Abstract
During courtship males often communicate information about their fitness to females. The matching of harmonic components of flight tone in male-female pairs of flying mosquitoes, or harmonic convergence, was recently described. This behaviour occurs prior to mating and has been suggested to function in mate selection. We investigated the hypothesis that harmonic convergence is a component of mosquito courtship. A key prediction of this hypothesis is that harmonic convergence should provide information to potential mates about fitness benefits. We measured the effect of harmonic convergence behaviour on the direct and indirect benefits obtained by females. We found that the sons of pairs that converged at harmonic frequencies prior to mating had increased mating success and that these offspring were themselves more likely to converge prior to mating. These results suggest that males may be able to signal information about their genetic quality to females prior to mating and that this signal may be heritable. These findings are important for our understanding of mosquito behaviour and have applications in the control of mosquito-borne disease. This study also contributes to the study of male-female interactions and signal coevolution.
In many organisms, female mating preferences are thought to have evolved in response to variation in the material or genetic resources offered by males (Andersson 1994). Males may offer females direct fitness benefits such as nutritional provisioning (Thornhill 1976; Gwynne 1984; Steele 1986; Markow 1988; Carlson 1989) or high quality territory for nesting and foraging (Holm 1973; Campanella & Wolf 1974; Jones 1981; Wells 1977). These benefits may increase fecundity (Simmons 1987; Moore 1994) and lifespan (Holm 1973; Thornhill 1983) to increase her overall reproductive rate. Female preference for males offering these benefits is maintained through natural selection (Neff & Pitcher 2005).
In some cases, however, males do not offer females any material resources, such as food or territory, and yet, females still display preferences among males. In this case, some researchers have postulated that female preference traits are maintained via indirect selection (Andersson 1994; Kokko et al. 2003; Neff & Pitcher 2005). Males offer females indirect or genetic benefits which increase the fitness of the female’s offspring and, therefore, her own fitness indirectly. Offspring of females mating with preferred males may enjoy higher survival (Moller 1990; Petrie 1994, Hasselquist et al. 1996) and growth rate (Moller 1990; Reynolds & Gross 1992; Petrie 1994; Welch et al. 1998). Females may also choose to mate with males that will increase offspring mating success (Welch et al. 1998).
In many instances, male courtship signals contain information about genetic characteristics that fathers may pass on to offspring (Neff & Pitcher 2005). Females that attend to signals and use them to distinguish between potential mates are able to achieve higher fitness by mating with males that offer the greatest indirect fitness benefit (Andersson 1994). There is evidence from various insects, including the cricket, Gryllus bimaculatus (Wedell & Tregenza 1999), and the sandfly, Lutzomyia longipalpis (Jones et al. 1998), that males are able to confer indirect benefits to their offspring. Further in many of these insects it has been determined that components of male signals are mechanisms by which females select males for these types of benefits. For example, Tregenza et al. (2006) found evidence that female field crickets (Teleogryllus oceanicus) are able to discriminate between males with higher innate immunity by their mating calls. Hoikkala et al. (1998) elegantly demonstrated that the frequency of male courtship song was correlated with offspring survival in Drosophila monatana. Additionally, the authors found that females were able to differentiate between these males and that they preferred them as mates. In many organisms, where large males have higher mating success, body size is heritable and females prefer the courtship signals of large males (Simmons 1987; Reynolds & Gross 1992; Gilburn & Day 1994; Gray 1997; Shaw & Herlihy 2000).
In the yellow fever mosquito, Aedes aegypti, mating occurs in aerial swarms, which form around the blood-meal host (Hartberg 1971). Ae. aegypti preferentially feeds on human blood (Harrington et al. 2001), and thus the host is typically a human. These aggregations are primarily composed of males with females entering the swarm singly. Mating occurs in flight. Males and females meet, form a copula in mid-air, and mate in a matter of seconds (Hartberg 1971; Moore 1979). Large male Ae. aegypti produce and transfer more sperm to females (Ponlawat & Harrington 2007, 2009), but there is no direct evidence from the field that these males achieve higher mating success. Until quite recently it was believed that there was no discernable courtship in swarming mosquito species (Yuval 2006).
Mosquito flight tone is non-sinusoidal and therefore it is composed of both fundamental and harmonic frequencies. Female Ae. aegypti produces a significantly lower fundamental frequency than the male. Flight tone frequency varies with temperature (Belton 1986), and studies have measured females producing fundamental frequencies from 330–544 Hz and males producing fundamental tones from 557–750 Hz (see Clements 1999). Roth (1948) demonstrated that male mosquitoes use the fundamental component of the flight tone signal of females to orient to and locate females in the swarm. In Cator et al. (2009), we described and demonstrated that when males and females are within 2–3 body lengths of one another, both males and females actively modulate their flight tone frequency to converge at harmonic frequencies. Harmonic convergence usually occurs between the female’s 3rd harmonic and male’s 2nd harmonic (approximately 1200 Hz) or at the male’s fundamental and female’s 2nd harmonic (approximately 800 Hz). Cator et al. (2009) suggested that convergence behaviour was related to mate selection. Harmonic convergence has now been described in several mosquito species including, the malaria vector, Anopheles gambiae (Cator et al. 2010; Pennetier et al. 2010) and the northern house mosquito (Culex pipiens) (Warren et al. 2009).
Here, we tested the hypothesis that harmonic convergence behaviours are courtship signals which inform females about potential benefits offered by mates. We measured and compared direct and indirect benefits for females that converged or did not converge with males at harmonic frequencies. We predicted that females mating with males with which they converged prior to mating would have higher direct or indirect fitness.
METHODS
Rearing Procedures
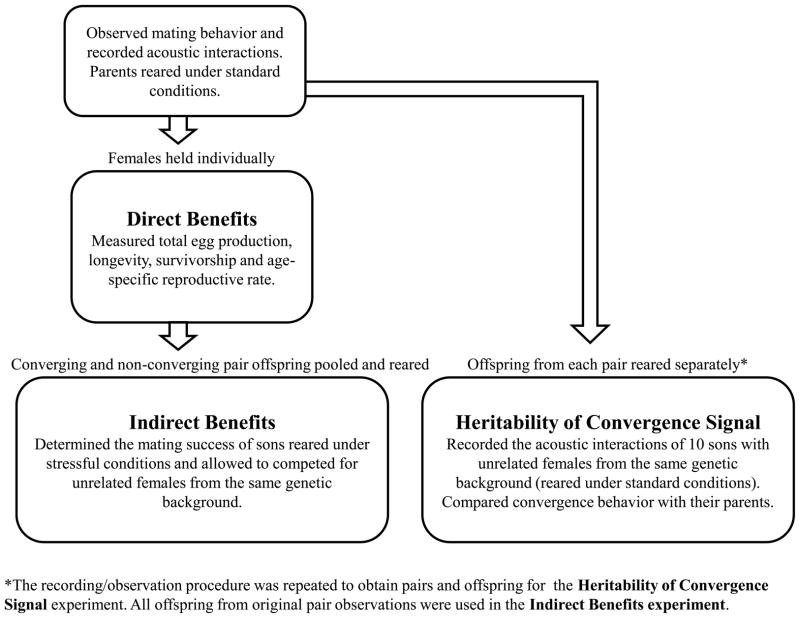
Three different rearing regimes were used for experiments. Parental generations were reared under standard conditions (Cator et al. 2009). In the indirect benefits portion of the study it was our aim to measure the genetic benefits males may have passed to their offspring. To observe genetic effects we reared larvae under moderate stress conditions (Cator 2011). Body size has been observed to have a tight correlation with gamete number in mosquitoes (Okanda et al. 2002; Ponlawat & Harrington 2007, 2009). We ensured that all of the individuals used in the parental generation and sons tested in the mating competition and heritability studies were all of the same body size to eliminate this variable from our study. We reared females of two distinct body sizes for the mating competition experiment (Ponlawat and Harrington 2007). These females were unrelated to the test males (although they were from the same genetic strain). We did this to measure not only which males were most likely to form a successful copula, but whether there was variation in the quality of the females with which these were able to form copulas with (Fig. 1).
Figure 1.
Diagram of experiments conducted.
Parental generation
We reared larvae (Ae. aegypti, Mexico Strain, Cornell Unviersity) as described in Cator et al. (2009). Larvae pupated on days 7–8, and adults eclosed on days 9–11. Pupae were separated to eclose individually, and we transferred newly emerged adult males and females into separate 500 ml cartons. Adults were provided with a 20% sucrose solution ad libitum and maintained in an environmental chamber set at a temperature range of 24 ± 4 C, 80% relative humidity and a photoperiod of 14 h L: 10 h D with a 2 h period of dusk/dawn conditions.
Rearing of sons for mating competition and heritability studies
The eggs laid by experimental females were allowed to embryonate under appropriate humidity conditions at 28 °C for a minimum of 1 week. We pooled egg papers into two groups (Fig. 1). One group contained offspring of parents that demonstrated harmonic convergence behaviours prior to mating, and the other group contained offspring from non-converging parents. Pooled egg papers were hatched under a vacuum for 15 min. We provided newly hatched larvae with 0.3 mg of Aedes diet and held them at 28 °C overnight.
We reared larvae under moderate stress conditions, which we had previously found cause a 10% increase in larval mortality (Cator, 2011). Adult virgin males were placed into cartons and held as described previously.
Rearing of females for male mating competition experiment
One large cohort and one small cohort of female Ae. aegypti were reared synchronously with experimental males as described in Ponlawat and Harrington (2007). This allowed us to test experimental males against females of the appropriate age. We collected virgin females daily and released them into a large bucket cage where they were held under standard conditions.
Rearing of females for heritability measurements
Females for heritability studies were reared under the conditions described for the parental generations and were unrelated to experimental males, but taken from the same genetic background.
Experiments
Initial Observations
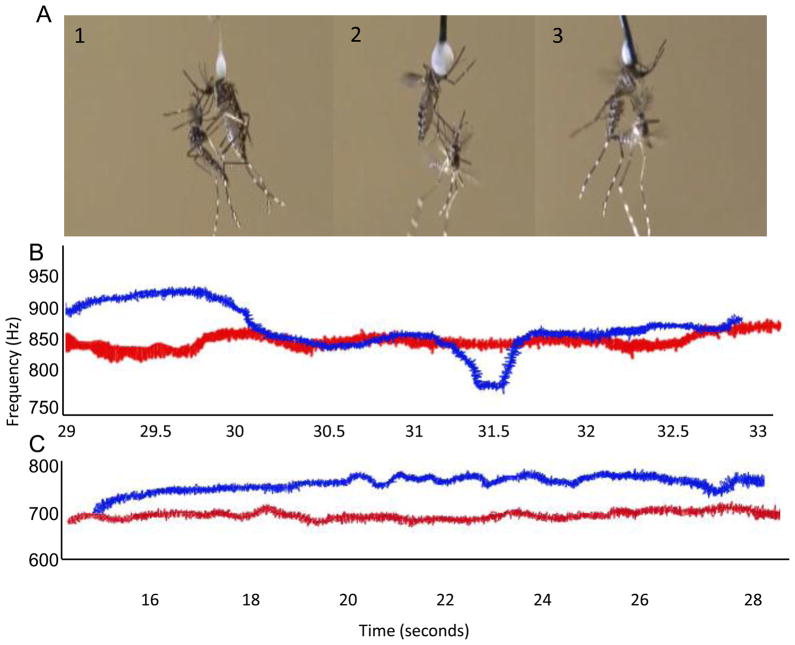
We observed the behaviour of 56 opposite sex pairs of virgin three-five day old Ae. aegypti while simultaneously recording any acoustic interactions that occurred between them (Fig 2). After we anesthetized females on wet ice for 5 min, we tethered females using Elmer’s glue to a 1–2 cm strand of human hair that had an insect pin attached to the opposite end (the “semi”-tether) as described in Cator et al. (2010). One female per trial was positioned in the center of a 12 × 6 × 8 cm mating arena and 4 cm above the sensitive face of a particle velocity microphone (NR-21358; Knowles, Itasca, IL). Host stimuli were provided by the close proximity of the observer (outside of arena and less than 30 cm). We stimulated flight using tarsal inhibition and gentle puffs of air. We released males into the arena three at a time and allowed males to mate with the female. Five of the six arena faces were opaque and the front face was clear plastic which allowed us to observe mosquito interactions.
Figure 2.
Harmonic convergence and mating behaviours of Ae. aegypti. A. Examples of observed mating behaviours in this study. Panel 1, female tilts abdomen away from male to prevent genital contact and semen transfer. Panel 2, female is holding male away from her body with her legs. Panel 3, the female and male are in successful copula. Females were tethered to strand of human hair (shown in 1). Video footage of these behaviours can be found in the supplemental materials. B. Spectrogram trace of a successful convergence of a male’s fundamental (blue trace) and female’s 2nd harmonic (red trace). C. Example of male (blue trace) and female (red trace) that did not converge during a mating attempt.
We recorded the behavioural outcome of each mating attempt. Instances of rejection were described as kicks (female physically dislodges the male with her legs) or holds (female uses her legs to hold male away from her body or tilts her abdomen to prevent genital contact). Acceptances were recorded when males were able to position themselves successfully venter-to venter and clasp the genitalia of the female (Fig. 2A and Supplemental Video). Trials in which males took longer than 5 min to initiate interaction with the female were discarded. We discarded 5% of trials. We removed the right wings of males and females for wing length measurements. Wing length is the standard approach to estimate adult body size in mosquitoes (Nasci 1990). Experiments were conducted between 0600 and 1800 hrs.
Acoustic recordings were taken from the time of male release to the termination of a successful copulation at the same time as behavioural observation. We analysed sound recordings of mosquito flight tone analysed using Raven 1.0 (Cornell Lab of Ornithology, Ithaca, NY). The definition of convergence was a matching of male and female harmonic frequencies during encounter with a potential mate. Frequencies were considered to be matching if they were within less than 4.95 Hz and lasted a minimum of 1 sec. If we detected convergence (Fig 2B), we then recorded the time of convergence in relation to paired interaction (s), the movement of male and female fundamental frequencies leading to convergence (Δ Hz), the rate of convergence (Δ Hz/Δ s), and the duration of converging (s). We recorded the initial flight tone of males and females from all copulas (Hz).
All statistical analyses for this part of the study and elsewhere were conducted using SPSS (Version 16, SPSS Inc., Chicago IL). A Generalized Linear Model with a Binary Logistic distribution was used to test for the significance of male and female body size, initial flight tone, convergence, and replicate effects in predicting the formation of a successful copula. When data were normally distributed, we tested for differences in wing length between converging and non-converging individuals using a Student’s t-test. A Mann-Whitney test was applied to non-parametric data.
Measurement of Female Benefits: Direct Benefits
In the next experiment, we tethered females as described above and released a single male into the mating arena. We initiated recordings with the entry of males into the arena and terminated them when we removed mating pairs. Copulas were not interrupted and males were not removed until they had released the female genitalia with their claspers. We cut the hair strand as close as possible to the female’s thorax using dissecting scissors to remove them from the tether. Females were transferred into a clean 500 ml carton to lay eggs.
Females were offered a blood meal (LJC, in accordance with Cornell University’s Institutional Review Board approved protocol #0906000050) every 3–5 days over the course of their lifespan, 25.05 ± 0.17 days. We checked for eggs each day. If eggs were found, we counted them and provided fresh filter paper. After removal, we allowed egg papers to dry overnight and then placed eggs from each female into a separate labeled plastic bag (Fig. 1). We documented female mortality each day until all females died.
The net reproductive rate (R0) was compared for converging and non-converging pairs using Cox regression (Cox 1972). We used a General Linear Model (GLM) to determine the effects of female body size and convergence behaviour on the number of eggs each female laid. Female survival from individual data was compared using Cox Regression (Cox & Oaks, 1984). We also used a GLM to determine the statistical difference between the longevity of females from the two groups (total number of days lived).
Measurement of female benefits: Indirect Benefits
We pooled together all eggs laid by converging and non-converging females and reared them under the conditions described above for rearing of sons for the mating competition study (Fig. 1). One-day-old males taken from each condition were marked with an identifying colour of florescent dust. On the following day, these males were used in experiments. Five sons of converging males and five sons of non-converging males were aspirated into a cylindrical cage (22 × 28 cm) for a total of 10 males in each mating trial. One large and one small female were aspirated into the cage (for a 1:5 ratio of males and females). Pairs engaged in copulation were collected and aspirated into individual tubes. Colour and parentage of the males found in copula were recorded. We replaced males and females removed in copulas with fresh males and females of the appropriate parentage and size. We wanted to ensure that newly released males did not have an advantage over males that had been in the cage previously. To minimize this effect, we replaced all individuals (male and female) every 15 min. At the conclusion of the experiment, we placed the mating cage into a 4 °C freezer, collected unmated males and females and removed the right wing to be measured. This experiment was replicated three times. Male mating competition was compared using a GLM with a BLR distribution.
Male and female body size, dust colour, replicate, and day effects were all tested for significance in predicting a successful mating. We removed predictor variables from the model when found not significant.
Heritability
To investigate the heritability of convergence behavior, we recorded the acoustic interactions of a new set of copulating pairs (Fig. 1). After copula formations, females were removed from semi-tethers as described in the direct benefits experiment and held in 500 mL cartons. Eggs were collected, labeled by adult pair ID, and held for maturation under the conditions previously described for a minimum of 1 week. The egg papers from each female were placed into a separate plastic dish (4.5 × 4 cm) and flooded with water. Dishes were placed into larger plastic containers (22 × 22 × 8 cm) with sealed lids and vacuum hatched for 15 min. We staggered hatches of egg clutches in groups of 9 clutches per day. We incorporated the day that we collected the data into analysis. Offspring from these recordings were reared under the same density, diet, and temperature conditions as their parents. Emerged males were released into a large bucket cage and provided with a 20% sucrose solution.
A standard cohort of Ae. aegypti taken from the original colony were reared synchronously with the offspring from recorded pairs (Cator et al. 2009). These females were not siblings of the males taken from recorded pairs. Pupae from these trays were sorted into individual tubes to obtain virgin females. Females were held with a 20% sucrose solution.
When sons were 2–5 days old, we assayed the harmonic convergence behaviour of 10 randomly selected sons when presented with an unrelated female from the same genetic background. We tested for harmonic convergence using the identical experimental set up as described above for their parents. After recording, the right wing of both males and females was removed and measured. This experiment was replicated three times.
A mixed model contains both fixed and random effects and is appropriate in situations where data are nested in groups (in this case days and replicates) (Fox 2002). We used a mixed model to determine the effect of parental convergence behaviour (fixed), replicate, day of recording (random effect), family identity (random effect), and both parental and son wing length (random effects). Factors found to have no significant main effect on offspring convergence behaviour were removed from the final model. Linear regression was used to determine the relationship between the rate of convergence (Δ Hz/Δ s) of fathers and sons
RESULTS
Initial Observations
We recorded 250 free flight interactions from 56 pairs with 41 of these forming successful copulas. Male and female body size, initial flight tone, and replicate effects were not significant in the model and were removed. The presence of harmonic convergence significantly predicted the formation of a successful copula (29 converging pairs of 41 successful copulas, Binary Logistic Regression: W1 = 14.54, P < 0.001). Inversely, females from converging pairs were less likely to exhibit rejection behaviour with only eight of 37 converging pairs not mating. Convergence between both the male 1st and female 2nd harmonic and the male 2nd and female 3rd harmonic was observed (Figure 2B).
Acoustic Recordings of Pairs Used to Measure Benefits
We recorded 141 pairs with 77 not converging prior to mating (54.6%) and 64 converging pairs (45.4%). Male flight tone averaged 711.98 Hz ± 13.82 (SE) and males responded to females at an average rate of 92.37 Hz/second ± 14.99 (SE). Convergence lasted an average of 3.18 ± 0.05 sec (SE). There was no significant difference between the wing lengths of converging and non-converging males and females (Student’s t-test: females, t47 = 0.98, P > 0.05; males, t77 = 0.06, P > 0.05).
Direct Benefits
We iteratively calculated age-specific R0, the ratio of individuals in a population at the end of a generation from the numbers present at the beginning of that generation (Begon et al. 1996). This value is determined by the number of expected daughters and survivorship of the females at each age in the population. We found no significant effect of convergence behaviour on R0 over all days in each replicate (see Table 1). When we analysed all replicates together, there was no significant effect of converging on R0 controlling for replicate (Cox Regression: W1 = 0.08, P = 0.77). There was no significant difference in the survivorship of females from the two groups (Cox Regression: X2 1 = 0.082, P = 0.77). We also analysed our data at an individual level. Data for the total number of eggs laid by each female were square root normalized. There was no significant effect of convergence behaviour on the total number of eggs laid by each female, controlling for wing length (GLM: F1 = 1.04, P = 0.31). Controlling for female wing length, replicate, and male wing length there was no significant effect of convergence on female longevity (GLM: W1 = 0.94, P = 0.33).
Table 1.
Variables for 3 replicates of converging and non-converging pairs in direct benefits experiment. Net reproductive rate (R0)= lxmx (lx, survivorship; mx, expected number of daughters), Generation time (Tc)= xlxmx/R0, and Intrinsic rate of increase (r)= lnR0/Tc. Significance was determined using a Wald Chi-Squared Test, d.f.=1. No significant differences between treatments were detected (p>0.05).
| Replicate | Convergence | N | R0 | Tc | r |
|---|---|---|---|---|---|
| 1 | Yes | 24 | 42.60 | 14.86 | 0.32 |
| No | 16 | 52.81 | 15.23 | 0.26 | |
| 2 | Yes | 14 | 73..46 | 15.07 | 0.29 |
| No | 28 | 57.52 | 23.89 | 0.62 | |
| 3 | Yes | 11 | 29.36 | 35.47 | 0.01 |
| No | 21 | 52.24 | 20.86 | 0.19 |
(d) Indirect Benefits
We observed 106 successful copulas with 71 of these involving the son of a converging pair. The successful convergence of parents significantly affected whether male offspring obtained matings (Binary Logistic Regression: W1 = 21.37 P = 0.003). Male offspring of converging pairs were more likely to achieve matings (Table 2). Controlling for wing length, there was no significant interaction between the color males were dusted and parental convergence behaviour (Binary Logistic Regression:W3 = 2.41, P = 0.49). The mean wing length of the females mated by converging and non-converging males also did not differ (Mann Whitney test, z = −0.411, P = 0.651).
Table 2.
Results of mating competition experiments between the sons of converging and non converging pairs. The columns give the number of males from each group that were captured just after a successful copula with an unrelated female.
| Replicate | Converging | Non-converging | ||
|---|---|---|---|---|
| mated | not mated | mated | not mated | |
| 1 | 26 | 154 | 10 | 170 |
| 2 | 21 | 164 | 16 | 169 |
| 3 | 24 | 141 | 9 | 156 |
(e) Heritability of Harmonic Convergence
The harmonic convergence behaviour of parents significantly predicted the harmonic convergence behaviour of their male offspring (Chi-Squared Test, χ22 = 8.20, P = 0.004). A greater percentage of the offspring of non-converging pairs did not converge with a potential mate (59%, n = 274), and a greater percentage of converging pairs converged with a potential mate (52%, n = 263). There was no significant effect of day, replicate, parent body sizes, or son body size. The rate of convergence (Δ frequency Hz/seconds) for both fathers and offspring was square root transformed to achieve normality. The rate of fathers did not predict the rate of offspring (GLM: F21 = 0.60, P = 0.88).
DISCUSSION
Our results show that harmonic convergence is likely involved in mosquito courtship and is correlated with mating success. Pairs that successfully converged prior to a mating attempt were more likely to form a successful copula. We observed female rejection behaviours less frequently when convergence preceded a mating attempt. Further, our findings indicate that females may derive an indirect benefit from preferentially mating with males with which they converge. The fitness of females from converging pairs was indirectly increased via increased mating success of sons. The sons of converging pairs were more successful in competition with the sons of non-converging pairs and more likely to converge with females prior to mating. Overall, these results suggest that there is a genetic component to male mating success in Ae. aegypti that is associated with harmonic convergence.
The evolution of indirect selection is likely widespread as there is evidence for positive correlation between preference and display traits in animals (Kokko et al. 2003). In some instances indirect benefits are facilitated by male parental care or provisioning, but in non-resource based systems lacking male parental care, males only pass genetic benefits to offspring. Sexual selection for indirect benefits is hallmarked by female preference for variable signal characteristics that are linked with an indirect fitness benefit. Further, signal traits must be inherited by the offspring (Kokko et al. 2003).
There are other examples of these types of signaling systems. In birds, plumage characteristics may correlate with male viability (Norris 1993) and parasite resistance (Moller 1990) and be heritable. Hasselquist et al. (1996) used cross-fostering experiments to determine that male great reed warblers (Acrocephalus arundinaceus) inherit song repertoires from their fathers, which has been linked to male mating success.
Evidence for signaling of indirect benefits has been reported in other insect species. Boake (1985) used genetic techniques to measure the heritability of male mating success and male pheromone traits in red flour beetles (Tribolium castaneum). Males inherited pheromone characteristics and males with preferred pheromone traits not only produced more grandchildren, but also passed on genes related to pheromone characteristics. In an elegant experiment, Moore (1994) presented evidence that genes associated with decreased development time for immature offspring were positively correlated with genes associated with increased male attractiveness in the cockroach, Nauphoeta cinerea. Multiple studies have demonstrated female preference for signal characteristics (Gwynne 1982; Tuckerman et al. 1993; Gray 1997) that are associated with indirect benefits (Zuk & Simmons 1997), and that both the signal and benefit traits are inherited (Simmons 1987; Hedrick 1988; Gray 1997; Shaw & Herlihy 2000).
We measured convergence as a property of a pair. Because convergence involves the modulation of both male and female flight tone and that these modulations occur synchronously, we were unable to determine which sex was driving convergence. It seems most likely, given what is known about the ecology of mosquitoes, that males are converging with females. This is supported by our finding that females are more likely to reject males when convergence does not occur. In an alternative scenario, females may converge with males as part of accepting them as a mate. Either interpretation suggests that females are somehow distinguishing between males and that convergence is an indicator of this process.
We did not detect any differences in the direct benefits associated with harmonic convergence. There are two explanations for this finding. First, it may be that there are no direct costs or benefits associated with harmonic convergence. This does not mean that direct selection is not involved in the mating system of Ae. aegypti. It simply means that males and females do not communicate about these types of costs and benefits using harmonic convergence. Mate choice in mosquitoes is likely informed by multiple inputs from multiple modalities and may include visual or chemical cues (Nijhout & Craig 1971, Polerstock et al. 2002, Zsemlye et al. 2005). Our conclusions are related only to information contained in acoustic signals.
A second explanation for our results is that there are differences in the direct benefits associated with harmonic convergence and we were simply unable to measure them using our techniques. Females were kept under ideal humidity and temperature conditions. Sugar was available to the mosquitoes for much of their lifespan, and they did not experience many of the metabolic and ecological stressors that they would under natural conditions. It may be that under such conditions, more natural than those used in our experiment, differences in fecundity and longevity would become apparent.
Body size is positively correlated with female fecundity in Ae. aegypti (Briegel 1990). Converging and non-converging sons mated with females of the same average body size. Therefore, the fitness advantage of the sons of converging pairs is based on their likelihood to mate and not the quality of that mate. If they achieved a successful copula, the sons of non-converging pairs were as likely to mate with large females as the sons of converging pairs. However these males were less likely to form a copula overall. A previous study with the malaria vector, An. gambiae, demonstrated that males and females respond to playbacks mimicking the flight tone of large potential mates with earlier and faster harmonic convergence behaviours (Cator et al. 2010). Future studies should be conducted to understand the relative roles of body size, which is largely determined by larval habitat, and convergence behavior, which our results have shown to have a heritable component, in determining mating success.
It should be noted that females in these experiments were tethered to a human hair and thus unable to exercise a complete range of behavioural responses to males. Females were unable to avoid flight and had limited ability to outmaneuver males, behaviours that have been described in previous experiments as allowing females to avoid mating (Jones & Pilitt 1973). If females were unable to exercise the full range of their rejection behaviours and if convergence is preferred by females, there may have been some instances in which females mated with males with which they would not have otherwise. If anything, tethering may have dampened our ability to measure differences and, thus, we do not believe that female tethering altered the conclusions of these specific experiments.
Future studies are necessary to clarify the association between harmonic convergence and the fitness of Ae. aegypti. Brooks & Endler (2001) found a trade-off between male sexual attractiveness and survival in a study performed in guppies (Poecilia reticulata). There may be a trade-off between harmonic convergence traits and other traits such as offspring survival. A cost-benefit analysis must be conducted to understand fully the importance of harmonic convergence signaling in male fitness. Such an assessment would ideally be conducted in the field, where these animals experience a full range of environmental stressors.
Harmonic convergence traits may be completely unlinked with another trait such as increased flight vigor or sensory sensitivity in offspring that facilitate their ability to converge. The function of this behaviour may be only to communicate to females that males carry a gene that will lead to “sexier” sons. Alternatively, it may be that these offspring inherit a sensory, metabolic, or physiological trait that makes them more likely to converge and that this trait is also associated with increased mating success. The subtle difference between these two scenarios is more important from a theoretical than from a practical standpoint, but nonetheless, determining the exact physiological mechanisms that dictate convergence behaviour would greatly improve our understanding of how it is inherited and how it relates to male mating success.
Further, convergence of offspring in this experiment was related to the convergence of both parents and not necessarily the convergence of their fathers. It would be interesting to conduct experiments to determine the relative importance of maternally and paternally inherited genes in determining convergence behaviour. Also, we measured convergence behaviours of sons and not of daughters. Future experiments should be conducted to determine the costs and benefits to daughters of converging pairs.
In general, the mating behaviour of mosquitoes is severely understudied, and many assume that there is no courtship behaviour in these insects. Our findings highlight a new level of complexity in the mating system of Ae. aegypti and argue for studying mosquitoes in the context of sexual selection. Further, our findings also add to the body of evidence for indirect benefits and demonstrate that indirect selection of this type operates in systems lacking obvious and elaborate courtship.
Not only do these results add considerably to our knowledge of mosquito pre-mating interactions, they have important applications to disease control efforts. Ae. aegypti is an important vector of several arboviruses including those that cause dengue and yellow fever. Our results indicate that mating success has a genetic component. This has important implications for transgenic control programs, understanding gene flow, and predicting the spread of insecticide resistance in wild populations. Continued study of mosquito mating interactions will improve current control strategies and will assist in the identification of new control targets.
Supplementary Material
Video footage of mating attempts in Ae. aegypti. Males attempted to mate with flying tethered females. In the first clip, the female held the male away from her body using her legs. In the next attempt, the male was able to position his body in the appropriate position for copulation, but the female tilted her abdomen away and prevented genital contact. In the third and forth clips, females rejected males by kicking them away in flight. In the final clip, the female accepted the male and there was firm and sustained genital contact, which is associated with seminal fluid transfer. All footage is shown at actual speed.
Highlights.
We tested the hypothesis that harmonic convergence is a component of mosquito courtship
We examined direct and indirect benefits of harmonic convergence to females
Sons of converging pairs had higher mating success
Sons of converging pairs were also more likely to show convergence before mating
Males may signal genetic quality to females before mating and this signal may be heritable
Acknowledgments
Funding was provided through a CDC Dissertations in Public Health Grant (1R36CK000130-01) and NIH/NIAID 1R21AI076828. The authors would like to thank M. Helenski, R. Hoy, K. Shaw, K. Reeve, B. Lazzaro and as well was three excellent reviewers for insightful comments.
Footnotes
To produce clear images, we used hard tethers for some photographs and videos. B. Example of converging pair. Spectrogram trace of a male (blue trace) converging with a female (red trace). Convergence occurred between the male 1st and female 2nd harmonic. C. Example of a non-converging pair. Male 1st harmonic (blue trace) and female 2nd harmonic (red trace) did not converge prior to mating.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we a re providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson M. Sexual selection. Princeton: Princeton University Press; 1994. pp. 184–205. [Google Scholar]
- Begon M, Mortimer M, Thompson TJ. Population Ecology. Oxford: Blackwell Science Ltd; 1996. pp. 52–188. [Google Scholar]
- Belton P. Sounds of insects in flight. In: Danthanararayana W, editor. Insect Flight: Dispersal and Migration. Berlin: Springer; 1986. pp. 61–70. [Google Scholar]
- Boake CR. Genetic consequences of mate choice: a quantitative genetic method for testing sexual selection theory. Science. 1985;227:1061. doi: 10.1126/science.227.4690.1061. [DOI] [PubMed] [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of Insect Physiology. 1990;36:165–172. [Google Scholar]
- Brooks R, Endler JA. Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata) Evolution. 2001;55:1002–1015. doi: 10.1554/0014-3820(2001)055[1002:daissa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Campanella PJ, Wolff LL. Temporal leks as a mating system in a temperate zone dragonfly (Odonata: Anisoptera). I. Plathemis lydia (Drury) Behaviour. 1974;51:49–87. [Google Scholar]
- Carlson A. Courtship feeding and clutch size in red-backed shrikes (Lanius collurio) American Naturalist. 1989;133:454–457. [Google Scholar]
- Cator LJ, Arthur BJ, Harrington LC, Hoy RR. Harmonic convergence in the love songs of the dengue vector mosquito. Science. 2009;323:1077–1079. doi: 10.1126/science.1166541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Ng’Habi KR, Hoy RR, Harrington LC. Sizing up a mate: variation in production and response to acoustic signals in Anopheles gambiae. Behavioral Ecology. 2010;21:1033–1039. [Google Scholar]
- Cator LJ. PhD thesis. Cornell University; 2011. The role of bioacoustics in the mating behavior of medically important mosquitoes. [Google Scholar]
- Clements AN. The Biology of Mosquitoes: Sensory Reception and Behavior. New York: CABI Publishing; 1999. [Google Scholar]
- Cox DR. Regression models and life-tables (with discussion) Journal of the Royal Statistical Society B. 1972;34:187–220. [Google Scholar]
- Cox DR, Oaks D. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- Fox J. Linear Mixed Models. In: Fox J, editor. An R and S-Plus Companion to Applied Regression. London: Sage Publications; 2002. Appendix. [Google Scholar]
- Gilburn AS, Day TH. Evolution of Female Choice in Seaweed Flies: Fisherian and Good Genes Mechanisms Operate in Different Populations. Proceedings of the Royal Society B. 1994;255:159–165. [Google Scholar]
- Gray DA. Female house crickets, Acheta domesticus, prefer the chirps of large males. Animal behaviour. 1997;54:1553–1562. doi: 10.1006/anbe.1997.0584. [DOI] [PubMed] [Google Scholar]
- Gwynne DT. Mate selection by female katydids (Orthoptera: Tettigoniidae, Conocephalus nigropleurum) Animal Behaviour. 1982;30:734–738. [Google Scholar]
- Gwynne DT. Courtship feeding increases female reproductive success in bush crickets. Nature. 1984;307:361–366. [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? Journal of Medical Entomology. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Hartberg WK. Observations on the mating behaviour of Aedes aegypti in nature. Bulletin of the World Health Organization. 1971;45:847–850. [PMC free article] [PubMed] [Google Scholar]
- Hasselquist D, Bensch S, Von Schantz T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature. 1996;381:229–232. [Google Scholar]
- Hedrick AV. Female choice and the heritability of attractive male traits: an empirical study. American Naturalist. 1988;132:267–276. [Google Scholar]
- Hoikkala A, Aspi J, Suvanto L. Male courtship song frequency as an indicator of male genetic quality in an insect species, Drosophila montana. Proceedings of the Royal Society of London B. 1998;265:503. doi: 10.1098/rspb.1998.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm CH. Breeding sex ratios, territoriality and reproductive success in the red-winged black bird (Agelaius pheoniceus) Ecology. 1973;54:356–365. [Google Scholar]
- Jones GP. Spawning-site choice by female Pseudolabrus celidotus (Pices: Labridae) and its influence on the mating system. Behavioral Ecology and Sociobiology. 1981;8:129–142. [Google Scholar]
- Jones J, Pilitt D. Observations on the sexual behavior of free-flying Aedes aegypti mosquitoes. Biological Bulletin. 1973;144:480–488. [Google Scholar]
- Jones TM, Quinnell RJ, Balmford A. Fisherian flies: benefits of female choice in a lekking sandfly. Proceedings of the Royal Society B: Biological Sciences. 1998;265:1651. doi: 10.1098/rspb.2000.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Brookes R, Jennions MD, Morely J. The evolution of mate choice and mating biases. Proceedings of the Royal Society B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow TA. Drosophila males provide a material contribution to offspring sired by other males. Functional Ecology. 1988;2:77–79. [Google Scholar]
- Moller AP. Effects of parasitism by a haematophagous mite on reproduction in the barn swallow. Ecology. 1990;71:2345–2357. [Google Scholar]
- Moore DF. Hybridization and mating behavior in Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 1979;16:223–226. doi: 10.1093/jmedent/16.3.223. [DOI] [PubMed] [Google Scholar]
- Moore AJ. Genetic evidence for the “good genes” process of sexual selection. Behavioral Ecology Sociobiology. 1994;35:235–241. [Google Scholar]
- Nasci RS. Relationship of wing length to adult dry weight in several mosquito species (Diptera: Culicidae) Journal of Medical Entomology. 1990;27:716–719. doi: 10.1093/jmedent/27.4.716. [DOI] [PubMed] [Google Scholar]
- Neff BD, Pitcher TE. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Molecular Ecology. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. [DOI] [PubMed] [Google Scholar]
- Nijhout HF, Craig GB. Reproductive isolation in Stegomyia mosquitoes. III Evidence for sexual pheromone. Entomological Experimentalis et Applicata. 1971;14:399–412. [Google Scholar]
- Norris KJ. Heritable variation in a plumage indicator trait of viability in male great tits Parus major. Nature. 1993;362:537–539. [Google Scholar]
- Okanda FM, Dao A, Njiru BN, Arija J, Akelo HA, Touré Y, Odulaja A, Beier JC, Githure JI, Yan G, Gouagna LC, Knols BGJ, Killeen G. Behavioural determinants of gene flow in malaria vector populations: Anopheles gambiae males select large females as mates. Malaria Journal. 2002;1:10. doi: 10.1186/1475-2875-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennetier C, Warren B, Dabiré KR, Russell IJ, Gibson G. “Singing on the wing” as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Current Biology. 2010;20:131–136. doi: 10.1016/j.cub.2009.11.040. [DOI] [PubMed] [Google Scholar]
- Petrie M. Improved growth and survival of offspring of peacocks with more elaborate trains. Nature. 1994;371:598–599. [Google Scholar]
- Polerstock AR, Eigenbrode SD, Klowden MJ. Mating alters the cuticular hydrocarbons of female Anopheles gambiae sensu stricto and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2002;39:545–552. doi: 10.1603/0022-2585-39.3.545. [DOI] [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2007;44:422–426. doi: 10.1603/0022-2585(2007)44[422:aabsim]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. Factors associated with male mating success of the dengue vector mosquito, Aedes aegypti. American Journal of Tropical Medicine and Hygiene. 2009;80:395–400. [PubMed] [Google Scholar]
- Reynolds JD, Gross MR. Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proceedings of the Royal Society B. 1992;250:57–62. [Google Scholar]
- Roth LM. A study of mosquito behavior: an experimental laboratory study of sexual behavior of Aedes aegypti (Linnaeus) The American Midland Naturalist. 1948;40:265–348. [Google Scholar]
- Shaw KL, Herlihy DP. Acoustic preference functions and song variability in the Hawaiian cricket Laupala cerasina. Proceedings of the Royal Society B. 2000;267:577–584. doi: 10.1098/rspb.2000.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons LW. Heritability of a male character chosen by females of the field cricket, Gryllus bimaculatus. Behavioral Ecology Sociobiology. 1987;21:129–133. [Google Scholar]
- Steele RH. Courtship feeding in Drosophila subobsura. I. The nutritional significance of courtship feeding. Animal Behaviour. 1986;34:1087–1098. [Google Scholar]
- Thornhill R. Sexual selection and nuptial feeding behavior in Bittacus apicalis (Insecta: Mecoptera) American Naturalist. 1976;110:529–548. [Google Scholar]
- Thornhill R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. American Naturalist. 1983;122:765–788. [Google Scholar]
- Tregenza T, Simmons LW, Wedell N, Zuk M. Female preference for male courtship song and its role as a signal of immune function and condition. Animal Behaviour. 2006;72:809–818. [Google Scholar]
- Tuckerman JF, Gwynne DT, Morris GK. Reliable acoustic cues for female mate preference in a katydid (Scudderia curvicauda, Orthoptera: Tettigoniidae) Behavioral Ecology. 1993;4:106–113. [Google Scholar]
- Warren B, Gibson G, Russell IJ. Sex Recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Current Biology. 2009;19:485–491. doi: 10.1016/j.cub.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Wedell N, Tregenza T. Successful fathers sire successful sons. Evolution. 1999;53:620–625. doi: 10.1111/j.1558-5646.1999.tb03797.x. [DOI] [PubMed] [Google Scholar]
- Welch AM, Semlitsch RD, Gerhardt HC. Call duration as an indicator of genetic quality in male gray tree frogs. Science. 1998;280:1928. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- Wells K. Territoriality and male mating success in the green tree frog (Rana clamitans) Ecology. 1977;58:750–762. [Google Scholar]
- Yuval B. Mating systems of blood-feeding flies. Annual Reviews in Entomology. 2006;51:413–440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- Zsemlye JL, Hancock RG, Foster WA. Analysis of a complex vertical copulatory-courtship display in the yellow fever vector Sabethes chloropterus. Medical and Veterinary Entomology. 2005;19:276–285. doi: 10.1111/j.1365-2915.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- Zuk M, Simmons LW. Reproductive strategies of the crickets (Orthoptera: Gryllidae) In: Choe JC, Crespi BJ, editors. The Evolution of Mating Systems in Insects and Arachnids. Cambridge: Cambridge University Press; 1997. pp. 89–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video footage of mating attempts in Ae. aegypti. Males attempted to mate with flying tethered females. In the first clip, the female held the male away from her body using her legs. In the next attempt, the male was able to position his body in the appropriate position for copulation, but the female tilted her abdomen away and prevented genital contact. In the third and forth clips, females rejected males by kicking them away in flight. In the final clip, the female accepted the male and there was firm and sustained genital contact, which is associated with seminal fluid transfer. All footage is shown at actual speed.