Abstract
The essential role of prolactin (PRL) in normal mammary gland growth and differentiation has implicated this hormone in the development and progression of breast cancer. Although Stat5 is the best-characterized mediator of PRL signals, PRL also activates multiple other signals, whose roles in normal and pathologic processes are not well understood. We have shown that PRL stimulates activating protein-1 (AP-1) activity in breast cancer cells, and can cooperate with estradiol in this pathway. AP-1 modulates many processes critical for carcinogenesis, including cell proliferation, survival, transformation, invasion and angiogenesis, and is elevated in many neoplasms, including breast tumors. Here, we investigated the relationship between PRL signals to AP-1 and Stat5. We found that PRL activation of Stat5a and Stat5b, but not Stat1 or Stat3, reduced PRL signals to AP-1, without altering estradiol-induced AP-1 activity. The truncation mutant, Stat5/Δ53C, but not Stat5Y699F, was an effective inhibitor, consistent with a requirement for Stat5 dimerization and nuclear accumulation, but not its C-terminal transactivation activity. The association of Stat5 with AP-1 proteins suggests that this underlies the inhibition. Predictably, the ability of PRL to activate Stat5 and AP-1 was inversely related in mammary cell lines. Further, reduction of Stat5 protein with siRNA in T47D cells, which contain elevated Stat5, increased PRL-induced AP-1 signals, transcripts for the AP-1 target, matrix metalloproteinase-2 and associated invasive behavior. This study points to the importance of cell context in determining the spectrum of PRL-induced actions, which is critical for understanding the contributions of PRL to breast cancer.
Keywords: prolactin, breast cancer, AP-1, Stat5
Introduction
The essential role of prolactin (PRL) in normal mammary gland development and differentiation, as well as the high PRL receptor (PRLR) expression in human breast tumors, local PRL production within the mammary epithelium, and correlation between circulating PRL and breast cancer, have implicated PRL in the development and progression of this disease (reviewed in Vonderhaar, 2000; Clevenger et al., 2003; Goffin et al., 2005; Tworoger and Hankinson, 2006). The PRLR activates signals through a complex web of kinases including src and protein kinase C family members, phosphatidylinositol 3′-kinase, multiple mitogen-activated protein kinases (MAPKs), and the best-studied pathway, Jak2/Stat5 (reviewed in Shemanko and Groner, 2001; Goffin et al., 2005). However, the different roles of these kinase cascades in PRL signals to the normal gland, and the effect of neoplastic changes on their relative importance, are not well understood.
Stat5, particularly Stat5a, mediates most of the actions of PRL in alveologenesis (reviewed in Ormandy et al., 2001; Hennighausen and Robinson, 2005). PRL-activated Jak2 rapidly phosphorylates Stat5 on Tyr694 (Tyr699 in a highly conserved position in Stat5b, and among different species) resulting in dimerization, translocation to the nucleus, and subsequent participation in transcriptional regulation (reviewed in Shemanko and Groner, 2001; Goffin et al., 2005). Stat5 contributes to the development of mammary cancer in several murine models (Humphreys and Hennighausen, 1999; Iavnilovitch et al., 2002; Ren et al., 2002). High levels of activated Stat5 also are found in a substantial proportion of human breast tumors, which interestingly correlate with a better prognosis (Cotarla et al., 2004; Nevalainen et al., 2004). Recent investigations of breast cancer cell lines in vitro have shown that activated Stat5 inhibits invasion and the epithelial to mesenchymal transition (Sultan et al., 2005; Nouhi et al., 2006). Together, these studies suggest that Stat5 may play complex roles in mammary oncogenesis, augmenting tumor development, but opposing tumor progression.
However, other PRL-activated signals may be important in mammary disease. We have shown that PRL-induced MAPK pathways can potently activate the transcription factor, activating protein-1 (AP-1) in breast cancer cells (Gutzman et al., 2004, 2005). AP-1 proteins, including Jun and Fos family members, have been implicated in many human cancers, including breast cancer (Bland et al., 1995; Johnston et al., 1999; Gee et al., 2000; Milde-Langosch et al., 2000). Target genes modulate many cellular processes important in neoplastic progression, such as proliferation, survival, transformation, invasion and angiogenesis (reviewed in Shaulian and Karin, 2002; Eferl and Wagner, 2003).
Because of the importance of Stat5 in PRL actions in the normal mammary gland, we examined interactions of this signaling pathway with PRL-induced activation of AP-1. We found that Stat5a and Stat5b dramatically inhibited PRL activation of AP-1. This required phosphorylation of Stat5 on Tyr694/699, but not its C-terminal transactivation domain. The predicted inverse Stat5 and AP-1 responses to PRL were confirmed in multiple human mammary cell lines, and reducing Stat5 expression with siRNA in T47D cells augmented PRL-induced AP-1 activity, elevated mRNA for the matrix metalloproteinase-2 (MMP-2), an AP-1 target gene important in tumor invasion, and increased PRL-induced invasive activity. This study demonstrates the complex actions of PRL in the mammary gland, necessary to elucidate its role in breast cancer and aid in the design of new diagnostics and therapies.
Results
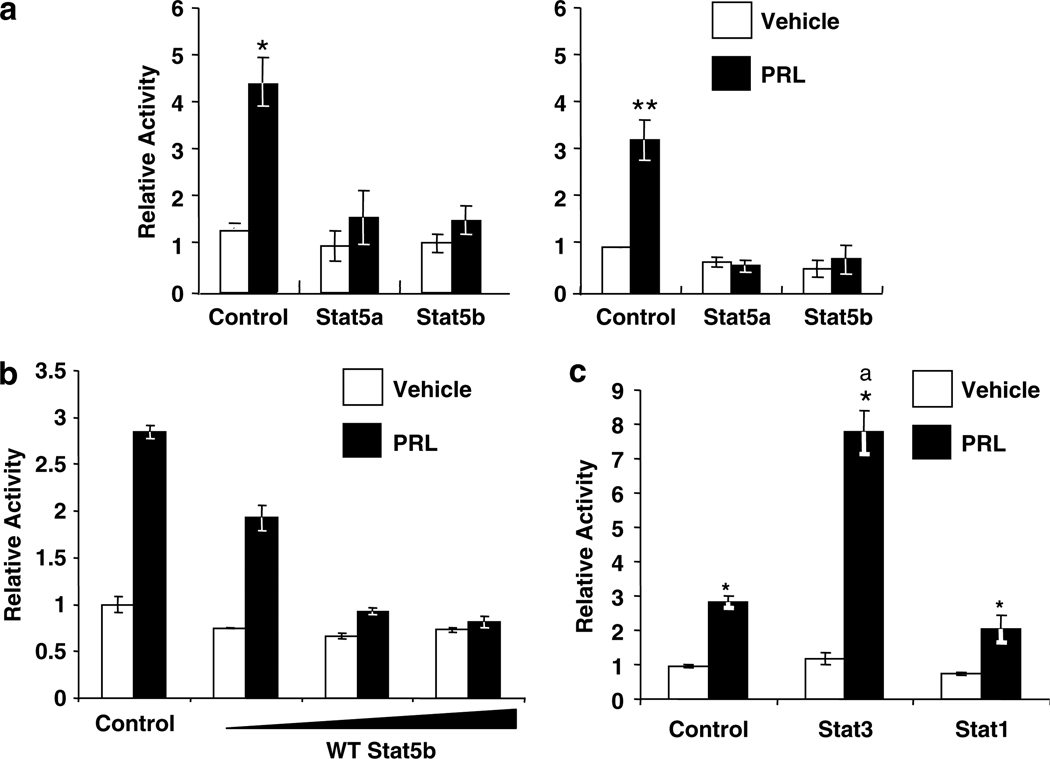
We have previously reported that PRL activates an AP-1 enhancer in PRL-deficient MCF-7 cells, which is detectable at 4 h and sustained for at least 24 h. To investigate the effect of PRL-activation of Stat5 on this activity, we overexpressed wild-type (WT) Stat5a or Stat5b, Stat1 or Stat3, which all can be activated by PRL (DaSilva et al., 1996), and examined the effect on PRL induction of a transiently co-transfected AP-1 reporter construct (4XAP-1-luc). As shown in Figure 1a, PRL strongly induced AP-1 activity, which was evident after both 6 and 24 h of exposure to the hormone, consistent with our previous results (Gutzman et al., 2004, 2005). Although normal mammary epithelial cells contain primarily Stat5a, MCF7 cells, like many other breast cancer cell lines including T47D cells, contain predominantly Stat5b (Weaver and Silva, 2006). Interestingly, overexpression of either WT Stat5a or Stat5b abolished the PRL-induced activity at both times, in a dose-dependent manner (Figure 1a and b). In contrast, WT Stat3 significantly enhanced PRL-induced activity, while WT Stat1 did not affect PRL action (Figure 1c), showing that the inhibitory action is specific for Stat5.
Figure 1.
Stat5 specifically inhibits PRL-induced AP-1 activity. MCF-7-derived cells were cotransfected with 4XAP-1-luc, lPRLR, β-galactosidase (β-gal), and eithervector DNA or (a) wild-type Stat5a (WT Stat5a) or wild-type Stat5b (WT Stat5b), (b) increasing concentrations of WT Stat5b, or (c) wild-type Stat3 (WT Stat3), or wild-type Stat1 (WT Stat1). Following transfection, cells were treated ±4 nm PRL for 6 (left) or 24 h (right) in (a), or 24 h in (b and c). Luciferase activity was determined, and normalized as described in the Materials and methods. (a and c) Relative activity indicates the mean of at least three independent experiments, shown as mean fold change relative to the vehicle treated control ±s.e.m. (b) Each bar represents the mean relative activity ±s.d. of triplicate wells from one representative experiment. Asterisks denote significant differences between vehicle and PRL-treated cells, and letters in (c) denote significant differences between PRL-treated cells transfected with different Stats using Student’s t-test (P < 0.05).
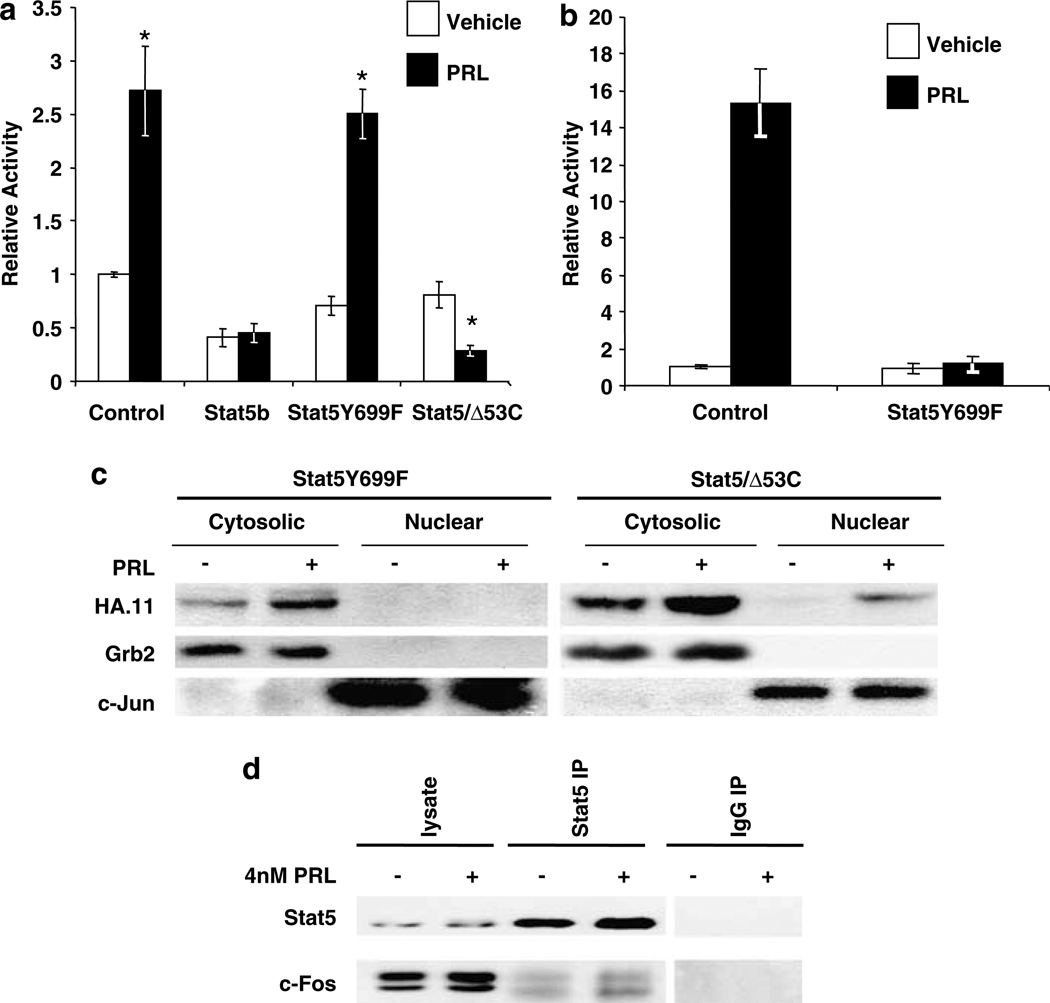
To determine how PRL-activated Stat5 mediates this inhibition, we examined the required structural determinants, employing Stat5 mutants. As shown in Figure 2a, Stat5Y699F, which is mutated at the site of Jak2 phosphorylation, the primary regulator of Stat5 dimerization and consequent nuclear translocation (Shemanko and Groner, 2001), failed to inhibit PRL-induced AP-1 activity. Control experiments confirmed that this mutant exerted dominant-negative effects on classical PRL activation of a GAS enhancer (Figure 2b), and that it was unable to translocate into the nucleus (Figure 2c). In contrast, a Stat5 mutant truncated prior to the C-terminal transactivation domain (Stat5/Δ53C) effectively blocked PRL activation of AP-1 (Figure 2a). This latter mutant is able to dimerize, translocate into the nucleus (Figure 2c) and bind DNA, but its inability to bind co-regulators also makes it a dominant negative at the GAS enhancer (Ilaria et al., 1999). Indeed, co-transfection with this mutant reduced AP-1 activity following PRL treatment to below unstimulated activity, suggesting that some Stat5 activity is required for maintaining basal AP-1 activity in these cells. Together, these mutants demonstrated that pTyr694/699 and nuclear accumulation were critical for Stat5 inhibitory activity.
Figure 2.
pTyr694/699-Stat5 is required for inhibition of PRL-induced AP-1 activity. (a) MCF-7-derived cells were co-transfected with 4XAP-1-luc, lPRLR, β-gal, and either vector DNA or WT Stat5b (Stat5b), dominant-negative Stat5b Y699F (Stat5Y699F), or truncated Stat5a Δ53C (Stat5/Δ53C). Relative activity indicates the mean of at least three independent experiments, shown as mean fold change relative to the vehicle treated control ±s.e.m. Asterisks denote significant differences between vehicle and PRL-treated cells using Student’s t-test (P < 0.05). (b) CHO cells were co-transfected with GAS-luc, lPRLR, β-gal, and either vector DNA or Stat5Y699F. Following transfection, cells were treated ± 4 nm PRL for 24 h. Luciferase activity was determined, and normalized as described in the Materials and methods. Each bar represents the relative activity ±s.d. of triplicate wells from one representative experiment. (c) Stat5Δ53C, but not Stat5Y699F, accumulates in the nucleus following PRL treatment. MCF-7 cells were transfected with either HA-Stat5 Δ53C or HA-Stat5Y699F. Following transfection, cells were treated ± 4 nm PRL for 30 min. Cytosolic and nuclear proteins were isolated as described in the Supplementary Information, and analysed by immunoblotting. Grb2 was used to mark the cytosolic fraction, and c-Jun, the nuclear fraction. Representative experiment shown. (d) Stat5 associates with c-Fos in MCF-7 cells. Serum-starved cells were treated ± 4 nm PRL for 60 min. Protein was immunoprecipitated (IP) with Stat5 or mouse IgG followed by Western analysis (WB) using c-Fos or Stat5 antibodies as shown. Representative experiment.
Both Stat1 and Stat3 have been shown to be functionally associated with AP-1 proteins (Shuai, 2000; Levy and Darnell, 2002 and references therein). Therefore, we postulated that a similar relationship with Stat5 might underlie our observations. As shown in Figure 2d, Stat5 was associated with c-Fos in unstimulated cells, and this was increased, but only slightly, in the presence of PRL. This is consistent with the low level of nuclear Stat5 observed in unstimulated cells (Luo and Yu-Lee, 2000), and the further activation following exposure to PRL. Similar results were obtained with c-Jun (data not shown). Overexpression of Stat5 did not alter PRL-induced phosphorylation and nuclear accumulation of ERK1/2 in these MCF-7-derived cells (data not shown).
We previously demonstrated that PRL and estradiol (E2) cooperatively enhance AP-1 in this system (Gutzman et al., 2005). Because E2 has been reported to modulate activity of Stat5 either positively or negatively in different cell types (Faulds et al., 2001; Bjornstrom and Sjoberg, 2002; Cao et al., 2004), we examined the effect of overexpressing Stat5 on E2 and E2/PRL signals to this transcription complex. Surprisingly, co-transfection with WT Stat5 did not affect the ability of E2 to stimulate AP-1 twofold basal levels, nor reduce the effect of E2 and PRL together below that of E2 alone (Supplementary Figure 1a), indicating that Stat5 inhibits only PRL signals without altering those initiated by E2. Since E2 has been shown to modulate phosphorylation of Stat5, increasing it in endothelial cells, but decreasing it in hepatocytes (Bjornstrom and Sjoberg, 2002; Cao et al., 2004), we also investigated the effects of these hormones on pTyr694-Stat5a/b. Our studies showed no effect of E2 alone or any interaction with PRL, consistent with our reporter gene experiments (Supplementary Figure1b), indicating that Stat5 and E2 signals to AP-1 are independent in these cells.
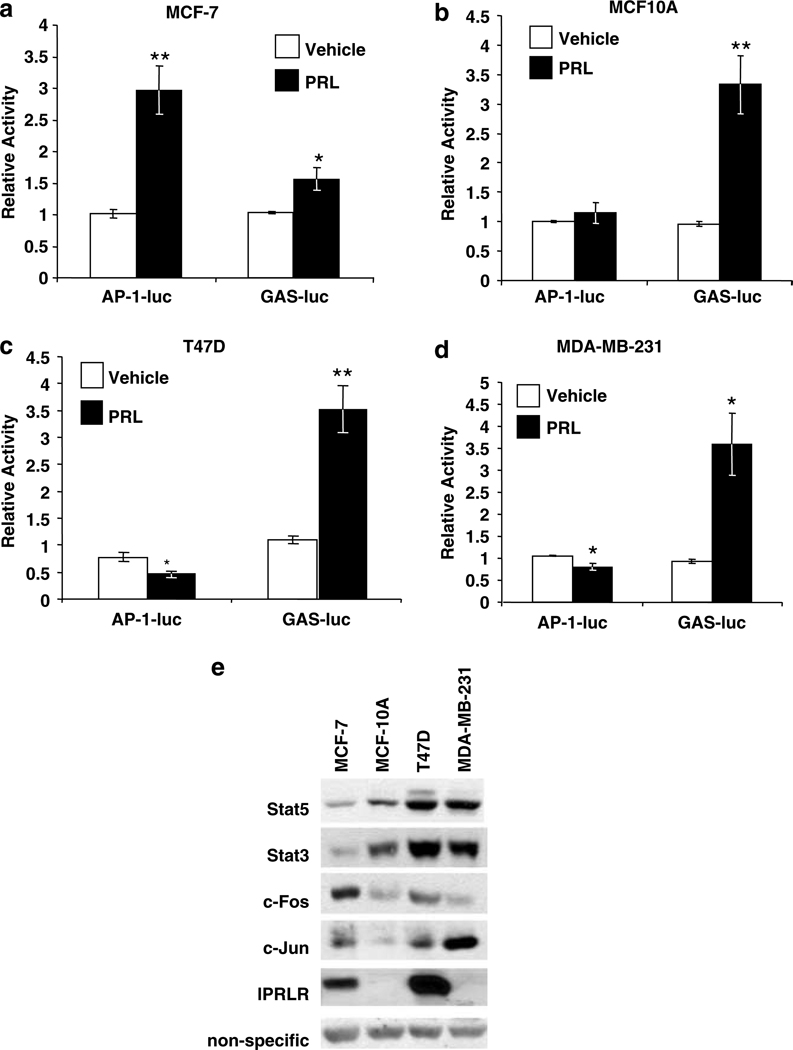
To understand the relationship between PRL-initiated Stat5 and AP-1 signals in mammary tumor cells of differing etiologies and phenotypes, including complement of AP-1 components and hormone responsiveness, we investigated PRL-induction of these pathways, as well as levels of endogenous Stat3 and Stat5, c-Fos and c-Jun in several other mammary cell lines. As shown in Figure 3, all these cells responded to PRL when transfected with lPRLR, including MCF-10A and MDA-MB-231 cells, which express only very low levels of endogenous lPRLR (not detectable by Western analysis, see Figure 3e, fifth panel). However, the strength of the PRL-induced signals to AP-1 and Stat5 pathways (AP-1- and GAS-luciferase, respectively), as well as expression of AP-1 components and Stat proteins, differed considerably. The MCF-7-derived cell line showed the largest AP-1 and lowest Stat5 response to PRL (Figure 3a). In contrast, the more ‘normal’ MCF-10A cell line, which normally expresses only low levels of lPRLR, displayed a strong Stat5-dependent response when PRLR levels were elevated by transfection, but did not activate AP-1 (Figure 3b). Both T47D and MDA-MB-231 cells, breast cancer cell lines which express endogenous PRLR and ERα at high and low levels, respectively, responded to PRL with strong activation of the GAS element, and a modest but significant decrease in AP-1 (Figure 3c and d). The latter may be due to activation of AP-1 proteins present in these cells which are capable of forming inhibitory dimers (Milde-Langosch et al., 2004). When cultured in the presence of serum, these cell lines also displayed variable levels of Stat3 and Stat5, as well as c-Fos and c-Jun (Figure 3e). Interestingly, relative levels of Stat3 and Stat5 in these cell lines tended to parallel one another. Higher amounts of these factors correlated with the ability of PRL to activate the Stat5-specific GAS enhancer, but not AP-1. Potential PRL-activation of AP-1 was associated with higher c-Fos, but not c-Jun levels, and tended to be inversely related to Stat3 and Stat5. These findings in a limited number of cell lines suggest inverse strength of PRL signals to GAS and AP-1 enhancers, which appears to depend in part on Stat5 levels.
Figure 3.
PRL activation of AP-1 activity is cell type dependent and is inversely related to PRL activation of Stat5. (a) MCF-7-derived cells; (b) MCF-10A cells; (c) T47D cells; or (d) MDA-MB-231 cells were cotransfected with 4XAP-1-luc or GAS-luc, and lPRLR, β-gal, and vector DNA as described in the Supplementary Information and treated ± 4 nm PRL for 24 h. Luciferase activity was determined, and normalized as described in the Materials and methods. Relative activity indicates the mean of at least three independent experiments, shown as mean fold change relative to the vehicle treated control ±s.e.m. Asterisks denote significant differences between vehicle and PRL-treated cells using Student’s t-test (*P < 0.04, **P < 0.009). (e) Cell lysates were analysed by immunoblotting as indicated.
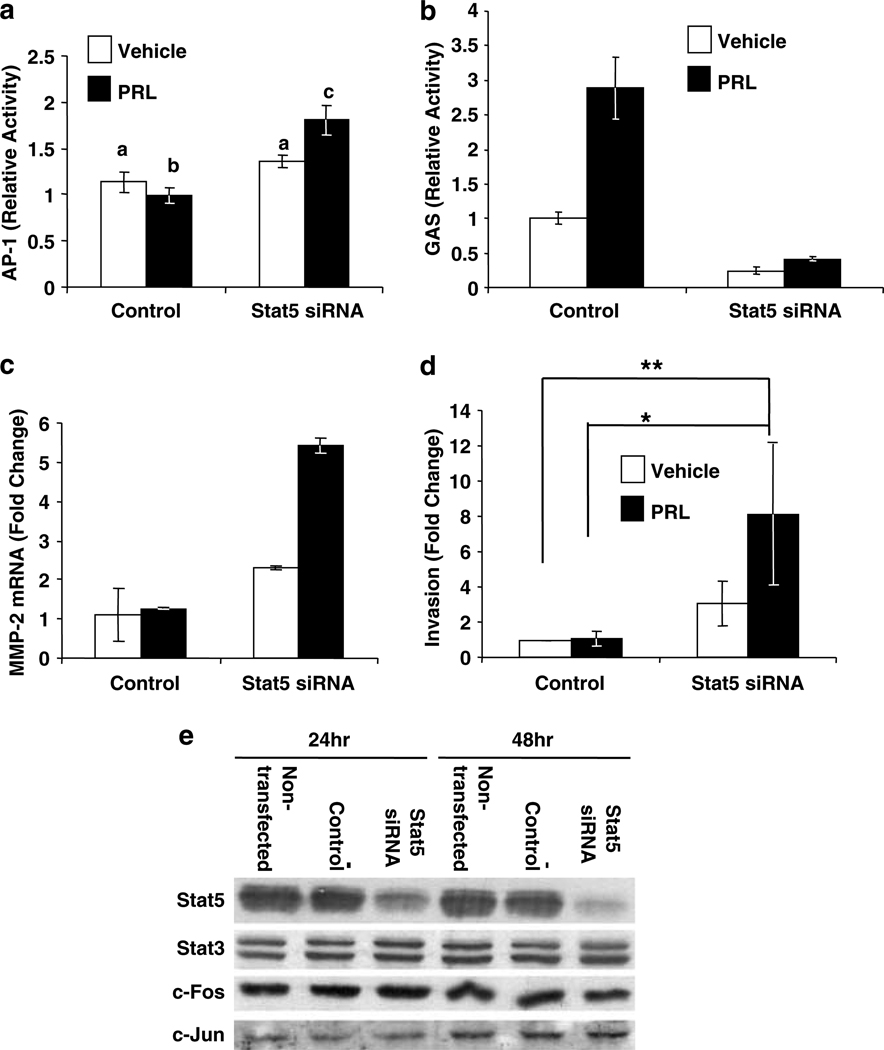
To further test the robustness of this relationship and the role of Stat5 levels in determining the strength of PRL-induced AP-1, we reduced Stat5 levels in T47D cells with siRNA, targeted to both Stat5a and Stat5b. T47D cells display higher PRL-activated GAS than AP-1 enhancer activity, the opposite of PRL signals in the MCF7 subline (compare Figure 3a and c). In contrast to the slight PRL-induced reduction in AP-1 activity observed with non-targeting siRNA in T47D cells (similar to Figure 3c), knockdown of Stat5 resulted in significant PRL-induced AP-1 activity (Figure 4a), as well as the expected dramatic reduction in GAS responsiveness (Figure 4b). This confirms that levels of Stat5 are important modulators of potential PRL-induced signals to AP-1. The enhanced PRL-induced AP-1 activity was associated with elevated transcripts for MMP-2 (Figure 4c), an AP-1 target that can degrade the basement membrane, a process essential for tumor invasion and metastasis (Vincenti, 2001), as well as increased PRL-induced invasive behavior (Figure 4d). As can be seen from Figure 4e, levels of c-Fos, c-Jun and Stat3 were not altered by Stat5 siRNA, indicating that global changes in these proteins do not mediate this modification of PRL signals.
Figure 4.
Knockdown of Stat5 increases PRL-induced AP-1 activity. (a and b) T47D cells were cotransfected with lPRLR, β-gal, either non-targeting (control) or Stat5 specific siRNA, and (a) 4XAP-1-luc or (b) GAS-luc. Following transfection, cells were treated ±4 nm PRL for 6 h. Luciferase activity was determined, and normalized as described in the Materials and methods. (a) Relative activity indicates the mean of three to five independent experiments, shown as mean fold change relative to the vehicle treated control ±s.e.m. Different letters denote significant differences among groups (P < 0.05), determined by one-way ANOVA followed by Neuman–Keuls post-test. (b) Each bar represents the mean relative activity ±s.d. of triplicate wells from one representative experiment. (c) Cells were transfected with non-targeting (control) or Stat5 specific siRNA for 24 h. Following transfection, cells were washed and treated ± 4 nm PRL for 48 h. Quantitative real-time PCR was performed for MMP-2 and Stat5 (to confirm knockdown, data not shown), using 18S as an internal control. Data was analysed using the ΔΔCt method. Representative experiment shown. (d) Cells were transfected as in (c). After 48 h, cells were placed into the upper chamber of a transwell apparatus containing ±2× 4 nm PRL, and their ability to invade a collagen I matrix toward RPMI containing 10% FBS evaluated after 30 h. Data represent fold change compared to vehicle-treated non-targeting siRNA-transfected cells from three independent experiments performed in triplicate, ±s.e.m. Asterisks denote significant differences among groups (*P < 0.05; **P < 0.01), determined after analysis of log transformed data by one-way ANOVA followed by Neuman–Keuls post-tests. (e) Cells were untransfected, or transfected with non-targeting (control), or Stat5 specific siRNA. Cell lysates were harvested at 24 or 48 h after transfection and analysed by Western blot as indicated.
Discussion
The correlation of circulating PRL with breast cancer risk independent of estrogen, and the high proportion of human breast tumors expressing PRLR, underscore the importance of understanding the contributions of PRL to this disease. While PRL signals via the Jak2/Stat5 pathway during mammary development are well recognized, relatively little is known about the role of other PRL-initiated signals, and their interrelationships in physiologic and pathologic processes. Recently, PRL-activated Stat5 has been reported to reduce processes associated with tumor aggression in vitro (Sultan et al., 2005; Nouhi et al., 2006), consistent with better prognoses in vivo (Cotarla et al., 2004; Nevalainen et al., 2004), prompting the suggestion that PRL signals may promote a more differentiated tumor phenotype. The present study extends our previous reports of PRL activation of another pathway, driving expression of AP-1 target genes associated with tumor progression. We demonstrate a reciprocal relationship between PRL signals to Stat5 and AP-1 in breast cancer cells, and show that the predominant PRL-initiated pathway is determined at least in part by Stat5 availability. Thus, depending on the cell context, PRL may have very different effects on markers associated with tumor aggression, emphasizing the importance of understanding the breadth of mechanisms whereby this hormone contributes to breast cancer.
Human breast tumors display diverse patterns of AP-1 proteins, which correlated with a negative prognosis in several studies (Bland et al., 1995; Gee et al., 2000; Johnston et al., 1999; Milde-Langosch et al., 2004). The consistent appearance of AP-1 target genes, such as metalloproteases and CD44, in transcript signatures associated with poor prognoses (Van’t Veer et al., 2002; Wang et al., 2005) further emphasizes the importance of this pathway in human neoplasms. Activities of the AP-1 and Stat5 pathways have not been compared in the same set of primary tumors. However, our data would predict an inverse relationship. Together, these data suggest that PRL can have different effects in neoplastic progression, depending on the signaling pathways available. Furthermore, it reveals a mechanism whereby PRL activation of Stat5 may ‘protect’ neoplastic cells from signals of local growth factors where possible, maintaining a better differentiated phenotype.
Stats have been shown to modulate transcriptional activity by multiple mechanisms. They can augment or inhibit promoter activity by binding to GAS sites, or form ‘enhanceosomes’ by cooperating with other factors bound nearby (Shuai, 2000; Shemanko and Groner, 2001; Levy and Darnell, 2002). They also can alter promoter activity by competing for limiting coactivators (Horvai et al., 1997; Luo and Yu-Lee, 2000; Shemanko and Groner, 2001). Interestingly, however, some outcomes vary with cell type (Nakamura et al., 2002). In the current study, inhibition of AP-1 required pTyr694/699-Stat5, but not the C-terminal transactivation domain which competes for limiting coactivators (Luo and Yu-Lee, 2000; Shemanko and Groner, 2001), and Stat5 associated with both c-Fos and c-Jun. The lack of efficacy of Stat5Y699F argues against Stat5-directed transcription of another mediatory factor. Together, these observations suggest yet another mechanism. Stat5 may alter the conformation or mask regions of AP-1 proteins, resulting in altered protein-protein interactions, and consequent decreased activity of the transcriptional complex. The regions of Stat3 that interact with c-Jun have been localized and critical residues identified; however, the amino-acid sequence of Stat5 is quite distinct at these loci (Zhang et al., 1999), suggesting a different relationship.
In contrast to Stat5, overexpression of Stat3 enhanced the PRL-induced AP-1 response in these studies. We have observed that exogenous WT Stat3 can enhance PRL signals to the c-fos promoter (data not shown), reminiscent of Stat3-mediated activation of the SIE within this promoter by IL-6 (Yang et al., 2003). This activity, and/or other actions such as potential direct interaction with the AP-1 complex as discussed above, may underlie our observations. Despite the ability of both Stat5 and Stat3 to promote processes linked to carcinogenesis (reviewed in Bromberg, 2002; Buettner et al., 2002), these related transcription factors exert opposing activities on alveolar development and invasion, in addition to the distinct effects on AP-1 activity reported here (reviewed in Ormandy et al., 2001; Watson, 2001; Hennighausen and Robinson, 2005; Sultan et al., 2005). Interestingly, Stat3 correlated with Stat5 expression in the limited number of mammary cell lines examined herein, and higher expression was associated with reduced AP-1 responses to PRL, not the increase predicted from the actions of Stat3 alone. Clearly, the importance of PRL signals to Stat3 in the normal gland and during tumorigenesis requires further study.
Our studies demonstrate that PRL activates at least two pathways in breast cancer cells that may contribute to carcinogenesis and differentially modulate tumor behavior. The observed inverse relationship between them results in part from direct inhibition of AP-1 activity by PRL-activated Stat5, which can be modified by Stat5 availability. This suggests that PRL can exert very different effects on disease outcome depending on the phenotype of the tumor, and that pathway specific, rather than ligand-based, approaches may prove a fruitful strategy in targeting PRL actions in this disease. In vivo models, such as our NRL-PRL transgenic mice, are required to explore these relationships in the dynamic processes of lesion development and progression, and interactions of PRL with other important cytokines, hormones and growth factors in breast cancer.
Materials and methods
Cell culture and transfection
Details of cell culture for each breast cell line are described in the Supplementary Information. For transient transfections, cells were serum starved for 24 h before transfection with constructs as indicated in the figure legends and described in the Supplementary Information. Total transfected DNA was equalized within each experiment with vector backbone. Transfections were optimized for each cell line as described in Supplementary Information. Luciferase values were corrected for transfection efficiency using β-gal, as described (Brockman et al., 2002). ‘Relative activity’ is the mean of at least three independent experiments represented as fold change relative to the vehicle control. We designed Stat5 specific siRNA using a region homologous to both the Stat5a and Stat5b isoforms (see Supplementary Information), in order to reduce both Stat5 isoforms, which can equally reduce AP-1 activity (Figure 1). Non-targeting siRNA (Dharmacon, Lafayette, CO, USA) was used as a control. 100 nm siRNA duplexes were transfected with Lipofectamine2000 for 24–48 h as indicated.
Immunoblotting
Cells grown in complete media (above) were analysed by Western blot as described previously (Schroeder et al., 2002). Primary antibody concentrations were as follows: c-Jun, 1:1000; c-Fos, 1:1000; hPRLR, 1:1000; Stat5 (sc-835X) 1:500 000; phospho-Stat5, 1:1000; HA.11 (1:1000), Grb2 (1:5000), and Stat3, 1:500 (See Supplementary Information for the source of the antibodies).
Real-time PCR
Cells were transfected with Stat5 or non-targeting siRNA for 24 h as described above, and treated −/+ 4 nm PRL for 48 h. Quantitative PCR for MMP-2, Stat5 and 18S RNA was performed as suggested by the manufacturer using a 7300 Real-time PCR System and analysed with the Sequence Detection Software Version 1.3 (Applied Biosystems, Foster City, CA, USA). Additional details are found in the Supplementary Information.
Invasion assays
Cells were transfected with non-targeting or Stat5-specific siRNA as above, and the ability to invade collagen I over 30 h was assessed as described (Keely, 2001).
Supplementary Material
Acknowledgements
We appreciate the plasmids provided by Drs C Clevenger, RL Ilaria, E Kabotyanski and J Rosen, and the assistance of Dr P Keely, A Guadarrama and Dr G Wiepz with the invasion assays. This work was supported in part by NIH R01 CA78312 and DK07623 (LAS), and DAMD17-01-1-0460 (JHG).
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- Bjornstrom L, Sjoberg M. STAT as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol. 2002;16:2202–2214. doi: 10.1210/me.2002-0072. [DOI] [PubMed] [Google Scholar]
- Bland KI, Konstadoulakis MM, Vezeridis MP, Wanebo HJ. Oncogene protein co-expression. Value of Ha-ras, c-myc, c-fos, and p53 as prognostic discriminants for breast carcinoma. Ann Surg. 1995;221:706–718. doi: 10.1097/00000658-199506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman JL, Schroeder MD, Schuler LA. Prolactin activates the cyclin D1 promoter via the JAK2-STAT pathway. Mol Endocrinol. 2002;16:774–784. doi: 10.1210/mend.16.4.0817. [DOI] [PubMed] [Google Scholar]
- Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- Cao JS, Wood M, Liu Y, Hoffman T, Hyde J, Park-Sarge OK, et al. Estradiol represses prolactin-induced expression of Na+/taurocholate cotransporting polypeptide in liver cells through estrogen receptor-α and STAT5a. Endocrinology. 2004;145:1739–1749. doi: 10.1210/en.2003-0752. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA. Role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotarla I, Ren SX, Zhang Y, Gehan E, Singh B, Furth PA. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer. 2004;108:665–671. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- DaSilva L, Rui H, Erwin RA, Howard OM, Kirken RA, Malabarba MG, et al. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol. 1996;117:131–140. doi: 10.1016/0303-7207(95)03738-1. [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- Faulds MH, Pettersson K, Gustafsson JA, Haldosen LA. Cross-talk between ERs and Stat5 is E2 dependent and involves two functionally separate mechanisms. Mol Endocrinol. 2001;15:1929–1940. doi: 10.1210/mend.15.11.0726. [DOI] [PubMed] [Google Scholar]
- Gee JM, Barroso AF, Ellis IO, Robertson JF, Nicholson RI. Biological and clinical associations of c-jun activation in human breast cancer. Int J Cancer. 2000;89:177–186. doi: 10.1002/(sici)1097-0215(20000320)89:2<177::aid-ijc13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Goffin V, Bernichtein S, Touraine P, Kelly PA. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev. 2005;26:400–422. doi: 10.1210/er.2004-0016. [DOI] [PubMed] [Google Scholar]
- Gutzman JH, Nikolai SE, Rugowski DE, Watters JJ, Schuler LA. Prolactin and estrogen enhance the activity of AP-1 in breast cancer cells: role of ERK1/2-mediated signals to c-fos. Mol Endocrinol. 2005;19:1765–1778. doi: 10.1210/me.2004-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzman JH, Rugowski DE, Schroeder MD, Watters JJ, Schuler LA. Multiple kinase cascades mediate prolactin signals to AP-1 in breast cancer cells. Mol Endocrinol. 2004;18:3064–3075. doi: 10.1210/me.2004-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Horvai AE, Xu L, Korzus E, Brard G, Kalafus D, Mullen TM, et al. Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc Natl Acad Sci USA. 1997;94:1074–1079. doi: 10.1073/pnas.94.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys RC, Hennighausen L. STAT5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. 1999;10:685–694. [PubMed] [Google Scholar]
- Iavnilovitch E, Groner B, Barash I. Overexpression and forced activation of Stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Cell Growth Differ. 2002;1:32–47. [PubMed] [Google Scholar]
- Ilaria RL, Jr, Hawley RG, Van Etten RA. Dominant negative mutants implicate STAT5 in myeloid cell proliferation and neutrophil differentiation. Blood. 1999;93:4154–4166. [PubMed] [Google Scholar]
- Johnston SR, Lu B, Scott GK, Kushner PJ, Smith IE, Dowsett M, et al. Increased AP-1 DNA binding and c-Jun NH2-terminal kinase activity in human breast tumors with acquired tamoxifen resistance. Clin Cancer Res. 1999;5:251–256. [PubMed] [Google Scholar]
- Keely PJ. Ras and Rho protein induction of motility and invasion in T47D breast adenocarcinoma cells. Methods Enzymol. 2001;333:256–266. doi: 10.1016/s0076-6879(01)33061-6. [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Luo GY, Yu-Lee LY. Stat5b inhibits NFkappaB-mediated signaling. Mol Endocrinol. 2000;14:114–123. doi: 10.1210/mend.14.1.0399. [DOI] [PubMed] [Google Scholar]
- Milde-Langosch K, Bamberger AM, Methner C, Rieck G, Loning T. Expression of cell cycle-regulatory proteins rb, p16/MTS1, p27/KIP1, p21/WAF1, cyclin D1 and cyclin E in breast cancer: correlations with expression of AP-1 family members. Int J Cancer. 2000;87:468–472. [PubMed] [Google Scholar]
- Milde-Langosch K, Roder H, Andritzky B, Aslan B, Hemminger G, Brinkmann A, et al. The role of the AP-1 transcription factors c-Fos, FosB, Fra-1 and Fra-2 in the invasion process of mammary carcinomas. Breast Cancer Res Treat. 2004;86:139–152. doi: 10.1023/B:BREA.0000032982.49024.71. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ouchida R, Kodama T, Kawashima T, Makino Y, Yoshikawa N, et al. Cytokine receptor common beta subunit-mediated STAT5 activation confers NF-kappa B activation in murine proB cell line Ba/F3 cells. J Biol Chem. 2002;277:6254–6265. doi: 10.1074/jbc.M109878200. [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, et al. Stat-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053–2060. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Nouhi Z, Chughtai N, Hartley S, Cocolakis E, Lebrun JJ, Ali S. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res. 2006;66:1824–1832. doi: 10.1158/0008-5472.CAN-05-2292. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Horseman ND, Naylor MJ, Harris J, Robertson F, Binart N, et al. Mammary gland development. In: Horseman ND, editor. Prolactin. Boston: Kluwer Academic Publishers; 2001. pp. 219–232. [Google Scholar]
- Ren S, Cai HR, Li M, Furth PA. Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene. 2002;21:4335–4339. doi: 10.1038/sj.onc.1205484. [DOI] [PubMed] [Google Scholar]
- Schroeder MD, Symowicz J, Schuler LA. Prolactin modulates cell cycle regulators in mammary tumor epithelial cells. Mol Endocrinol. 2002;16:45–57. doi: 10.1210/mend.16.1.0762. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Shemanko CS, Groner B. Transcription factors, cofactors and target genes mediating prolactin signals. In: Horseman ND, editor. Prolactin. Boston: Kluwer Academic Publishers; 2001. pp. 381–404. [Google Scholar]
- Shuai K. Modulation of STAT signaling by STAT-interacting proteins. Oncogene. 2000;19:2638–2644. doi: 10.1038/sj.onc.1203522. [DOI] [PubMed] [Google Scholar]
- Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene. 2005;24:746–760. doi: 10.1038/sj.onc.1208203. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Hankinson SE. Prolactin and breast cancer risk. Cancer Lett. 2006;243:160–169. doi: 10.1016/j.canlet.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Van’t Veer LJ, Dai HY, Van de Vijver MJ, He YDD, Hart AAM, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Vincenti MP. The matrix metalloproteinase and tissue inhibitor of metalloproteinase genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol Biol. 2001;151:121–148. doi: 10.1385/1-59259-046-2:121. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK. Prolactin in Human Breast Cancer Development. In: Ethier SP, editor. Endocrine Oncology. Totowa, NJ: Humana Press; 2000. pp. 101–120. [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Watson CJ. Stat transcription factors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:115–127. doi: 10.1023/a:1009524817155. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Silva CM. Modulation of Stat5b activity in breast cancer cells by mutation of tyrosines within the transactivation domain. Mol Endocrinol. 2006;20:2392–2405. doi: 10.1210/me.2005-0418. [DOI] [PubMed] [Google Scholar]
- Yang E, Lerner L, Besser D, Darnell JE., Jr Independent and cooperative activation of chromosomal c-fos promoter by STAT3. J Biol Chem. 2003;278:15794–15799. doi: 10.1074/jbc.M213073200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wrzeszczynska MH, Horvath CM, Darnell JE., Jr Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation. Mol Cell Biol. 1999;19:7138–7146. doi: 10.1128/mcb.19.10.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.