Abstract
Nicotine influences cognition and behavior, but the mechanisms by which these effects occur are unclear. By using positron emission tomography, we measured cognitive activation (increases in relative regional cerebral blood flow) during a working memory task [2-back task (2BT)] in 11 abstinent smokers and 11 ex-smokers. Assays were performed both after administration of placebo gum and 4-mg nicotine gum. Performance on the 2BT did not differ between groups in either condition, and the pattern of brain activation by the 2BT was consistent with reports in the literature. However, in the placebo condition, activation in ex-smokers predominated in the left hemisphere, whereas in smokers, it occurred in the right hemisphere. When nicotine was administered, activation was reduced in smokers but enhanced in ex-smokers. The lateralization of activation as a function of nicotine dependence suggests that chronic exposure to nicotine or withdrawal from nicotine affects cognitive strategies used to perform the memory task. Furthermore, the lack of enhancement of activation after nicotine administration in smokers likely reflects tolerance.
Nicotine affects cognition and behavior (1). In smokers and nonsmokers, it produces small improvements in finger-tapping rate, motor response on tests of focused and sustained attention, and recognition memory (2). In smokers, tobacco deprivation can impair cognitive performance, and subsequent nicotine administration or smoking can reverse these deficits (3–5). Thus, nicotine-reversible performance deficits in withdrawal may help sustain nicotine dependence (1). To elucidate the neuroanatomical substrates of the effects of nicotine on cognition, we used a test of working memory and positron emission tomography (PET), which permits the visualization of regional brain function (6). Our ultimate objective is to clarify the effects of nicotine on brain function in the hope that such information might improve our knowledge of the mechanisms of nicotine dependence.
Methods
Subjects.
A total of 11 smokers and 11 ex-smokers completed the study. All subjects gave written informed consent after receiving an explanation of the study and its procedures. Inclusion criteria were: age between 21 and 45 years, IQ > 85 (assessed by the Shipley Institute of Living Scale; ref. 7), and right handedness. Smokers had a history of smoking for at least 2 years, with current use > 20 cigarettes per day and a score >5 on the Fagerström test for nicotine dependence (range 0–10; ref. 8). For ex-smokers, prior exposure to nicotine without current smoking (nicotine abstinence for more than 1 month and no evidence of nicotine dependence for more than 3 years before the study) was required. History of exposure to nicotine was required to minimize the occurrence of side effects from nicotine gum. Exclusion criteria were current psychopathology (symptom checklist-90; ref. 9), history of psychiatric disorders (diagnostic interview schedule; ref. 10) including substance-abuse disorders other than nicotine dependence, and evidence of acute or chronic medical problems (medical history, physical examination, and routine blood screen).
Study Design.
Each subject participated in two PET assays 1-week apart. In one PET session, subjects received nicotine gum; in the other, they received placebo gum. Administration of the gum was double-blind with order randomized across subjects. In each session, regional cerebral blood flow (rCBF) was measured during the performance of the 2-back task (2BT) and a control task. Smokers were instructed not to smoke for 12 h and avoid taking nonprescription medications for 24 h before the PET study. To prevent caffeine withdrawal, caffeine users were instructed to drink only one-half cup of a caffeine-containing beverage no less than 2 h before the study.
Nicotine Administration.
Before each test session (20 min), subjects received two pieces of polacrilex gum to chew for 15 min at a rate of one chew every 3 sec. Each piece of gum contained either nicotine (2 mg Nicorette) or taste-matched placebo. Peak plasma concentrations of nicotine using this procedure are similar to those achieved by smoking a typical American cigarette (11).
Expired-air CO levels were recorded on arrival at the PET center. A level >10 ppm, which reflected recent smoking, was the criterion for disqualifying a subject. No subject had a CO level >5 ppm. Vital signs, nicotine withdrawal (Minnesota nicotine-withdrawal scale (MNWS; ref. 12), and side effects [subjective treatment emergent-symptom scale (STESS); ref. 13] were recorded before and after drug administration. Of the 32 items of the STESS, four symptoms were selected on the basis of previous reports of side effects from nicotine gum: difficulty paying attention, stomachaches, dizziness, and shakiness. A measure of state anxiety [state trait anxiety index (STAI); ref. 14] was obtained before gum administration. Blood samples were collected before and 30 min after the 15-min period of chewing the gum for assays of plasma cotinine and nicotine concentration. Assays were performed by liquid extraction and gas chromatography (Labstat, Ontario, Canada).
Working-Memory Task.
The 2BT required subjects to keep in memory a series of three letters that were being updated constantly. The letters were 2.5 cm high and presented one at a time in the middle of a screen placed at 45 cm from the subject's eyes. They appeared for 500 msec at intervals of 1,000 msec. The subjects were instructed to press a “target” button (Psyscope button box, Research Methods, Pittsburgh, PA) whenever a letter was repeated with exactly one intervening letter. When any other letter appeared, the instruction was to press a “nontarget” button. This way, the number of finger movements remained constant, independent of performance. The button was always pressed with the right hand. In the control condition, subjects performed a sensorimotor control task, similar to the 2BT except that the target button is pressed in response to the letter “x,” and the nontarget button for all other letters. Subjects were trained to criterion (75–80% correct responses) on the 2BT in an earlier session to obviate a learning effect. Scans were acquired under three conditions: resting (eyes fixated on a dot at the center of the screen), active task (2BT), and control task. The resting condition was included for use in a future protocol that would measure absolute rCBF but was not used in the present data analysis. In each PET session, six 1-min scans were acquired. The order of conditions was: rest, 2BT, control, 2BT, control, rest or rest, control, 2BT, control, 2BT, and rest. This order was counterbalanced across subjects but kept constant for each subject. Each task began 30 sec before an i.v. bolus injection of 30 mCi (1 Ci = 37 GBq) of O-15-labeled water and continued for 4.5 min.
PET.
The determination of rCBF by PET involves the administration of a freely diffusible positron-emitting radiotracer such as O-15-labeled water. Because of the short half-life of 15O (2.05 min), repeated PET measurements can be performed every 10–12 min. H215O is administered by i.v. bolus, and a 60-sec scan is obtained after the radiotracer reaches the brain (15, 16). The relationship between radioactivity counts in brain and rCBF is almost linear, and the PET image closely reflects perfusion differences between brain regions, providing a measure of relative rCBF. PET scans were performed on a Scanditronix (Milwaukee, WI) PC2048–15B brain tomograph. This instrument produces images of 15 contiguous transaxial slices 6.5-mm thick each. The within-plane resolution (full width at half maximum) is 6.5 mm. A thermoplastic mask, custom-made for each subject, was used to minimize head movement. Radioactive counts were recorded for 1 min after brain activity reached a threshold value of 8,000 counts. Scan data were reconstructed with corrections for attenuation (measured with transmission scans). Because arterial blood was not sampled, absolute rates of rCBF were not determined.
Data Analysis.
Demographic, physiological, subjective, and cognitive variables.
Demographic and subjective variables were compared between smokers and ex-smokers by using Student's t test. Physiological variables (blood pressure and pulse) were analyzed by a multivariate ANOVA testing the effects of “group” (between-subjects factor, smokers and ex-smokers), “drug” (within-subjects factor, placebo and nicotine), and “time” (within-subjects factor, pregum vs. 30 min postgum). Plasma nicotine and cotinine concentrations at baseline and 30 min after gum administration were analyzed by two ANOVAs with group and time as between-subjects and within-subjects factors, respectively.
For the 2BT, three different sets of performance scores were analyzed: accuracy (percentage of correct responses and percentage of incorrect responses), reaction time (RT to all stimuli and RT to correct responses), and RT variability (RT variability to all stimuli and RT variability to correct responses). The standard deviation of the mean RT for each subject was used as a measure of variability. This measure tracks sustained attention and reflects consistency in psychomotor speed. Multivariate ANOVA was used to estimate the effect of group and drug on cognitive performance.
Brain-imaging data.
To correct for head motion between scans, all PET data for each subject were coregistered by using the AUTOMATED IMAGE REGISTRATION program (17). The mean of the counts of all voxels common to all registered scans of an individual (i.e., global counts) was used to normalize regional data (proportional scaling). By using statistical parametric mapping (18), the registered data were resized and reshaped to a stereotaxic atlas (19) to facilitate interscan analysis. Data were then smoothed with a three-dimensional Gaussian filter (10 mm, 10 mm, and 10 mm in the x, y, and z planes) to reduce high-frequency noise and the effects of individual differences in gyral anatomy. Finally, a voxel-by-voxel analysis was performed for all planes common to all subjects (from 28 mm below to 54 mm above the intercommissural line). Activations were analyzed by testing the rCBF differences between the 2BT and the control task for each drug condition in each group. For each planned analysis, the value of t for each voxel was calculated and transformed to a normal standard distribution. Maps of the z statistics showing all voxels significantly activated at P < 0.005 within a cluster of an extent threshold of P < 0.05 were computed, and stereotaxic coordinates of the epicenters (i.e., maxima) of areas of significance were determined. Prefrontal, cingulate, and parietal areas were predicted to be activated by the 2BT (20, 21).
Correlation analyses were performed between activated pixels and clinical variables (performance scores, anxiety and nicotine-withdrawal ratings, and plasma concentration of nicotine and cotinine). Scores of nicotine withdrawal, not experienced by ex-smokers, were not used for analysis in ex-smokers; similarly, nicotine and cotinine concentrations were not used for analysis in ex-smokers during placebo, because they were nicotine-free. For each subject, four mean images of the two repeated scans of each condition (placebo-control task, placebo 2BT, nicotine-control task, and nicotine 2BT) were computed by using the statistical parametric mapping adjusted-mean module. Then, by using MEDx (Sensor Systems, Sterling, VA) calculator function, difference images (2BT minus control task) were calculated for each subject. Finally, maps of Pearson coefficient correlation (r) and their corresponding z maps were computed for each group in each drug condition by using MEDx correlation-analysis module. Maxima of peak correlations were collected only in the regions that were activated by the 2BT (prefrontal, cingulate, and parietal cortices). The criterion for significance was set at P < 0.05, corrected for the number of tests (five correlations in each group, P < 0.01, z < 2.33, r > 0.68). Because of the relatively small sample sizes and the absence of corrections for the number of voxels tested, these correlations are exploratory and must be interpreted with caution. Although these analyses may be helpful in understanding the functional correlates of the areas activated during placebo, their significance becomes complex in the nicotine condition and will not be discussed to avoid excessive speculation.
Results
Sample.
The 11 smokers (age 31.8 ± 6.8 years; male/female 5:6; socioeconomic status 2.4 ± 3.0) and 11 ex-smokers (age 30.2 ± 7.1 years; male/female 6:5; socioeconomic status 2.9 ± 3.7) did not score significantly differently on the Shipley IQ test (smokers 30.6 ± 5.4, ex-smokers 33.6 ± 5.1) and psychopathology index (symptom checklist-90: smokers 70.6 ± 29, ex-smokers 59.8 ± 38). Mean score of nicotine dependence (Fagerström test for nicotine dependence) was 6.9 ± 1.5 for smokers. Smokers reported smoking, on average, 33.9 cigarettes per day (SD 9.7, range 20–45) for a mean duration of 15.9 years (SD 6.7, range 6–24 years). Ex-smokers had smoked previously an average of 3.2 cigarettes per day (SD 6.4, range <1–10) for a mean duration of 8.6 years (SD 8.2, range <1–23 years) and had been abstinent for an average of 6.4 years (SD 8.3).
Plasma Nicotine and Cotinine.
Plasma concentrations of nicotine and cotinine were in the expected range for 12-h nicotine-abstinent smokers (nicotine 4.4 ± 3.5 ng/ml, cotinine 195.4 ± 106.3 ng/ml) and ex-smokers (nicotine 2.3 ± 3.4 ng/ml, cotinine 9.2 ± 11.6 ng/ml) during the placebo condition (11, 22). Although the mean plasma concentration of nicotine in smokers exceeded that in ex-smokers during placebo, this difference was not significant. Plasma nicotine concentration essentially doubled from baseline to 30 min after nicotine administration in ex-smokers (3.0 ± 3.1–6.2 ± 3.6 ng/ml, P = 0.004) and smokers (4.5 ± 4.3–8.9 ± 5.1 ng/ml, P = 0.037). There was no statistical difference of these increases between smokers and ex-smokers. Plasma cotinine concentration increased significantly between baseline and 30 min after nicotine administration in ex-smokers (5.9 ± 7.3–11.5 ± 9.9 ng/ml, P = 0.03) but not in smokers (210.6 ± 115.1–201.6 ± 122.9 ng/ml).
Autonomic and Subjective Measures (Table 1).
Table 1.
Mean ± SD of physiological and self-report measures
| Placebo
|
Nicotine
|
|||
|---|---|---|---|---|
| Pre-gum | Post-gum | Pre-gum | Post-gum | |
| Smokers (n = 11) | ||||
| SYS | 114.2 ± 10.7 | 119.3 ± 10.6 | 118.3 ± 10.7 | 120.1 ± 11.6 |
| DIAS | 69.3 ± 9.5 | 75.9 ± 5.8 | 74.7 ± 4.0 | 77.2 ± 8.1 |
| Pulse | 72.0 ± 10.8 | 69.7 ± 9.4 | 74.2 ± 9.2 | 72.8 ± 10.7 |
| Anxiety* | 38.0 ± 11.0 | —† | 39.9 ± 11.6 | —† |
| SE‡ | 0.3 ± 0.5 | 0.5 ± 0.8 | 0.4 ± 0.5 | 0.3 ± 0.4 |
| WD§ | 28.1 ± 18.7 | 31.6 ± 19.5 | 29.8 ± 16.2 | 26.3 ± 11.4 |
| Ex-Smokers (n = 11) | ||||
| SYS | 115.0 ± 10.7 | 118.4 ± 12.4 | 114.6 ± 10.7 | 113.9 ± 12.3 |
| DIAS | 70.4 ± 7.3 | 72.4 ± 7.2 | 71.2 ± 5.9 | 70.0 ± 8.2 |
| Pulse | 67.4 ± 8.3 | 66.4 ± 13.1 | 64.0 ± 11.2 | 65.9 ± 9.4 |
| Anxiety | 23.7 ± 6.4 | —† | 24.5 ± 7.2 | —† |
| SE | 0.3 ± 0.3 | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.3 ± 0.5 |
| WD | 3.6 ± 4.6 | 4.0 ± 4.0 | 3.7 ± 5.0 | 6.0 ± 4.7 |
SYS, systolic BP; DIAS, diastolic BP; SE, side effects; WD, withdrawal.
State trait anxiety index.
Not measured.
Subjective Treatment Emergent Symptom Scale.
Minnesota nicotine-withdrawal scale.
Vital signs.
Drug or group effects, or drug × group interaction on systolic and diastolic blood pressure were not significant statistically. However, there was an interaction of time × group on pulse (F = 7.6, df = 1,18, P = 0.013), indicating that after nicotine administration, pulse increased in ex-smokers and decreased in smokers.
Side effects of nicotine [four-item subjective treatment emergent-symptom scale (STESS)].
There was a group × drug × time interaction on the STESS (F = 6.36, df = 1,20, P = 0.02): after placebo, side effects decreased in ex-smokers and increased in smokers between baseline and 1 h after gum administration, whereas after nicotine, side effects increased in ex-smokers and decreased in smokers.
STAI.
Smokers scored significantly higher than ex-smokers on the STAI (F = 14.5, df = 1,20, P = 0.001), which was administrated only before the gum. Because STAI scores were highly correlated with MNWS-withdrawal scores in smokers (0.80 < r < 0.93), the STAI scores likely reflected effects of nicotine deprivation.
Subjective ratings of withdrawal (smokers only).
There was a trend for a time × gum interaction (F = 3.84, df = 1,10, P = 0.078) on the MNWS: scores increased after placebo but decreased after nicotine in smokers.
Memory performance (Table 2).
Table 2.
Mean ± SD of accuracy and reaction time (msec) on 2BT
| Measure | Placebo | Nicotine |
|---|---|---|
| Smokers (n = 11) | ||
| PCT correct† | 88.3 ± 4.3 | 89.9 ± 5.5* |
| PCT incorrect‡ | 11.2 ± 4.6 | 9.8 ± 5.3* |
| RT all§ | 456.3 ± 78.7 | 431.7 ± 59.8 |
| SD-RT all¶ | 96.9 ± 29.7 | 85.7 ± 19.3 |
| RT c‖ | 529.6 ± 118.0 | 517.4 ± 121.3 |
| SD-RT c** | 162.8 ± 41.9 | 149.2 ± 30.1* |
| Ex-smokers (n = 11) | ||
| PCT correct | 89.0 ± 9.1 | 85.5 ± 15.6 |
| PCT incorrect | 12.4 ± 7.5 | 10.7 ± 6.6 |
| RT all | 447.7 ± 80.5 | 440.3 ± 76.3 |
| SD-RT all | 106.6 ± 41.3 | 92.2 ± 27.0 |
| RT c | 481.9 ± 137.1 | 512.4 ± 128.2 |
| SD-RT c | 151.7 ± 62.5 | 157.6 ± 59.5 |
, P < 0.05, placebo vs. nicotine.
Percentage of correct responses.
Percentage of incorrect responses.
Reaction time to all stimuli.
Variability (SD) of reaction time to all stimuli.
Reaction time to correct responses.
Variability (SD) of reaction time to correct responses.
Overall, the effects of history of smoking (group) and nicotine (drug) on memory performance were weak. Across groups, nicotine improved performance compared with placebo: it reduced the percentage of incorrect responses (drug: F = 5.1, df = 1,20, P = 0.04) and the variability of RT to all stimuli (drug: F = 4.6, df = 1,20, P = 0.05). The other variables, percentage of correct responses and RT, did not show significant differences in the effects of nicotine compared with those of placebo. In other words, nicotine improved performance accuracy and consistency of RT, but not RT per se. Across drug treatment, differences in performance between ex-smokers and smokers were not significant. The drug × group interaction on RT to correct responses did not reach statistical significance (F = 3.7, df = 1,20, P = 0.07): compared with placebo, nicotine increased RT in ex-smokers and decreased it in smokers. Differences in performance scores between the nicotine and placebo conditions were significant only for smokers, i.e., nicotine improved accuracy and reduced variability in RT (P < 0.05 and P < 0.03, respectively by post hoc Student's t tests). These findings suggest that nicotine improved accuracy and consistency in RT of memory performance of smokers but not of ex-smokers.
Brain Activation During 2BT Performance (Table 3 and Fig. 1).
Table 3.
Statistical parametric mapping activation (2BT minus control task)
| Cluster P | Cluster size, no. of voxels | Peak P | Coordinates (x, y, z) | Location | |
|---|---|---|---|---|---|
| Placebo | |||||
| Ex-smokers | 0.009 | 386 | 0.001 | 8, 14, 30 | 24 *CING right |
| 0.002 | 0, 12, 52 | 6 GFD right | |||
| 0.002 | −2, 18, 44 | 6 GFD left | |||
| 0.016 | 314 | 0.000 | −48, 6, 34 | 9 GFM left | |
| 0.001 | −46, 12, 26 | 44 GFI left | |||
| 0.004 | −38, 16, 20 | ||||
| 0.022 | 281 | 0.000 | −42, 30, 30 | 9 GFM left | |
| 0.037 | 226 | 0.000 | −30, −2, 54 | 6 GFM left | |
| Smokers | 0.002 | 610 | 0.000 | 10, 26, 32 | 32 CING right |
| 0.000 | 2, 26, 44 | 8 GFD right | |||
| 0.000 | 10, 8, 46 | 32 CING right | |||
| 0.015 | 337 | 0.000 | 38, 44, 18 | 46 GFM right | |
| 0.022 | 291 | 0.000 | 50, −50, 42 | 40 LPI right | |
| 0.032 | 247 | 0.000 | 44, 10, 34 | 9 GFM right | |
| Nicotine | |||||
| Ex-smokers | 0.000 | 804 | 0.000 | 34, −4, 52 | 6 GFM right |
| 0.000 | 46, −8, 44 | 4 GPRC right | |||
| 0.001 | 52, 6, 30 | 44 GFI right | |||
| 0.002 | 598 | 0.000 | 40, 32, 30 | 40 GFM right | |
| 0.000 | 40, 44, 16 | 46 GFM right | |||
| 0.003 | 44, 50, 4 | 10 GFM right | |||
| 0.002 | 570 | 0.000 | −44, 2, 34 | 6 GFM left | |
| 0.001 | −26, 0, 21 | 6 GFM left | |||
| 0.003 | −40, −2, 48 | ||||
| 0.004 | 480 | 0.000 | 48, −48, 40 | 40 LPI right | |
| 0.000 | 32, −72, 38 | 19 CU right | |||
| 0.000 | 34, −52, 40 | ||||
| 0.005 | 468 | 0.000 | −28, −54, 42 | 40 LPI left | |
| 0.000 | −28, −62, 38 | 19 LPI left | |||
| 0.000 | −46, −34, 42 | ||||
| 0.01 | 373 | 0.000 | −40, 34, 24 | 9/46 GFM left | |
| 0.001 | −34, 48, 18 | 46 GFM left | |||
| 0.03 | 233 | 0.000 | −4, 18, 48 | 8 GFD left | |
| Smokers | 0.015 | 333 | 0.000 | 48, 6, 28 | 44 GFI right |
| 0.001 | 50, 16, 24 | 44 GFI right | |||
| 0.015 | 338 | 0.000 | −44, 6, 28 | 44 GFI left | |
| 0.001 | −38, 28, 26 | 46 GFM left |
CING, cingulate cortex; GFM, middle frontal gyrus; CU, cuneus; GFS, superior frontal gyrus; GFD, medial superior frontal lobe; GORC, precentral gyrus; GFI, inferior frontal gyrus; LPI, inferior parietal lobule.
Brodmann areas.
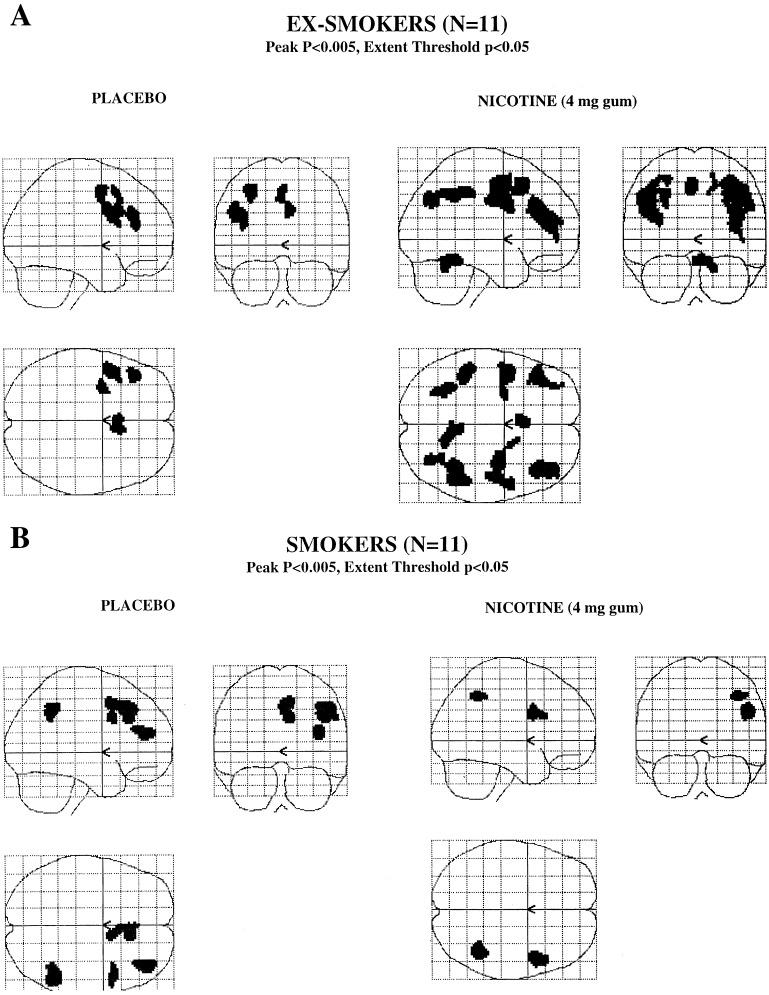
Figure 1.
Statistical parametric maps showing brain activation during performance of the 2BT by ex-smokers (Upper) and smokers (Lower) (n = 11 per group) when given placebo or 4-mg nicotine gum (Left and Right, respectively). Maps of the z statistics show all voxels significantly activated at P < 0.005 (peak) within a cluster of an extent threshold of P < 0.05.
In ex-smokers, the memory component of the 2BT (2BT minus control task) activated the right anterior cingulate gyrus and the left prefrontal cortex [Brodmann areas (BA) 6, 8, and 9] after placebo gum. After nicotine gum, the anterior cingulate gyrus ceased to be activated significantly. In contrast, activation in the prefrontal cortex was enhanced both in extent (number of voxels activated: during placebo, 1,207; during nicotine, 3,526) and strength of statistical significance (see Table 3). The left and right inferior parietal areas (BA 40) also were recruited after nicotine.
Similarly, smokers showed prefrontal activation (BA 8,9,46) after placebo gum (i.e., during nicotine abstinence). However, activation was restricted to the right hemisphere, in contrast to the findings in ex-smokers, in whom activation was restricted to the left hemisphere. As in ex-smokers, the right anterior cingulate gyrus was activated. In addition, smokers showed recruitment of the right inferior parietal cortex (BA 40). After nicotine gum, cognitive activation was limited mainly to the inferior frontal gyrus (BA 44); and as in ex-smokers, the cingulate cortex was no longer activated. Unlike ex-smokers, in whom nicotine enhanced the activation during 2BT, smokers showed a diminished activation (number of voxels activated: during placebo, 1,485; during nicotine, 671).
Correlations Between Clinical Variables and Brain Activation During 2BT.
Performance scores.
In ex-smokers during placebo, percentage of correct responses was correlated with left midprefrontal activation (BA 46, z = 3.77). Mean RT was not correlated with any activated areas. During nicotine, the percentage of correct responses was correlated with right anterior cingulate activation (BA 24/32, z = 2.71). Mean RT was correlated negatively with activation of right and left mid- and inferior prefrontal (BA 44,45, and 46,9,10, z = −2.35 to −3.16) and right inferior parietal cortices (BA 40, z = −2.85). In smokers during placebo, percentage of correct responses was correlated positively with right inferior frontal activation (BA 44, z = 3.09). Mean RT was not correlated with any activated areas. During nicotine, neither percentage of correct responses nor mean RT was correlated significantly with any brain activations.
Anxiety (ex-smokers only) and nicotine withdrawal (smokers only).
In ex-smokers during placebo, STAI scores were not correlated with any of the left prefrontal or anterior cingulate activations. During nicotine, STAI scores were correlated negatively with right inferior prefrontal activation (BA 46, z = −2.54). In smokers during placebo, MNWS scores were correlated with right cingulate activation (BA 32, z = 2.92) and negatively with right midprefrontal (BA 46,10, z = −3.31) and right inferior parietal activations (BA 40, z = −3.12). During nicotine, MNWS scores were not correlated with any brain activations.
Plasma nicotine and cotinine.
In ex-smokers during nicotine, plasma nicotine concentration was correlated negatively with left midprefrontal activation (BA 9,44, z = −3.12). Cotinine concentration was not correlated with activation of any brain regions. In smokers during placebo, no significant correlations were found between nicotine concentration and brain activations. Cotinine concentration was correlated negatively with right midprefrontal activation (BA 46,10, z = −2.63). During nicotine, plasma nicotine concentration was correlated with right inferior parietal cortex (BA 40, z = 2.73). Cotinine concentration was not correlated with any brain activations.
Discussion
Cognitive Performance.
Overall, effects of nicotine and smoking history on memory performance were weak. However, abstinent smokers but not ex-smokers showed significantly improved performance on the 2BT after nicotine gum compared with their performance after placebo gum. Such enhanced cognitive processing is consistent with reports that nicotine improved recognition memory in overnight-abstinent smokers (23). It is unclear, however, whether the nicotine-induced improvement observed in this study represents an enhancement of performance above a basal level or a relief from withdrawal, because smokers were abstinent from nicotine overnight before being tested.
The lack of effect of nicotine on working memory in ex-smokers conflicts with reports that in nonsmokers, nicotine improved recognition memory (23) and enhanced response time in a digit recall test (24). Training our subjects on the working memory task to a criterion of 75–80% correct responses before the PET sessions may have produced a ceiling effect, making it difficult for subjects to manifest improvement.
Brain Activation During Placebo.
Overall, the regions activated during performance of the working memory were consistent with those reported in the PET and functional MRI literature (20, 21), i.e., dorsolateral prefrontal, anterior cingulate, and inferior parietal areas. In support of the functional link of prefrontal cortical activation with task performance, accuracy was better as activation was stronger in the prefrontal cortex in both ex-smokers and smokers. In addition, higher level of recent smoking (plasma cotinine concentration) predicted lower right midprefrontal activation in smokers, suggesting that cigarette smoking might hinder prefrontal activation, potentially resulting in depressed cognitive performance.
Regional differences in activation were seen between ex-smokers and smokers during placebo, particularly with respect to hemispheric lateralization. Whereas ex-smokers showed activation predominantly in the left hemisphere, smokers showed activation in the right hemisphere. Several factors can account for this difference: (i) use of different cognitive strategies, (ii) interaction of neural circuits involved in withdrawal symptoms with those subserving cognitive processes, and (iii) lateralization of neural activity associated with chronic exposure to nicotine.
Cognitive Strategies.
Hemispheric and regional specialization has been observed for different aspects of memory processes (see review in ref. 20). For example, attentional processes, components of working memory (e.g., retrieval vs. encoding), and processing of specific features of stimuli (e.g., lexical vs. visuospatial) all show hemispheric lateralization. Attentional processes generally are lateralized to the right hemisphere (25) and engage anterior cingulate, right prefrontal, and right parietal areas (26, 27). It is possible that smokers placed more effort on the attentional system to perform the task than ex-smokers. In addition, memory performance recruits neural networks of the left hemisphere for the processing of language-based stimuli (e.g., lexical and phonological; ref. 28) and those of the right hemisphere for the processing of visuospatial stimuli (29, 30). Ex-smokers may employ a different strategy in the 2BT than smokers, e.g., they may use a phonological strategy, whereas smokers may use a visual imagery strategy.
The anterior cingulate gyrus was the area most strongly activated in both ex-smokers and smokers. This region is engaged in tasks of attention, particularly those with conflicting information (26, 31) and sustained attention (27) and tasks of memory of time order (32). The latter cognitive function is critical in the 2BT, which requires subjects to maintain in memory and to update constantly the order in which letters appear. In contrast to the cingulate activation common to both groups, the right parietal cortex (BA 40) was recruited only in smokers. This discrepancy further supports the notion that smokers performed the task by drawing resources preferentially from the neural network that mediates sustained attention and visuospatial processes comprising right prefrontal and parietal regions (25), whereas ex-smokers used resources from the phonological loop of working memory (33).
Withdrawal Symptoms.
In light of substantial evidence that emotional states involve a lateralization of brain activity (34), differences in affective states between smokers and ex-smokers may contribute to the different activation in the two groups. The emotional background that accompanies the performance of a task may influence which brain regions subserve the cognitive processes. For instance, tasks performed in depressed subjects may recruit right-sided networks more readily than tasks performed during positive mood states (34). Anxiety also is associated with right- more than left-brain activity (35–37). In the present study, anxiety in ex-smokers was not associated with any brain activation, suggesting that in basal conditions (placebo condition in control subjects), anxiety did not influence cognitive networks significantly. It is possible, however, that the state of withdrawal, characterized by negative affect and highly correlated with anxiety levels, could increase the participation of the right hemisphere in cognitive demands. An association, although negative, was found between severity of nicotine withdrawal in smokers and activation of the right midprefrontal cortex and right inferior parietal cortex. These negative correlations indicate a functional link between withdrawal and cognitive activation of the right hemisphere as well as a potential deleterious influence of withdrawal on cognitive processing, because lower activation predicted worse performance. Of interest, the anterior cingulate was activated in both smokers and ex-smokers, but the activation was associated positively with severity of withdrawal in smokers. This finding supports the view that the anterior cingulate is recruited in tasks with substantial demands on attention, given that task performance is likely more taxing in withdrawal than in smoking satiety.
Chronic Exposure to Nicotine.
The cognitive demands of the task and the history of chronic exposure to nicotine also can affect which neurotransmitter systems are recruited. Indeed, noradrenergic and cholinergic systems are involved in the modulation of attention and vigilance, and the dopaminergic system is involved in the regulation of higher executive function (see review in ref. 38). Chronic exposure to nicotine alter these neurochemical systems (39) and thereby can affect the neural substrates of working memory. The putative lateralization of neurotransmitter systems (40, 41) could contribute to the present finding of differential lateralization of cognitive activation in function of history of nicotine exposure.
Brain Activation After Nicotine Gum.
The diminished activation in smokers compared with enhanced activation in ex-smokers after nicotine gum is the first evidence of tolerance to nicotine measured directly in the human brain. Previous studies have shown autonomic and behavioral tolerance to nicotine, indicated by reduced cardiovascular, subjective, and behavioral responses to nicotine in smokers compared with nonsmokers (23).
Whereas tolerance was specific to smokers, the blockade of the cingulate activation after nicotine gum occurred in both smokers and ex-smokers. This finding is consistent with the reports of two studies of rCBF measurements paired with cognitive tasks during cholinomimetic drug challenges. These studies tested the effects of nicotine in 24-h abstinent smokers (42) and of physostigmine in subjects with unspecified smoking history (43). Both studies reported deactivation or reduced activation in the cingulate cortex when the cholinomimetic drug was given. In contrast, an fMRI study assessing the effects of i.v. nicotine on rCBF showed a dose-dependent increase in rCBF of cingulate cortex (44). This study was performed in nonabstinent smokers at rest without a cognitive challenge. Taken together, these findings indicate that cholinergic transmission influences the neural activity of the cingulate cortex. Although the direction and exact location of these effects vary across studies, our results suggest that the smoking status of the subjects (abstinent smokers vs. ex-smokers) does not influence these effects. However, the direction of the effects of nicotine on the cingulate cortex may depend on the state of activation of this region, whether it is recruited during a cognitive process or latent during rest.
In closing, it is important to address the potential direct vascular effects of nicotine on rCBF, which could confound the interpretation of changes in rCBF. Although nicotine does have vasoactive properties, purely vascular changes would not be specific to those brain regions that predictably were activated by the 2BT. Given that we found only circumscribed and functionally meaningful effects (consistent with previous studies of similar cognitive activation), we assume that these rCBF alterations were of neural origin.
Acknowledgments
We thank Drs. A. Zametkin and P. Herscovitch and the staff of the Department of Nuclear Medicine, National Institutes of Health Clinical Center, for help in performing the study.
Abbreviations
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- 2BT
2-back task
- MNWS
Minnesota nicotine-withdrawal scale
- STAI
state trait anxiety index
- RT
reaction time
- BA
Brodmann area
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Heishman S J, Taylor R C, Henningfield J E. Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- 2.Heishman S J. Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- 3.Bell S L, Taylor R C, Singleton E G, Henningfield J E, Heishman S J. Nicotine Tob Res. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- 4.Snyder F R, Henningfield J E. Psychopharmacology (Berlin) 1989;97:17–22. doi: 10.1007/BF00443406. [DOI] [PubMed] [Google Scholar]
- 5.Parrott A C, Roberts G. J Psychopharmacol. 1991;5:404–409. doi: 10.1177/026988119100500435. [DOI] [PubMed] [Google Scholar]
- 6.Posner M I, Raichle M E. Proc Natl Acad Sci USA. 1998;95:763–764. doi: 10.1073/pnas.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zachary R A, Paulson M J, Gorsuch R L. J Clin Psychol. 1985;41:820–831. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Heatherton T F, Kozlowski L T, Frecker R C, Fägerström K O. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 9.Derogatis L R, Rickels K, Rock A F. Br J Psychiatry. 1976;128:280–289. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- 10.Robins L N, Helzer J E, Croughan J, Ratcliff K S. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz N L, Porchet H, Sheiner L, Jacob P. Clin Pharmacol Ther. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 12.Hughes J R, Hatsukami D. Tob Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy W. ECDEU Assessment Manual for Psychopharmacology (revised) Education, and Human Welfare, Rockville, MD: Department of Health; 1976. Publication No. (ADM) 76-338, pp. 76–338. [Google Scholar]
- 14.Spielberger C D, Gorsuch R L, Lushene R E. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 15.Herscovitch P, Markham J, Raichle M E. J Nucl Med. 1983;24:782–789. [PubMed] [Google Scholar]
- 16.Raichle M E, Martin W R W, Herscovitch P, Mintun M A, Markham J. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 17.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Friston K J, Frith C D, Liddle P F, Frackowiak R S J. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 19.Talairach J, Tournoux P. Co-Planar Sterotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- 20.Smith E E, Jonides J. Proc Natl Acad Sci USA. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J D, Forman S D, Braver T S, Casey B J, Servan-Schreiber D, Noll D C. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 22.Leischow S J, Sachs D P, Hansen M D, Bostrom A G. Psychopharmacology (Berlin) 1995;117:125–129. doi: 10.1007/BF02245177. [DOI] [PubMed] [Google Scholar]
- 23.Perkins K A, Grobe J E, Fonte C, Goettler J, Caggiula A R, Reynolds W A, Stiller R L, Scierka A, Jacob R G. J Pharmacol Exp Ther. 1994;270:628–638. [PubMed] [Google Scholar]
- 24.Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell M A. Psychopharmacology (Berlin) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- 25.Heilman K M, Voeller K K, Nadeau S E. J Child Neurol. 1991;6:S76–S81. doi: 10.1177/0883073891006001s09. [DOI] [PubMed] [Google Scholar]
- 26.Pardo J V, Pardo P J, Janer K W, Raichle M E. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen R M, Semple W E, Gross M, Holcomb H H, Dowling M S, Nordahl T E. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:3–20. [Google Scholar]
- 28.Paulesu E, Frith C D, Frackowiak R S J. Nature (London) 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 29.Jonides J, Smith E E, Koeppe R A, Awh E, Minoshima S, Mintun M A. Nature (London) 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 30.Frackowiak R S J. Trends Neurosci. 1994;17:109–115. doi: 10.1016/0166-2236(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 31.Bench C J, Frith C D, Grasby P M, Friston K J, Paulesu E, Frackowiak R S, Dolan R J. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- 32.Nyberg L, McIntosh A R, Cabeza R, Habib R, Houle S, Tulving E. Proc Natl Acad Sci USA. 1996;93:11280–11285. doi: 10.1073/pnas.93.20.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coull J T, Frith C D, Frackowiak R S, Grasby P M. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 34.Davidson R J. In: Brain Asymmetry. Davidson R J, Hugdahl K, editors. Cambridge, MA: MIT Press; 1995. pp. 361–387. [Google Scholar]
- 35.Reivich M, Alavi A, Wolf A, Greenberg J H, Fowler J, Christman D, MacGregor R, Jones S C, London J, Shiue C, Yonekura Y. J Cereb Blood Flow Metab. 1982;2:307–319. doi: 10.1038/jcbfm.1982.32. [DOI] [PubMed] [Google Scholar]
- 36.Stapleton J M, Morgan M J, Liu X, Yung B C K, Phillips R L, Wong D F, Shaya E K, Dannals R F, London E D. J Cereb Blood Flow Metab. 1997;17:704–712. doi: 10.1097/00004647-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Reivich M, Gur R, Alavi A. Hum Neurobiol. 1983;2:25–33. [PubMed] [Google Scholar]
- 38.Coull J T. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen K, Czachura J F. Psychopharmacology (Berlin) 1997;133:343–346. doi: 10.1007/s002130050411. [DOI] [PubMed] [Google Scholar]
- 40.Barneoud P, Le Moal M, Neveu P J. Int J Neurosci. 1991;56:283–294. doi: 10.3109/00207459108985426. [DOI] [PubMed] [Google Scholar]
- 41.Glick S D, Ross D A, Hough L B. Brain Res. 1982;234:53–63. doi: 10.1016/0006-8993(82)90472-3. [DOI] [PubMed] [Google Scholar]
- 42.Ghatan P H, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Psychopharmacology (Berlin) 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- 43.Furey M L, Pietrini P, Haxby J V, Alexander G E, Lee H C, VanMeter J, Grady C L, Shetty U, Rapoport S I, Schapiro M B, Freo U. Proc Natl Acad Sci USA. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein E A, Pankiewicz J, Harsch H H, Cho J K, Fuller S A, Hoffmann R G, Hawkins M, Rao S M, Bandettini P A, Bloom A S. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]