconstruction. Z.Med Phys. 2006;16:31–46. doi: 10.1078/0939-3889-00290. [DOI] [PubMed] [Google Scholar]
- 14.Kinahan PE, Rogers JG. Analytic 3D image reconstruction using all detected events. IEEE Trans Nucl Sci. 1989;36:964–968. [Google Scholar]
- 15.Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–1434. [PubMed] [Google Scholar]
- 16.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, Larson S, Mankoff DA, Siegel BA, Van den Abbeele A, Yap J, Sullivan D. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, Kudomi N, Shiomi M, Magata Y, Iida H, Saji H. (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–1250. [PubMed] [Google Scholar]
- 19.Wu YW, Kao HL, Chen MF, Lee BC, Tseng WY, Jeng JS, Tzen KY, Yen RF, Huang PJ, Yang WS. Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase-1. J Nucl Med. 2007;48:227–233. [PubMed] [Google Scholar]
- 20.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J.Clin.Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- 22.Loftus IM, Naylor AR, Goodall S, Crowther M, Jones L, Bell PR, Thompson MM. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31:40–47. doi: 10.1161/01.str.31.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tahara N, Kai H, Nakaura H, Mizoguchi M, Ishibashi M, Kaida H, Baba K, Hayabuchi N, Imaizumi T. The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J. 2007;28:2243–2248. doi: 10.1093/eurheartj/ehm245. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109:1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- 26.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin.Chim.Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu M, Ohfusa H, Aizawa T, Hashizume K. Adiponectin Inversely Correlates with High Sensitive C-reactive Protein and Triglycerides, but not with Insulin Sensitivity, in Apparently Healthy Japanese Men. Endocr.J. 2007;54:553–558. doi: 10.1507/endocrj.k07-032. [DOI] [PubMed] [Google Scholar]
- 28.Richardson J, Viswanathan K, Lucas A. Serpins, the vasculature, and viral therapeutics. Front Biosci. 2006;11:1042–1056. doi: 10.2741/1862. [DOI] [PubMed] [Google Scholar]
- 29.Tahara N, Kai H, Yamagishi S, Mizoguchi M, Nakaura H, Ishibashi M, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am.Coll.Cardiol. 2007;49:1533–1539. doi: 10.1016/j.jacc.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Dunphy MP, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med. 2005;46:1278–1284. [PubMed] [Google Scholar]
- 31.Mahmud E, Cavendish JJ, Salami A. Current treatment of peripheral arterial disease: role of percutaneous interventional therapies. J Am Coll Cardiol. 2007;50:473–490. doi: 10.1016/j.jacc.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 32.Calkin AC, Forbes JM, Smith CM, Lassila M, Cooper ME, Jandeleit-Dahm KA, Allen TJ. Rosiglitazone attenuates atherosclerosis in a model of insulin insufficiency independent of its metabolic effects. Arterioscler Thromb Vasc Biol. 2005;25:1903–1909. doi: 10.1161/01.ATV.0000177813.99577.6b. [DOI] [PubMed] [Google Scholar]
- 33.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 34.Zhuang HM, Cortés-Blanco A, Pourdehnad M, Adam LE, Yamamoto AJ, Martínez-Lázaro R, Lee JH, Loman JC, Rossman MD, Alavi A. Do high glucose levels have differential effect on FDG uptake in inflammatory and malignant disorders? Nucl Med Commun. 2001;22:1123–1128. doi: 10.1097/00006231-200110000-00011. [DOI] [PubMed] [Google Scholar]