Abstract
Previous theories have proposed that the amygdala is hyper-responsive to novel stimuli in persons with an inhibited temperament—a biologically based predisposition to respond to novelty with wariness or avoidance behavior. In the current study, we used functional magnetic resonance imaging (fMRI) to assess amygdala blood oxygenation level-dependent (BOLD) response when viewing novel or recently familiarized faces in persons with an extreme inhibited or uninhibited temperament. In persons with an inhibited temperament, the amygdala showed increased BOLD response when viewing both novel and recently familiarized faces. In contrast, in persons with an uninhibited temperament, BOLD response in the amygdala was increased only when viewing novel faces. These findings suggest that inhibited temperament is characterized not by a simple exaggerated response to novel faces, but rather by a sustained increase in amygdala response to faces even after the faces have become familiarized. In individuals with an inhibited temperament, this sustained response may be related to the wariness of social situations that persists beyond initial exposure.
Keywords: temperament, amygdala, novelty, familiarity, social anxiety, fMRI
INTRODUCTION
Neuroimaging studies of adults with psychiatric disorders reveal the neurobiology of current disease states; however, to identify the neurobiological precursors of psychiatric disease, we must broaden our research focus to include studies of developmental risk factors. Temperament—defined as individual differences in emotions, cognitions, and behaviors arising in infancy—is a crucial developmental factor for further study because it is biologically based (Kagan et al., 1988), heritable (Wilson and Matheny, 1986) and confers risk for adult psychiatric disorders (Caspi et al., 1996; Windle and Windle, 2006). Of the many temperament dimensions, inhibited temperament, a biologically based predisposition to respond to novelty with wariness or avoidance behaviors (Kagan et al., 1987), may be especially important because of its well-established phenotype and demonstrated risk for both social anxiety (Hirshfeld et al., 1992; Schwartz et al., 1999; Chronis-Tuscano et al., 2009; Muris et al., 2009) and major depression (Caspi et al., 1996; Beesdo et al., 2007). While behavioral and physiological features of inhibited temperament have been well described (for a review see reference Fox et al., 2005), researchers are in the early stages of identifying the underlying brain regions that mediate these features.
The amygdala has been proposed as a neural substrate of inhibited temperament (Kagan et al., 1987, 1988; Kagan and Snidman, 2004) because the amygdala mediates animal and human physiological responses during fear and anxiety (Davis and Whalen, 2001). Relevant to inhibited temperament, the human amygdala also responds to novel stimuli such as new or unusual objects (Blackford et al., 2010) and novel human faces (Schwartz et al., 2003b; Zald, 2003). Consistent with a sensitivity to novelty, the amygdala response to faces habituates with repeated exposure in healthy adults (Breiter et al., 1996). Preliminary evidence for temperament differences in amygdala responses to novel faces comes from two studies. In a pioneering functional magnetic resonance (fMRI) study, Schwartz and colleagues (2003a) examined a group of young adults that had been characterized as extremely inhibited or uninhibited as toddlers. Inhibited participants had larger amygdala blood oxygenation level-dependent (BOLD) response to novel relative to newly familiarized faces, whereas the uninhibited participants had smaller amygdala BOLD responses to both novel and familiarized faces. Blackford and colleagues (2009) examined amygdala BOLD response to novel versus newly familiarized faces in young adults who reported both past and current extreme inhibited or uninhibited temperament. Like the Schwartz study (Schwartz et al., 2003a), individuals with an uninhibited temperament had similar amygdala BOLD responses to the novel and familiarized faces. In contrast to the finding of Schwartz et al. (2003a), the Blackford (2009) study suggested that individuals with an inhibited temperament showed increased BOLD signal in the amygdala, relative to the uninhibited participants, to both novel and familiarized faces, rather than a selectively increased response to novel faces. This finding raises the provocative idea that persons with an inhibited temperament may show an increased amygdala response to both novel and familiarized faces.
Whether inhibited temperament is characterized by a selectively greater response to novel faces versus a broader heightened responsiveness to both novel and newly familiarized faces has significant implications for understanding the construct of inhibited temperament. Previous theories of the amygdala’s role in inhibited temperament have focused on a heightened response to novelty, as avoidant response to novel stimuli is key to the temperament construct (Kagan et al., 1987, 1988; Kagan and Snidman, 2004). However, individuals with an inhibited temperament often describe prolonged social concerns that are not limited to situations in which they are meeting new people. Rather, they show a hyper-vigilance to social information and potential negative evaluations as reflected in the increased incidence of social anxiety disorder in persons with an inhibited temperament (Schwartz et al., 1999; Biederman et al., 2001; Chronis-Tuscano et al., 2009). Given such hyper-vigilance, an alternative theory may be proposed that in inhibited temperament, the amygdala continues to show an increased response to social stimuli, such as faces, even after an initial familiarization. If supported, this theory would influence current conceptualizations of the role of amygdala neurobiology in inhibited temperament and related conditions.
While both prior studies of amygdala function in inhibited temperament (Schwartz et al., 2003a; Blackford et al., 2009) suggested important directions for further study, the studies had relatively small sample sizes and employed different experimental designs. Given that the only two published fMRI studies directly addressing this topic reached different conclusions, it is essential to conduct additional studies to more definitively address the critical role of the amygdala in inhibited temperament. In the current study, we use a larger sample size and improved experimental design to further test the hypothesis that persons with an inhibited temperament have heightened amygdala BOLD responses to both novel and familiarized faces.
METHODS
Participants
Thirty-three young adults with continuous, extreme inhibited (n = 18) or uninhibited (n = 15) temperament in childhood and adulthood participated in the study. Participants had a mean age of 23.0 years (s.d. = 2.77 years, range 18–30) and 64% were female. The inhibited and uninhibited temperament groups were significantly different on both childhood and adult measures of inhibited temperament, but did not differ in age, gender, ethnicity or handedness (Table 1).
Table 1.
Participant characteristics by temperament group
| Inhibited temperament |
Uninhibited temperament |
||
|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | P-value | |
| Temperament | |||
| Retrospective | 3.17 (0.50) | 1.48 (0.17) | <0.0001 |
| Current | 3.14 (0.38) | 1.64 (0.20) | <0.0001 |
| Demographics | |||
| Age | 23.44 (2.91) | 22.47 (2.59) | ns |
| N (%) | N (%) | P-value | |
| Gender, female (%) | 12 (67) | 9 (60) | ns |
| Handedness, right (%) | 16 (89) | 13 (87) | ns |
| Ethnicity (%) | |||
| Caucasian | 14 (78) | 14 (93) | ns |
| African-American | 3 (17) | 0 (0) | |
| Asian | 1 (6) | 0 (0) | |
| Other | 0 (0) | 1 (7) | |
| Mean (s.d.) | Mean (s.d.) | P-value | |
| Recognition memory | |||
| Familiar face accuracy | 95 (8) | 99 (4) | ns |
| Novel face accuracy | 69 (17) | 72 (16) | ns |
ns, not significant.
The Vanderbilt University Institutional Review Board approved the study and we obtained written informed consent after providing participants with a complete description of the study.
Participant selection
Potential study participants responded to advertisements seeking people who were ‘especially shy or outgoing as a child’ for a larger study of temperament. We assessed child and adult temperament using two questionnaires: the Retrospective Self-Report of Inhibition (RSRI) and Current Self-Report of Inhibition (CSRI). The RSRI is a 30-item instrument which asks about behaviors during childhood (1–5 Likert scale, 1 = uninhibited, 5 = inhibited). The CSRI, a 31-item instrument, asks about current behaviors (1–5 scale). The RSRI and CSRI have good reliability and construct validity (Reznick et al., 1992) and high internal consistency in the present sample (Cronbach’s α = 0.94 for both). We used guidelines based on available normative data from the general population (Reznick et al., 1992) to select the top and bottom 15% of potential participants (inhibited ≥2.6, uninhibited ≤1.9) for each of the instruments. Of the 163 participants who completed the screening, 77 (47%) were eligible, and the majority (n = 65, 84%) completed the larger study of temperament.
From this larger study, we selected participants for the fMRI study who passed an MRI safety screen (e.g. no metal in body, no claustrophobia) and were free of: any current psychoactive medications, substance abuse in the past 6 months, major medical illness and history of brain trauma. Participants were not excluded for presence or history of psychiatric illness because both inhibited and uninhibited temperaments are associated with increased rates of psychiatric disorder (Schwartz et al., 1996). Of the 65 participants in the larger study, 51 (78%) met these inclusion criteria for the fMRI study and 37 (73%) of those completed this study. Four participants were later removed prior to analysis (see fMRI data section below) due to technical scanner issues or excessive movement, providing a final analytic sample of 33 participants.
A trained clinical interviewer assessed psychiatric history using the Structured Clinical Interview for DSM-IV (SCID; Spitzer et al., 1992). Consistent with prior studies (Schwartz et al., 1996; Blackford et al., 2009), persons in the inhibited group had higher rates of current internalizing disorders compared with the uninhibited group. Of the inhibited participants, six met criteria for at least one anxiety disorder: Social Anxiety Disorder (n = 5), Generalized Anxiety Disorder (n = 3), Specific Phobia (n = 1) and Anxiety Not Otherwise Specified (NOS; n = 2). Two of these also met criteria for Dysthymia and one participant met criteria for only Dysthymia. Of the uninhibited participants, one met criteria for Post Traumatic Stress Disorder and one met criteria for Major Depression NOS and Anxiety NOS.
fMRI task
Stimuli
Stimuli were black and white human face images with neutral expressions, selected from two standard sets of emotional expressions (Lundqvist et al., 1998; Gur et al., 2001). All stimuli were edited to ensure uniform face size, eye position and nose position, and all extraneous features (e.g. shirt collars, hair) were removed. Stimuli were randomly selected for the novel or familiar group and balanced across gender and stimulus set. We used Eprime software (Version 1.1, Psychology Software Tools, Pittsburgh, PA, USA) to present the stimuli.
fMRI procedure
The fMRI protocol included a familiarization phase and a test phase. During the familiarization phase, participants were repeatedly shown a set of six faces while in the scanner. Faces were presented in four 18-s blocks, each separated by a 10-s break (e.g. fixation cross, face block, fixation cross) for a total of 122 s. Each block consisted of 12 1-s face presentations (two of each of the six familiarized faces per block) separated by a 0.5 s blank screen, with face order randomly determined.
In the test phase, we randomly presented the novel and familiarized faces during four separate runs of 12 novel and 12 familiarized face presentations each (for a total of 48 novel and 48 familiar faces). Each of the six familiarized faces was presented twice within each run and each novel face was presented only once across all runs. Faces were presented for 0.5 s with a 12 s average, jittered, inter-stimulus interval. Each run lasted ∼300 s.
In this study, we made three adaptations to the experimental design used in our prior study. First, due to our concerns of attentional overload, we changed the familiarization procedure from a single 96-s stream of faces to four blocks of faces interspersed with break periods to provide periods with low attentional demand. Second, in our previous study, the interstimulus interval consisted of a blank screen with a fixation cross prompt prior to the face presentation. Because the sudden appearance of a visual cue might produce an orienting response that itself increases amygdala activity, here we used a grey circle to maintain visual orientation throughout the interstimulus interval, with a color shift (to white) as the cue. Finally, we increased the number of stimulus presentations to enhance statistical power.
Recognition memory procedure
To assess facial recognition memory for the presented faces and to verify that the participants were actively engaged in the task, participants completed a recognition memory task after the fMRI procedure. Participants were presented with 26 faces previously seen in the scanner (six familiarized faces and 20 faces that were seen once), and asked to determine whether each face was seen once or many times before.
MRI data acquisition
Anatomical and echo planar imaging (EPI) images were collected on a 3 Tesla Phillips magnet (Philips Healthcare, Inc., Best, The Netherlands). High resolution T1-weighted anatomical images were collected (256 mm FOV, 170 slices, 1-mm slice thickness, 0-mm gap). EPI images were acquired using a sequence optimized for the amygdala: 2 s TR, 22 ms TE; 90° flip angle; 1.8 SENSE, 240 mm FOV; 3 × 3 mm in plane resolution using an 80 × 80 matrix (reconstructed to 128 × 128), and higher order shimming to limit susceptibility artifacts. Each volume contained 36 2.5 mm (0.25 gap) axial oblique slices (titled 15° anterior higher than posterior relative to the intercommissural plane), which provided complete anterior–posterior coverage and inferior–superior coverage from the bottom of the temporal lobe to the top of the cingulate gyrus.
MRI data processing
MRI data were pre-processed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/) and Matlab (Version 7.1, The MathWorks, Inc, Natick, MA, USA). Data were slice time corrected, realigned to the first slice, resampled to 3 × 3 × 3 mm voxels, spatially normalized into standard stereotactic space (MNI EPI template), and high pass filtered (128 s). Data were smoothed with a 6-mm FWHM Gaussian kernel to account for individual differences in brain anatomy.
For each participant, motion was assessed and EPI images were visually inspected for artifacts, signal dropout, and coverage of the amygdala region of interest. Two participants had excessive motion (>3 mm) and were removed from further analysis. For three participants, the last functional run was removed due to motion. The last two functional runs for two other participants were removed due to artifacts.
MRI data modeling
Using SPM5, the first-level (participant) general linear model (Friston et al., 1994) was estimated using the two stimulus types (novel and familiar) as regressors, with temporal and dispersion derivates included (Friston et al., 1998; Henson et al., 2001). First-level contrast images were created for each of the conditions compared to baseline. Next, the second-level (group) general linear model was estimated with temperament group (inhibited/uninhibited) and face type (novel/familiar) as factors.
Data analysis
Demographic and behavioral data
To test for differences in demographic and behavioral variables, chi-square analyses were used for categorical variables and independent t-tests for continuous variables (two-tailed, α = 0.05). SAS statistical software (Version 9.1, SAS Institute Inc., Cary, NC, USA) was used to perform these statistical analyses.
Amygdala region of interest
The bilateral amygdala region of interest (ROI) was defined using the AAL templates (Tzourio-Mazoyer et al., 2002) implemented in WFU Pick Atlas (Version 2.4; Maldjian et al., 2003). SPM5 was used to test for the interaction of temperament group and face type within the amygdala ROI. Cluster-based thresholding provided Type I error control. Based on simulations performed with AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf), a voxel P-value of 0.05 and contiguous cluster size of 11 controlled for family-wise error across the amygdala at α = 0.05.
For significant clusters identified in the main analysis, percent signal change values were extracted using MarsBar (Brett et al., 2002). Post hoc analyses were performed using SAS (Version 9.1, SAS Institute Inc., Cary, NC, USA). First, repeated measures ANOVAs were performed to confirm the SPM findings, with temperament as the between-subjects factor and face type (novel/familiar) as the within-subjects factor. Second, to examine possible behavior–brain relationships, correlation analyses were performed between the continuous temperament scores (child and adult) and the amygdala percent signal change values for the novel–familiar contrast. Finally, repeated measures ANOVAs were performed to test for effects of gender or temperament on lateralization of amygdala percent signal change.
Whole-brain analysis
To determine if other brain regions also showed an interaction between temperament group and face type on BOLD response, exploratory whole-brain analyses were performed. As with the ROI analyses, SPM5 was used to test for an interaction of temperament group and face type. For the whole brain, a voxel P-value of 0.005 and a cluster size of 25 provided family-wise error correction at α = 0.05. Statistically significant regions are presented in the Results, but are only discussed briefly because the analyses are exploratory.
RESULTS
Recognition memory
To test for temperament differences in recognition memory, participants performed a recognition task following the scanning session. On average, participants had 97% accuracy in recognizing the familiarized faces and 71% accuracy in recognizing the novel faces. Performance was similar in the two temperament groups (Table 1).
Amygdala region of interest
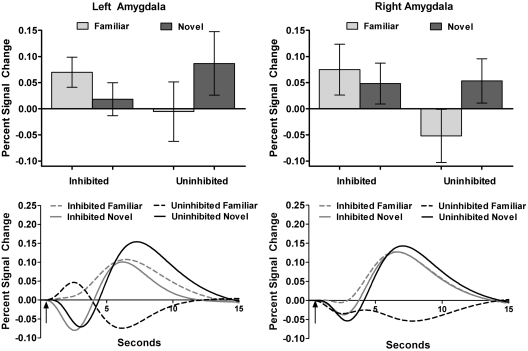
To examine temperament differences in amygdala responses when viewing faces, we compared change in amygdala BOLD signal when viewing novel or familiar faces in persons with an inhibited or an uninhibited temperament. The two temperament groups had significantly different patterns of bilateral amygdala BOLD signal change when viewing novel compared to familiar faces (temperament × face type interaction, P < 0.05 corrected, Figure 1). Post hoc analysis of variance for percent signal change confirmed the significant interaction of temperament group by face type in both the left and right amygdala, F(1,31) = 4.36, P = 0.045 and F(1,31) = 8.21, P = 0.01, respectively. Specifically, the inhibited participants had similar increases in left and right amygdala BOLD signal when viewing both novel and familiar faces (Figure 2; both P > 0.10). In contrast, the uninhibited participants showed a significantly greater BOLD response when viewing the novel compared to the familiar faces in the right amygdala, T(14) = 3.13, P = 0.01. BOLD response was also greater for novel faces in the left amygdala for the uninhibited participants, but the difference failed to reach significance. It should be noted that for the uninhibited participants, average amygdala BOLD response to the familiar faces was lower than BOLD response to the baseline condition (i.e. deactivation), although the difference failed to differ significantly from baseline for either amygdala (both P’s > 0.30).
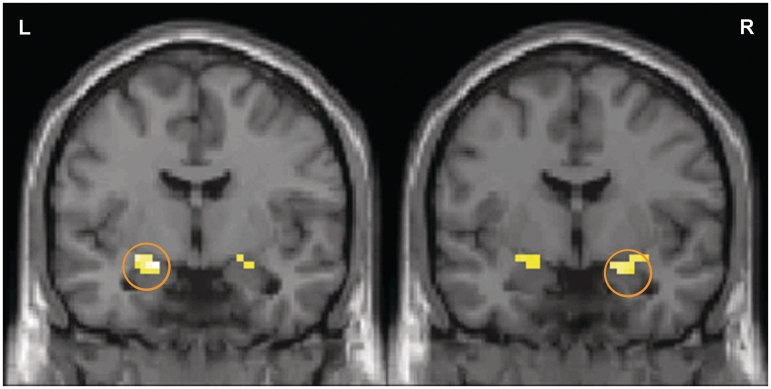
Fig. 1.
Temperament modulates amygdala BOLD response to novel and newly familiar faces. Amygdala BOLD signal increased when viewing both novel and familiar faces for the inhibited participants, but only when viewing novel faces for the uninhibited participants. In both the left and right amygdala there were significant clusters for the contrast of uninhibited > inhibited for novel > familiar faces (cluster size = 22 voxels each). Activation maps for the left and right amygdala are superimposed on a coronal section of a single standard brain image (MNI canonical T1 image; left, y = –6; right, y = –3). The activation map is thresholded at P < 0.05 and cluster >11 voxels for the amygdala region of interest.
Fig. 2.
Inhibited temperament group shows similar activation to novel and newly familiar faces. In the top panel, bar graphs show average percent signal change values (relative to baseline) for the familiar and novel faces for each temperament group. Lines represent standard error or the mean. In the bottom panel, the time course line graphs illustrate the average hemodynamic response functions by temperament group and face type, with arrows indicating when the face was presented (0.5 s).
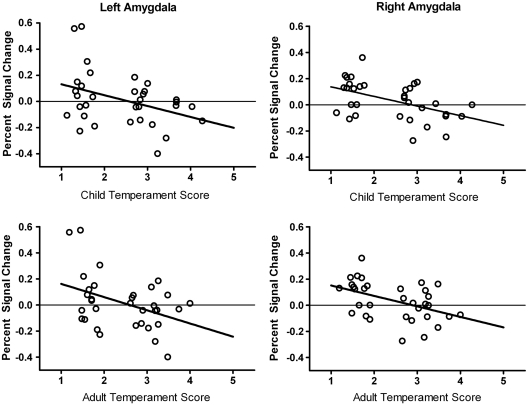
Next, we examined the relationship between temperament scores and amygdala percent signal change scores (extracted from significant clusters; see Methods section). Amygdala percent signal change was inversely correlated with both child temperament scores (left: r = –0.38, P = 0.03; right: r = –0.48, P = 0.005) and adult temperament scores (left: r = –0.41, P = 0.02; right: r = –0.46, P = 0.01). Thus, larger differences in amygdala BOLD response to novel relative to familiar faces were correlated with lower (more uninhibited) temperament scores (Figure 3), consistent with the between-groups analysis.
Fig. 3.
Temperament scores correlate with amygdala BOLD response. Both child and adult temperament scores inversely correlate with amygdala percent signal change values for novel relative to familiar faces. Scatterplots illustrate the relationship for child temperament (top row) and adult temperament (bottom row) scores for both the left (left column) and right (right column) amygdalae.
Finally, because previous studies have reported amygdala lateralization differences by gender (Zald, 2003; Cahill et al., 2004) or personality (Baeken et al., 2009), we tested for gender and temperament effects on amygdala lateralization. There were no main effects or interactions with gender or temperament on lateralization (all P’s > 0.45).
Whole-brain analysis
We performed exploratory whole-brain analysis to identify other brain regions that showed temperament differences in BOLD signal when viewing novel compared to familiar faces. Increased activation for novel relative to familiar faces differed by temperament group (temperament group × face type interaction, P < 0.05 corrected; Table 2). Similar to the ROI analysis, the uninhibited group showed increased BOLD signal when viewing novel compared to familiar faces across multiple brain regions. The brain regions included occipital cortex, prefrontal cortex, temporal cortex, insula, thalamus, caudate and cerebellum, consistent with brain regions that show increased activation to novel stimuli (Kiehl et al., 2005). There were no brain regions where the BOLD signal for novel versus familiar faces was greater for the inhibited participants.
Table 2.
Statistically significant brain regions for the interaction of temperament group uninhibited > inhibited) by face type (novel > familiar)
| Cluster |
Peak voxel |
|||||
|---|---|---|---|---|---|---|
| Brain regions (hemisphere) | Cluster Size | P-value | t-score | x | y | z |
| Frontal lobes | ||||||
| Lateral prefrontal cortex (L) | 2247 | <0.001 | 5.14 | –45 | 33 | 9 |
| Dorsolateral prefrontal cortex (R) | 149 | <0.001 | 3.79 | 30 | 42 | 15 |
| Inferior frontal gyrus (R) | 542 | <0.001 | 4.88 | 54 | 21 | 12 |
| Cerebellum and brainstem | ||||||
| Cerebellum dentate and declive (R) | 254 | <0.001 | 4.86 | 15 | –54 | –30 |
| Cerebellum dentate and culmen (L) | 222 | <0.001 | 3.85 | –12 | –48 | –33 |
| Cerebellum VIII and Crus I (L) | 80 | <0.001 | 4.04 | –24 | –60 | –39 |
| Brainstem (R/L) | 78 | <0.001 | 4.14 | 9 | –33 | –45 |
| Temporal lobes | ||||||
| Middle temporal gyrus (R) | 84 | <0.001 | 3.94 | 45 | –66 | 9 |
| Middle temporal gyrus (L) | 38 | <0.001 | 3.62 | –45 | –60 | 0 |
| Amygdala (L)a | 22 | 0.004 | 2.72 | –18 | –6 | –15 |
| Amygdala (R)a | 22 | 0.013 | 2.29 | 21 | 0 | –18 |
| Occipital lobes | ||||||
| Occipital cortex/BA19 (R) | 46 | <0.001 | 3.60 | 36 | –78 | 21 |
| Occipital cortex/BA19 (L) | 104 | <0.001 | 3.98 | –45 | –75 | 18 |
| Occipital cortex/BA18 (R) | 43 | 0.001 | 3.30 | 6 | –78 | –9 |
| Fusiform gyrus (R) | 27 | 0.001 | 3.34 | 39 | –36 | –27 |
| Calcarine sulcus/Precuneus (R) | 28 | 0.001 | 3.00 | 15 | –60 | 18 |
| Calcarine sulcus/Precuneus (L) | 80 | <0.001 | 3.90 | –6 | –69 | 21 |
| Sub-cortical regions | ||||||
| Insula (L) | 979 | <0.001 | 5.51 | –39 | 3 | 0 |
| Insula (R) | 45 | <0.001 | 4.08 | 36 | –3 | 12 |
| Caudate (R) | 52 | <0.001 | 4.64 | 18 | 18 | 3 |
| Thalamus (L) | 31 | <0.001 | 3.68 | –9 | –12 | 18 |
aSmall volume corrected.
DISCUSSION
The present study demonstrates that inhibited temperament is characterized by a sustained increase in amygdala BOLD response to newly familiar human faces, and not an exaggerated amygdala response to novel faces. Both inhibited and uninhibited groups had an increased amygdala BOLD response to novel faces, consistent with a prior study of typical adults (Schwartz et al., 2003b). However, inhibited participants also showed an increased BOLD response to the familiarized faces, whereas uninhibited participants showed deactivation of the amygdala BOLD response, consistent with amygdala habituation (Wright et al., 2001). Thus, it was the familiarized faces, the ‘weaker’ condition, and not the stronger, novel face condition, that elicited temperament differences in amygdala BOLD response. Similarly, clinical populations with anxiety disorders are characterized by anxious responses to small or ambiguous threats and not by overreactions to clear, unambiguous threats (Lissek et al., 2006).
Previous theories of inhibited temperament have proposed a hyper-reactive amygdala response to novel faces, consistent with the definition of inhibited temperament as wariness or avoidance responses to novelty. We propose instead that in inhibited temperament the amygdala fails to respond normally to recently familiarized stimuli; that is, the amygdala continues to show an increased response to familiar stimuli as though they were still novel. Importantly, recognition memory was not impaired, suggesting that the sustained response to the familiar faces was not due to memory deficits.
Reeb-Sutherland and colleagues (2009) also report that individuals with an inhibited temperament have a sustained physiological response. In a study of fear-potentiated startle, both inhibited and uninhibited adolescents had an increased startle to the threat cues. However, when safety cues were presented, only the individuals with an inhibited temperament continued to have an increased startle response. Together with the findings from the present study, this suggests that individuals with an inhibited temperament have a sustained autonomic nervous system response, reflected by increased startle and amygdala activation, even after the stimuli have been demonstrated to be relatively safe.
Our findings differ from the findings of Schwartz et al. (2003a). In the present study and our prior study, the inhibited participants had increased amygdala BOLD responses to both novel and familiarized faces, whereas the inhibited participants in the Schwartz study (2003a) had a heightened amygdala BOLD response only to the novel faces. Differences in study design or samples may account for the disparity. In the Schwartz study (2003a), faces were presented in alternating novel and familiarized blocks, which may have reduced novelty both within a block (once participants saw the first few faces in a block, they knew the remaining face types) and within the study (once participants determined that blocks were alternating, they could anticipate the next type of faces). In our study, we randomly presented a single face at a time, removing potential confounding effects of predictability inherent in the design. If predictability is a critical source of differences in the two studies, it would suggest that the amygdala responses of those with an inhibited temperament may be directly linked to contextual factors such as predictability or uncertainty. Study samples also differed. In our study, we used retrospective and current self-report whereas Schwartz and colleagues (2003a) assessed temperament during childhood. While childhood assessments may more closely reflect an underlying biology due to less opportunity for environmental impact, adding current temperament measures has the advantage of selecting only those participants who are still inhibited, the group at highest risk for later psychopathology (Hirshfeld et al., 1992; Chronis-Tuscano et al., 2009). It is also possible that the selection procedures for participants may critically impact the observed biological responses. Participants in the Schwartz study (2003a) were classified as behaviorally inhibited based on short-term responses to novelty as toddlers, which may have led to a sample with particularly high responses to novelty. In contrast, the self-report measures provide a broad assessment of characteristics related to behavioral inhibition in both childhood and adulthood which, in contrast, place more emphasis on long-term, continued avoidance of novelty.
There are several limitations to the present study. First, as in the study by Schwartz (2003a) we experimentally manipulated familiarity to create familiarized faces. This procedure has the advantage of providing fully standardized stimuli across participants, but limits the stimuli to a superficial level of familiarization as participants do not learn any other characteristics about the individuals. In contrast, Beaton and colleagues (2008) used personally familiar faces versus novel faces in their study of a similar temperament dimension (shy/bold). Yet, consistent with the present study, shy people had increased amygdala BOLD response to both the personally familiar and novel faces. This makes it unlikely that the heightened response to recently familiarized faces in the present study are an artifact of the familiarization procedure. Second, we only examined amygdala response to human faces. It will be important for future studies to test responses to novel objects to determine whether the increased amygdala response is specific to faces or reflects a broader deficit to any stimulus. Third, both this study and prior studies have only compared two extreme temperament groups. It will be important to include a third group of average temperament in future studies to further clarify how these extreme groups differ from a typical group.
In conclusion, by studying brain function in two extreme groups of temperament, we may begin to identify the underlying mechanisms through which inhibited temperament confers risk for social anxiety. In the present study, the inhibited participants did not have an exaggerated amygdala response to novel faces as previously thought, but instead showed a sustained amygdala response to familiarized faces. Although the amygdala typically habituates following repeated face presentations, in inhibited individuals the habituation did not occur. Thus, a sustained amygdala response to newly familiar people may be one cause of elevated social anxiety in inhibited individuals. This finding may also have implications for prevention or intervention. For individuals with temperament-based risk for social anxiety, increased exposure to human faces through traditional exposure therapy or through computer-based training may enhance amygdala habituation and reduce social anxiety.
Conflict of Interest
None declared.
Acknowledgments
Research was supported in part by funding from the National Institute of Mental Health (K01-MH083052 to J.U.B.; R01-MH074567 to D.H.Z.); the National Institute of Drug Abuse (DA015137, DA020149 and DA00366 to R.L.C.); the Vanderbilt Institute for Clinical and Translational Research (1-UL1-RR024975 NCRR/NIH); Vanderbilt University Institute of Imaging Science. Portions of this work were presented at the American College of Neuropsychopharmacology Annual Meeting, Hollywood, FL, December 2009. The authors thank Jerome Kagan for helpful comments on an earlier version of the manuscript and Kristi Simmons and Zak Millman for research assistance.
REFERENCES
- Baeken C, De Raedt R, Ramsey N, et al. Amygdala responses to positively and negatively valenced baby faces in healthy female volunteers: Influences of individual differences in harm avoidance. Brain Research. 2009;1296:94–103. doi: 10.1016/j.brainres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Schmidt LA, Schulkin J, Antony MM, Swinson RP, Hall GB. Different neural responses to stranger and personally familiar faces in shy and bold adults. Behavioral Neuroscience. 2008;122:704–9. doi: 10.1037/0735-7044.122.3.704. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–12. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, et al. Further evidence of association between behavioral inhibition and social anxiety in children. American Journal of Psychiatry. 2001;158:1673–9. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the amygdala in novelty detection. Neuroimage. 2010;50:1188–93. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] Neuroimage. 2002;16:s497. [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An fMRI investigation. Learning & Memory. 2004;11:261–66. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53:1033–9. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1994;2:189–210. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–76. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg MD, Friston KJ. The choice of basis functions in event-related fMRI. Neuroimage. 2001;13:149. [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, et al. Stable behavioral-inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:103–11. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–73. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–71. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. The Long Shadow of Temperament. Cambridge, MA: Harvard University Press; 2004. [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n=100) MRI study of an auditory oddball task. Neuroimage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: a potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–70. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A The Karolinska Directed Emotional Faces - KDEF. 1998. Department of Clinical Neuroscience, Psychology section, Karolinska Institute. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Muris P, Bos AER, Mayer B, Verkade R, Thewissen V, Dell’Avvento V. Relations among behavioral inhibition, Big Five personality factors, and anxiety disorder symptoms in non-clinical children. Personality and Individual Differences. 2009;46:525–9. [Google Scholar]
- Reeb-Sutherland BCP, Helfinstein SMB, Degnan KAP, et al. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:610–7. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Development and Psychopathology. 1992;4:301–21. [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Early childhood temperament as a determinant of externalizing behavior in adolescence. Development and Psychopathology. 1996;8:527–37. [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–15. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003a;300:1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, et al. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003b;53:854–62. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The structured clinical interview for Dsm-Iii-R (Scid) .1. History, rationale, and description. Archives of General Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wilson R, Matheny A. Behavior genetics research and infant temperament: the Louisville twin study. In: Plomin R, Dunn J, editors. The Study of Temperament: Changes, Continuities, and Challenges. Hillsdale, NJ: Erlbaum; 1986. pp. 81–98. [Google Scholar]
- Windle M, Windle RC. Adolescent temperament and lifetime psychiatric and substance abuse disorders assessed in young adulthood. Personality and Individual Differences. 2006;41:15–25. [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney S, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]