Abstract
Two neurodevelopmental disorders, Williams syndrome (WS) and autism, are both commonly described as having opposite social profiles: social avoidance in autism vs hypersociability in individuals with WS. The goal of this study was to contrast the brain activity associated with language processing in these two populations, in order to understand the very likely interplay between the use of language and the sociability dimension, on which these disorders diverge. Towards this aim, the N400 component of the event-related potentials was used to quantify the processing of semantic integration in these two populations. Results revealed that individuals with WS showed a significantly larger N400 effect, as compared to both typical controls and individuals with autism, while the latter group demonstrated the smallest N400 effect. The findings demonstrate quite opposite profiles of neural correlates of language processing in WS and autism, mirroring their contrasting social phenotypes.
Keywords: Williams syndrome, autism spectrum disorders, sociability, event-related potentials, N400
INTRODUCTION
Williams syndrome (WS) and autism (or, generally, autism spectrum disorders, ASD) are both neurodevelopmental disorders associated with atypical social and communication profiles. While individuals with WS are typically described as extremely sociable, friendly, empathic and possessing socially engaging language (Doyle et al., 2004; Järvinen-Pasley et al., 2008), those with ASD are characterized by impaired social interaction and communication skills. Although it is widely recognized that autism is a constellation of cognitive and behavioral deficits, impairments in social functioning and in verbal communication are consistently observed in all individuals with autism, regardless of intelligence quotient (IQ) level or severity of symptoms. On the other hand, despite the multifactorial nature of WS and its complex cognitive and behavioral profile, excessive sociability and a keen interest in interacting with people are the most robust behavioral characteristics of WS (cf. Järvinen-Pasley et al., 2008), a neurodevelopmental disorder caused by a hemizygous deletion of 25–30 genes on chromosome 7q11.23 (Korenberg et al., 2000).
In line with this polar social orientation of the two syndromes are their distinct language skills, which follow a remarkably similar divide. Although linguistic abilities can vary significantly across the autistic spectrum, from a complete absence of receptive and expressive speech to mild impairment in semantics (Tager-Flusberg, 2003, 2004), an overall deficit in the ‘communicative use of language’ is a defining feature of ASD and one of the triad of symptoms at the core of the ASD diagnosis [American Psychiatric Association (APA), 2000]. On the other hand, despite mild to moderate mental retardation, individuals with WS appear to have ‘relative’ proficiencies in linguistic skills (cf. Mervis et al., 1999), including elaborate and rich vocabularies and pictorial, affect-rich expressive language (Reilly et al., 2004; Brock et al., 2007; Gothelf et al., 2008), that make their speech engaging and facilitate—rather than impede, as in ASD—the likelihood of interaction and communication with others. It is this divide in language skills and use, which mirrors the opposite social profiles that led us to explore how individuals with WS and ASD process language.
One common factor that may underlie a wide range of language abnormalities in ASD can be conceptualized as a difficulty in using semantic context to understand and predict meaning (Tager-Flusberg, 2003, 2004; Harris et al., 2006; Walenski et al., 2006). Even individuals with high-functioning ASD have shown deficits in utilizing sentence context to determine the context-dependent pronunciation of words with several meanings (Firth and Snowling, 1983; Happe, 1997) and to comprehend idioms, an ability heavily relying on interpreting language in context (Kerbel and Grunwell, 1998). Furthermore, it has been demonstrated that a prototypical IQ profile for high-functioning autism is characterized by a relatively depressed score on tasks of comprehension, in the face of an otherwise preserved and even IQ profile (Siegel et al., 1996; Goldstein et al., 2002), underscoring that deficit in understanding language is one of the core features of ASD.
On the other hand, while much controversy has ensued in the field regarding which aspects of language—syntax, semantics, phonology or pragmatics—and how much of it is ‘spared’ in WS (see Karmiloff-Smith et al., 2003 for a review), there seems to be a general consensus that compared to the severe anomalies and deficiencies in non-verbal, visuospatial cognitive functioning, the expressive language of WS individuals is ‘relatively’ proficient (Mervis et al., 1999; Bellugi et al., 2000), even if distinct linguistic skills are not uniformly intact. When compared to their mental-age and, often, chronological-age peers, WS individuals as a group are unusually loquacious and highly expressive (Udwin and Yule, 1990), use a wide variety of affective and social engagement devices in their narratives, such as character speech, sound effects, intensifiers, etc. (Reilly et al., 2004), and give accurate and detailed verbal descriptions of objects (Bellugi et al., 1994) despite being unable to draw the same objects. On standardized vocabulary tests such as the Peabody Picture Vocabulary Test (PPVT), WS subjects perform well above their respective mental ages (Bellugi et al., 2000) and on fluency tasks they produce at least as many items as chronological-age controls, albeit generating more low-frequency words (Bellugi et al., 1994). Additionally, WS individuals produce associates related to the primary meaning of the homonym words as well as unimpaired controls do (Bellugi et al., 2000). Thus, notwithstanding the disagreements regarding the degree of impairment of the morphosyntactic aspects of language, which are beyond the scope of this project, it appears that the vocabulary and semantic organization are ‘relative’ strengths in WS, standing out in stark contrast to their overall intellectual disability and spatial deficits. Yet, it is important to note that the peculiarity of the choice of words generated on fluency tasks, coupled with experimental evidence indicating that individuals with WS (i) produce definitions compatible with both primary and secondary meanings of homonyms on definition tasks, and (ii) provide an equal number of primary and secondary associates to homonyms on a similarity judgment task (Bellugi et al., 2000), suggest an ‘atypical’ (rather than plainly impaired) semantic organization (cf. Karmiloff-Smith et al., 2003).
Thus, the goal of the current study was to contrast the semantic processing in WS and ASD, in order to understand the very likely interplay between the ability to derive meaning out of linguistic context and the sociability dimension, on which WS and ASD seem to represent two ends of the continuum (cf. Tager-Flusberg et al., 2006; Brock et al., 2008; Riby and Hancock, 2008). To quantify the contextual integration ability, we examined the degree to which an electrophysiological index of semantic processing, the N400 component of the event-related potentials (ERPs), distinguished individuals with WS from those with ASD. Considered a robust marker of contextual integration, N400 is sensitive to variations in the semantic content and is thought to reflect the extent to which an individual word is expected, or semantically plausible, given its current linguistic—sentential or discourse—context (Kutas and Hillyard, 1980, 1984).
In the general population, the occurrence of a semantically incongruent word at the end of a sentence is associated with a large N400, a negative-going potential occurring ∼400 ms after the critical word onset (Kutas and Hillyard, 1980). The N400 magnitude is inversely related to the degree of semantic fit between a word and its context, such that a word more predictable from the preceding context generates a smaller N400 than a word that is less expected given the preceding words in the sentence (Kutas and Hillyard, 1980, 1984). Overall, it appears that the N400 indexes the ease of integration of stimuli into an ongoing context. When this integration is easier—that is, when a word fits with, or is predicted by the context—the amplitude of N400 is smaller (Kutas and Federmeier, 2000).
Given the behavioral evidence reviewed above, we have predicted that individuals with ASD will be impaired in their abilities to make use of context to establish a semantic expectation against which a target word is judged, manifested in smaller N400 amplitudes, as compared to typically developing (TD) controls. On the other hand, because individuals with WS show relatively preserved albeit atypical semantics, we expected them to exhibit N400 amplitudes comparable to those of TD controls. We have tested this 2-fold prediction using a sentence paradigm based on that described by Holcomb et al. (1992), in which auditory sentences are presented one word at a time, with the final word being either semantically congruent or incongruent with the meaning of the rest of the sentence. Using an auditory paradigm (which is used less commonly than visual/reading tasks in studying the N400 in adults) where participants are listening to ‘spoken’ language was critical given the primacy of this modality in both expressive language of individuals with WS and in impaired verbal communication in ASD. We also examined whether IQ differences affected their N400 responses or moderated the group differences on the N400.
METHODS
Participants
Sixteen individuals with WS (seven males; mean age 21 years, range 17–30 years) were recruited as part of an ongoing multicenter research program based at the Salk Institute. Genetic diagnoses of WS were established using fluorescent in situ hybridization (FISH) test for elastin (ELN), a gene invariably associated with the WS microdeletion (Korenberg et al., 2000). Twelve individuals with ASD (11 males; mean age 31 years, range 17–46 years) were recruited from the area clinics. Participants with ASD were all classified as having the social and communicative deficits of autism, including abnormal eye contact, according to the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994) as young children. As adults, all met the criteria for autism on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000), as well as the DSM-IV criteria for autism (APA, 2000) as diagnosed by a licensed clinical psychologist. Eighteen healthy individuals (eight males; mean age 30 years, range 19–41 years) were recruited through advertisements in local newspapers and fliers posted in the community and constituted the TD control group. All TD participants were screened for the level of education, and only those with no more than 2 years of college-level education were included in the study.
All potential participants were screened for current and past psychiatric and/or neurological problems, including history of acquired brain injury and seizure disorder. All participants were native speakers of American English and had no known hearing deficits. Data were also collected from two additional TD participants but not included in the analyses because of the excessive motion artifacts in the EEG data.
Demographic and psychometric information for each group is summarized in Table 1. One-way analysis of variance (ANOVA) showed significant age differences between the groups [F(2,45) = 9.40, P < 0.001), due to the WS group being younger than the ASD (P = 0.003) or the TD (P = 0.001) group.
Table 1.
Demographic, psychometric and behavioral data for WS, ASD and TD groups
| IQ |
ERP Task | ||||||
| Gender | Age | Full Scale | Verbal | Performance | Accuracy Rate | ||
|---|---|---|---|---|---|---|---|
| Group | N | F/M | M (SD; range) | M (SD) | M (SD) | M (SD) | % |
| WS | 16 | 9/7 | 21 (3.9; 17-30) | 68 (6.5) | 72 (6.1) | 66 (7.6) | 96.1 |
| ASD | 12 | 1/11 | 31 (8.0; 17-46) | 86 (14.7) | 84 (17.5) | 91 (15.6) | 81.9 |
| TD | 18 | 8/10 | 30 (8.8; 19-41) | 100 (11.8) | 101 (11.0) | 100 (11.2) | 96.9 |
Note. The WS group was significantly younger than both ASD and TD groups (both P < 0.01); there were no significant differences in age between the ASD and TD groups (P = 0.98).
Stimuli
Participants listened to a series of 80 highly contextually constrained sentences, presented over headphones one word at a time. Naturally spoken sentences were digitized at 12 kHz and presented at a rate of one word/1000 ms. Half of the 80 sentences ended with a last word judged by an independent sample to be the best completion ending for that sentence (e.g. ‘Kids learn to read and write in “school” ’), and half ended with an anomalous last word (e.g. ‘Kids learn to read and write in “finger” ’). The participant’s task was to indicate, by a button press, whether or not the preceding sentence made sense. As described in detail elsewhere (Holcomb et al., 1992; Röder et al., 2000), 160 highly constrained sentences were originally created (a cloze probability >0.8) and were divided into two lists of 80 sentences each. In each list, 40 sentences were presented in their original version that is with their best, highly constrained completion, while in the remaining 40 sentences the last words were exchanged with the words from another list, creating sentences with incongruent endings while retaining the same sentence stems. Each participant heard one list of 80 sentences, with the two lists counterbalanced across participants.
Procedure
ERP task
Participants were first exposed to 10 practice trials; behavioral and electrophysiological responses to these practice stimuli were not included in later analyses. Each trial ended with an onset of a visual prompt, which followed 3 s after the final word and indicated that the participant should make a judgment. The prompt remained on the computer screen until the subject made a button response indicating whether or not the sentence made sense. This delayed response was designed to prevent any contamination of ERPs associated with processing of the final word by brain activity associated with motor response. There was a 2- to 3-min self-paced break after every 20 trials.
Cognitive testing
At the conclusion of the ERP testing, participants were administered a battery of standardized cognitive tests, in order to ascertain the average range of cognitive abilities in each group, to rule out potential confounding factors for predicted ERP differences. As a measure of general cognitive ability, all participants were administered the Wechsler Adult Intelligence Scale Third Edition (WAIS-III; Wechsler, 1997) from which verbal, performance and overall (i.e. full scale) intelligence quotients (VIQ, PIQ and FSIQ) were derived. The IQ scores achieved by each group are displayed in Table 1. A repeated measures ANOVA between the groups revealed a significant main effect of group [F(2,45) = 34.9, P < 0.001], such that the TD group obtained significantly higher scores (on VIQ, PIQ and FSIQ) than both WS and ASD groups (all P < 0.01), and the ASD group had higher IQ scores than the WS group (all P < 0.01), as was expected given the well-documented cognitive phenotypes of WS and ASD.
EEG recording and off-line processing
The electroencephalogram (EEG) was recorded using a 16-channel tin electrode cap (ElectroCap International Inc., Eaton, Ohio) at 250 Hz, with a bandpass of 0.01–100 Hz. Recordings included eight sites from standard 10/20 placements over left- and right-fronto-central (Fp1/Fp2), frontal (F7/F8), occipital (O1/O2) and mid-line positions (Cz and Pz) and six non-standard sites including left- and right-anterior temporal (L/R22, situated one-half the distance between F7/F8 and T3/T4), temporal (L/R41, situated 33% of the distance from T3/T4 to C3/C4) and temporo-parietal (WL/WR, situated 50% of the distance between T3/T4 and P3/P4). Additionally, the electroocculogram (EOG) was recorded from over (Fp1) and under the left eye (Le) to monitor blinks and vertical eye movements, and from the right outer canthus (He) to monitor horizontal eye movements. Impedances were maintained below 5 kilo ohms. During data acquisition, all electrodes were referenced to A1 (right mastoid).
Off-line, the EEG was low-pass filtered at 30 Hz, re-referenced to an average mastoid reference, and subjected to an Independent Component Analysis (ICA; Jung et al., 2000) for correction of eye blinks and lateral eye movement artifacts. The ICA-based artifact rejection procedure allowed retention of most of the trials for analysis. After excluding trials associated with large biological or technical artifacts, a mean of 34/40 trials (85%) per condition (83% for the WS group; 83% for the ASD group; 92% for the TD group) were retained for analysis (the proportion of artifact-free trials per condition was not significantly different between the three groups; P = 0.10). Artifact-free data were segmented into 1000-ms-long ERP epochs time-locked to the onset of the final word, for each trial associated with the correct response. The 1000-ms-long segments, baseline-corrected using a 100-ms baseline preceding the final word, were averaged separately for congruent and incongruent conditions.
ERP component extraction
For objective, data-driven, measurements of the ERP components, ERPs were subjected to a principal components analysis (PCA), a formal multivariate procedure which has a number of advantages over peak and area measures (cf. Spencer et al., 2001). PCA is a factor-analytic statistical approach used with ERP data to capture variance across electrode sites or across time points, thereby distinguishing between consistent patterns of electrocortical activity and separating latent components that may not be readily apparent in the ERP averages (Spencer et al., 2001; Fishman et al., 2008). A PCA over time, or so-called temporal PCA, was conducted, with the data matrix consisting of voltage readings at each of the 250 time points (1000-s epochs, sampled every 4 ms) as variables and all the channels (16) × conditions (2) × participants (48) as cases. PCA decomposition was based on covariance association matrix and the resulting solution was rotated using the Varimax procedure to maximize the amount of variance associated with the smallest number of variables (Donchin and Heffley, 1978). The number of components to be rotated was determined by the Scree test (Cattell, 1966).
The resulting PCA factor scores, reflecting the magnitude of activity of a given temporal factor (i.e. time window) for each combination of participant, sentence category and recording site, served as dependent variables and were analyzed for variance across groups and experimental conditions. The N400 was assumed to be embodied by the temporal factor falling in the window corresponding to the N400 latency range (i.e. 400–600 ms) at the vertex, Cz (in accordance with the well-established scalp distribution of the N400 elicited by auditory stimuli). These PCA-derived N400 values were analyzed using repeated measures ANOVA with three between-subjects levels (group: WS, ASD, TD) and two within-subject levels (sentence category: congruent vs incongruent). Tukey HSD was used to test for differences between pairs of groups.
RESULTS
Behavioral performance on the ERP task (i.e. accuracy of judgment whether the preceding sentence made sense or not) was first analyzed across the groups. TD participants correctly accepted 97.8% of the semantically appropriate sentences and correctly rejected 96% of semantically anomalous sentences; WS participants correctly accepted 96.8% of the semantically congruent sentences and correctly rejected 95.4% of semantically incongruent sentences; and ASD group correctly accepted 82.7% of the congruent sentences and correctly rejected 81% of incongruent sentences (Table 1). ANOVA conducted on three levels of group revealed significant effect for group [F(2,45) = 10.10, P < 0.001, η2 = 0.33]. Pair-wise comparisons revealed that ASD had significantly lower accuracy rates than either TD or WS group (P < 0.001 for both). The WS group’s accuracy was not significant different than that of TD (P = 0.96). The accuracy rate was uncorrelated with verbal IQ (r = 0.13, P = 0.39 for the overall sample; P ranging from 0.29 to 0.68 for within-group correlations).
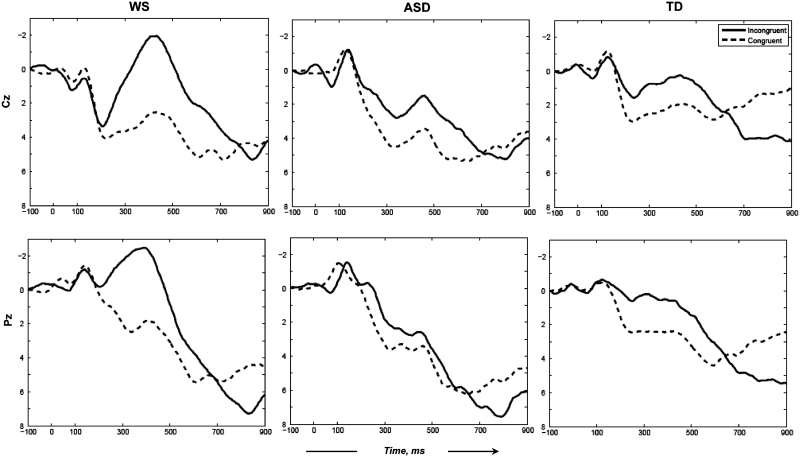
Averaged ERP waveforms for congruent and incongruent conditions, prior to PCA, are plotted by group at Cz (vertex electrode, where the auditory N400 is typically at its maximum) and Pz (centro-parietal midline electrode) in Figure 1. There is a characteristic negative deflection with a peak latency of ∼400–450 ms after the target word (N400), which varies as a function of two sentence categories, i.e. incongruent vs congruent. Specifically, it appears that all groups, to an extent, exhibit the typical N400 effect with a larger negativity elicited by the incongruent sentence endings. However, a clear difference between the groups is also apparent in that this effect appears to be markedly larger in individuals with WS and almost negligent in the ASD group. To quantify these differences, N400 amplitude values were derived by applying PCA to the data, as described in ‘Methods’ section. The Scree test suggested retention of 15 factors accounting for 92.8% of the variance, which were then rotated to simple structure using Varimax. Thus, the temporal PCA reduced the dimensionality of the dataset from 250 time points to 15 temporal factors presumed to reflect 15 underlying processes/ERP components.
Fig. 1.
Mid-line electrodes (Cz and Pz) waveforms averaged for individuals with WS, ASD and TD. Zero time signifies stimulus (last word of the sentence) onset. Negative voltages are plotted as upward deflections.
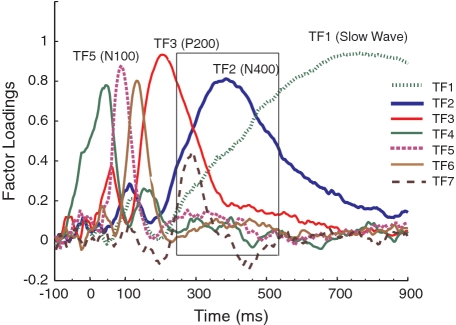
Figure 2 represents factor loadings for the first seven temporal factors (the remaining factors accounted for a negligible amount of variance, <1% each). Factor loadings, which are the correlations between the original variables (i.e. time points) and the factors, signify the extent to which a factor has an influence on each time point; hence, higher loadings indicate time points when the factor is strongly active, whereas smaller loadings indicate time points when the factor is relatively inactive. In other words, Figure 2 illustrates the time course of the first seven factors extracted by the PCA. The first temporal factor (TF1), which accounted for 44% of the variance, appeared to reflect the classical Slow Wave, which typically emerges among the first factors in temporal PCAs (Spencer et al., 2001). The second factor, TF2, loaded highly in the 400 ms (300–500) range, the time window corresponding to N400 in which the differences between sentence categories and groups emerged in the raw averaged data (Figure 1). Thus, TF2 was presumed to represent the N400 component. The third factor, TF3, which loaded highly in the 180–220 ms range of the epoch, was attributed to the P200. TF2 and TF3 accounted for 16 and 12% of the variance, respectively.
Fig. 2.
Factor loadings for seven temporal factors (higher loadings indicate time points when the factor is strongly active). Remaining factors not plotted here were negligible in size.
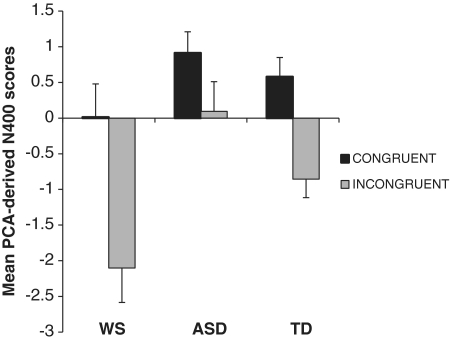
Ultimately, the PCA resulted in a finite set of factors corresponding to the ERP components visible in the average waveforms (Figure 1). Based on the temporal variance accounted for, the primary candidates for further analyses were TF2 factor scores at Cz, where the N400 elicited by auditory stimuli is typically at its maximum. Next, the means of the TF2 factor scores at Cz (referred to as N400 in future analyses, in the interest of parsimony) were analyzed with regards to the group membership and sentence category. A 3 (group) × 2 (sentence category) mixed ANOVA with repeated measures of the sentence category revealed a main effect of congruity (i.e. N400 effect), such that incongruent sentence endings elicited a significantly larger N400 than congruent stimuli [F(1,43) = 29.02, P < 0.001, η2 = 0.409; Figure 3]. In addition, a significant group effect was also observed [F(2,43) = 6.37, P = 0.004]. Pair-wise comparisons revealed that WS had significantly larger N400 effect than either TD group (P = 0.03) or ASD group (P = 0.001). The ASD group’s N400 effect was nearly significantly smaller than that of TD (P < 0.07).
Fig. 3.
Means of the PCA-derived N400 component, as a function of group and sentence category. Error bars represent ±1 s.e.m.
Next, because the WS and ASD groups differed in IQ, and had virtually non-overlapping distributions, the correlation between IQ and the N400 amplitude was examined to rule out the IQ confound in the N400 group differences. There were no significant correlations between IQ (FSIQ, VIQ or PIQ) and the N400 amplitude (all r < 0.10; all P > 0.28). Furthermore, to rule out the potential confound of age, given the younger mean age of the WS group, the correlation between age and N400 amplitude was examined. There was no significant correlation between age and N400 in the overall sample (P = 0.18). Likewise, Pearson correlations assessed separately within each group revealed no significant results (all r < 0.18; all P > 0.21).
DISCUSSION
The current research represents the only known to us study designed to compare the electrophysiological correlates of language comprehension in two populations with opposite communication and social profiles—WS, a syndrome characterized by unusual social interest, and ASD, a syndrome characterized by social withdrawal. The primary intent of the study was to contrast the ERP index of semantic processing in these two populations, with an underlying assumption that the ability to derive meaning from linguistic context might be related to social phenotype. As predicted, there was a systematic group difference between WS and ASD participants with respect to the magnitude of the ERP component known to be sensitive to variations in semantic content. Specifically, individuals with WS showed a significantly larger N400 effect, as compared to both typical controls and ASD individuals, while the latter group demonstrated the smallest N400 effect. These effects were not driven by age or overall cognitive abilities, as measured by IQ.
The N400 indexes the cognitive demand incurred by the integration of a meaningful stimulus (such as a word) into a more general semantic context (such as a sentence). Consequently, smaller N400 effect found in the ASD group suggests that they make less use of contextual information, which could be due to a less elaborate or less densely connected semantic network. Because of the N400 sensitivity to semantic context, it is considered a metric of the pattern of spreading activation in the semantic network of the language user. Additionally, it is thought to provide insight into an individual’s ability to integrate this semantic information into a cohesive discourse representation (Osterhout and Holcomb, 1999). Thus, a plausible interpretation of the diminished N400 effect in ASD participants is that they rely less on integrating processes that bring the words of a sentence together into an integrated semantic representation. This interpretation is inline with other functional evidence regarding language comprehension and use in ASD. For instance, participants with autism have been found to show greater activation in visual primary and association cortices on tasks of sentence comprehension, suggesting that they routinely recruit visual imagery for comprehending sentences rather than comprehending them on a purely linguistic basis (Kana et al., 2006). Moreover, the diminished N400 effect found in ASD adults in this study is in line with the findings by Dunn and colleagues (Dunn et al., 1999; Dunn and Bates, 2005) who demonstrated a failure to show an N400 effect in children with autism (notably, using a different, single-word experimental paradigm), underscoring the notion that this effect is not simply an artifact of delayed development in ASD as it persists into adulthood.
As for WS, the finding of a greatly enhanced N400 effect was not necessarily expected given their relative proficiency in language skills, reviewed in detail in the Introduction section. If one accepts the notion that semantic processing is relatively unimpaired in WS, the N400 amplitudes observed in this group should have been comparable to those of TD, as was hypothesized. However, the empirical data indicate a significantly greater effect than that found in TD, which sets a stage for a more challenging and less transparent interpretation. ERP components (e.g. P300 or N400) seen in typical control individuals are presumed to be a baseline response, relative to which other populations (or the same individuals but under certain experimental manipulations) most often exhibit a ‘reduced’ effect. In fact, despite the N400 being conceivably one of the most widely studied ERP components, no other adult population has been found to have a ‘larger’ N400 effect than that observed in healthy controls. A clue might be found in the developmental literature indicating that increased N400 amplitudes have been well documented in childhood and early adolescence. According to a large-scale (N = 130) developmental ERP study (Holcomb et al., 1992), increased N400 effect is seen from 5 to 16 years of age, after which the effect is stabilized at adult-like levels. This age effect on N400 is thought to signify that sentence-level contextual factors play a greater role in language comprehension in children, than they do in more experienced language users (i.e. adults). This is presumed to be either due to the continued functional specialization of neural systems that take place throughout development or, alternatively, due to the plain fact that anomalous sentence endings become more expected with age, because an adult has had a lengthier experience with language and, as a result, more exposure to individual words.
However, the same reasoning is not easily applied when attempting to interpret the marked increase in the N400 effect found in the WS group. First, the continued brain development and specialization account can be ruled out given the adult age of the WS participants and the lack of correlation of N400 with age in our sample. It is similarly unlikely that adult individuals with WS would have had less exposure and less experience with language than age-matched TD controls (which could have led to reduced expectancies for incongruent words and, consequently, to a larger N400 effect). Although this latter account cannot be ruled out entirely without a targeted empirical investigation, it is likely that, given the keen interest in interacting with people and relative proficiency with language, individuals with WS probably have as much, if not more, experience as language users as their TD counterparts do.
Consequently, one prudent interpretation of the markedly enhanced N400 effect in WS is that, even as adults, individuals with WS continue to heavily rely on sentence-level contextual cues in order to derive meaning and comprehend language. Moreover, the larger than TD’s magnitude of the N400 effect, not seen in any other clinical adult population, is in line with the notion of the ‘atypically’ developed semantic organization and language in general, which appears to be the consensual view regarding WS (Karmiloff-Smith, 1998; Klein and Mervis, 1999).1
With regards to the potential source of these divergent results—the diminished N400 effect indicating a decrease in reliance on contextual factors in ASD, and the enhanced N400 effect signifying an increased use of contextual cues in WS—one must consider the possibility that alterations in early sensory processing within the auditory modality might influence the operation of higher order language-relevant systems. Specifically, the hypersensitivity of the auditory system in WS is well documented (Klein et al., 1990; Blomberg et al., 2006) and may in part underlie the relative proficiency in language seen in WS, as was first suggested by Neville et al. (1994). On the other hand, diminished sensitivity to perception of speech prosody, as measured by decreased mismatch negativity (MMN) of auditory ERPs, has been identified in ASD (Korpilahti et al., 2007) and might underlie the impaired language comprehension in ASD. Thus, the low-level sensory processes, which appear to be differentially affected in WS and ASD, might be contributing to the discrepant profiles of language processing in the brain.
Furthermore, these results need to be considered in light of the well-documented effects of experience in the fine-tuning of brain functional organization during development (cf. Kandel et al., 2000). It is possible that divergent patterns of brain activity associated with language processing found in WS and ASD may reflect the brain’s differential plasticity trajectories in response to distinct perceptual inputs resulting from distinct attention, given the WS and ASD’s divergent social-behavioral tendencies. That is, different developmental paths (early attention to people and increased social interactions in WS vs social avoidance in ASD) may manifest as differential information processing abilities when it comes to interpreting context and extracting meaning from what is said by other individuals.
Overall, while WS and ASD had been contrasted with regard to their discrepant attention to social stimuli, often manifested as differential fixation patterns when scanning images including human faces (Riby and Hancock, 2008, 2009), their divergent language processing received less attention in the literature, despite behaviorally distinct use of language by the two populations. Thus, the results of this study further contribute to the systematic picture of discrepant functioning at the levels of behavior, cognitive processing and, now, brain processing of information, emerging between WS and ASD.
In conclusion, the results of this study demonstrate contrasting patterns of brain activity associated with processing contextual information in individuals with WS and ASD, indicating that people with these two neurodevelopmental disorders process spoken language qualitatively differently, paralleling their divergent social and communication behavior. These findings can be used to generate more specific hypotheses regarding language perception and its neural correlates in both WS and ASD, as well as the core processes involved in the development of social-communicative functioning.
Acknowledgments
We wish to thank all the participants and their families who so generously gave their time to take part in this study. This research was supported by the National Institute of Child Health and Human Development (NICHD) grant (P01 HD033113) awarded to U.B. and D.M. and National Institute of Mental Health (NIMH) fellowship (5 T32 MH20002) awarded to I.F. We thank Lawrence G. Appelbaum, Marie St. George and Debbie Rull for their help with data collection.
Footnotes
1Of interest, this finding of an atypically large ERP component existing in tandem with what seems to be a relatively proficient ability (language comprehension) typically indexed with this component is reminiscent of other socially relevant skills in WS. Specifically, there is evidence that on some tasks involving processing of socially relevant information, such as face processing, the near typical performance in individuals with WS is also associated with ‘atypical’ neural processing (Mills et al., 2000; Haas et al., 2009).
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC: Author; 2000. [Google Scholar]
- Bellugi U, Lichtenberger L, Jones W, Lai Z, St. George M. I. The neurocognitive profile of Williams syndrome: a complex pattern of strengths and weaknesses. Journal of Cognitive Neuroscience. 2000;12(Suppl. 1):1–29. doi: 10.1162/089892900561959. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Wang P, Jernigan T. Williams syndrome: an unusual neuropsychological profile. In: Broman S, Grafman J, editors. Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function. Hillsdale, NJ: Erlbaum Press; 1994. pp. 23–56. [Google Scholar]
- Blomberg S, Rosander M, Andersson G. Fears, hyperacusis and musicality in Williams syndrome. Research in Developmental Disabilities. 2006;27:668–80. doi: 10.1016/j.ridd.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Brock J, Einav S, Riby DM. The other end of the spectrum? Social cognition in Williams syndrome. In: Reid V, Striano T, editors. Social Cognition: Development, Neuroscience, and Autism. Oxford: Blackwell; 2008. pp. 281–300. [Google Scholar]
- Brock J, Jarrold C, Farran EK, Laws G, Riby DM. Do children with Williams syndrome really have good vocabulary knowledge? Methods for comparing cognitive and linguistic abilities in developmental disorders. Clinical Linguistics & Phonetics. 2007;21(9):673–88. doi: 10.1080/02699200701541433. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivariate Behavioral Research. 1966;1(2):140–61. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Donchin E, Heffley E. Multivariate analysis of event-related potential data: a tutorial review. In: Otto D, editor. Multidisciplinary Perspectives in Event-related Potential Research. Washington, DC: US Government Printing Office; 1978. pp. 555–72. [Google Scholar]
- Doyle TF, Bellugi U, Korenberg JR, Graham J. “Everybody in the world is my friend”: hypersociability in young children with Williams Syndrome. American Journal of Medical Genetics. 2004;124A:263–73. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- Dunn M, Bates J. Developmental changes in neural processing of words by children with autism. Journal of Autism and Developmental Disorders. 2005;35:361–76. doi: 10.1007/s10803-005-3304-3. [DOI] [PubMed] [Google Scholar]
- Dunn M, Vaughan H, Kreuzer J, Kurtzberg D. Electrophysiological correlates of semantic classification in autistic and normal children. Developmental Neuropsychology. 1999;16:79–99. [Google Scholar]
- Firth U, Snowling M. Reading for meaning and reading for sound in autistic and dyslexic children. Journal of Developmental Psychology. 1983;1:329–42. [Google Scholar]
- Fishman I, Goldman M, Donchin E. The P300 as an electrophysiological probe of alcohol expectancy. Experimental and Clinical Psychopharmacology. 2008;16(4):341–56. doi: 10.1037/a0012873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Mills D, Yam A, Hoeft F, Bellugi U, Reiss A. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. Journal of Neuroscience. 2009;29(4):1132–9. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe FGE. Central coherence and theory of mind in autism: reading homographs in context. British Journal of Developmental Psychology. 1997;15:1–12. [Google Scholar]
- Harris GJ, Chabris CF, Clark J, et al. Brain activation during semantic processing in autism spectrum disorders via function al magnetic imaging. Brain Cognition. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holcomb P, Coffey S, Neville H. Visual and auditory sentence processing: a developmental analysis using event-related brain potentials. Developmental Neuropsychology. 1992;8(23):203–41. [Google Scholar]
- Goldstein G, Minshew NJ, Allen DN, Seaton BE. High-functioning autism and schizophrenia: a comparison of an early and late onset neurodevelopmental disorder. Archives of Clinical Neuropsychology. 2002;17(5):461–75. [PubMed] [Google Scholar]
- Gothelf D, Searcy YM, Reilly J, et al. Association between cerebral shape and social use of language in Williams syndrome. American Journal of Medical Genetics Part A. 2008;146A:2753–61. doi: 10.1002/ajmg.a.32507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Bellugi U, Reilly J, et al. Defining the social phenotype in Williams syndrome: a model for linking gene, brain, and cognition. Development and Psychopathology. 2008;20:1–35. doi: 10.1017/S0954579408000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski T. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111(10):1745–58. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(9):2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Jessell TM, Sanes JR. Sensory experience and the fine tuning of synaptic connections. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th edn. New York: Elsevier; 2000. pp. 1115–30. [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences. 1998;2(10):389–98. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A, Brown JH, Grice S, Paterson SJ. Dethroning the myth: cognitive dissociations and innate modularity in Williams syndrome. Developmental Neuropsychology. 2003;23:227–43. doi: 10.1080/87565641.2003.9651893. [DOI] [PubMed] [Google Scholar]
- Kerbel D, Grunwell P. A study of idiom comprehension in children with semantic–pragmatic difficulties. Part II: between-groups results and discussion. International Journal of Language and Communication Disorders. 1998;33(1):23–44. doi: 10.1080/136828298247910. [DOI] [PubMed] [Google Scholar]
- Klein AJ, Armstrong BL, Greer MK, Brown FR. Hyperacusis and otitis media in individuals with Williams syndrome. Journal of Speech and Hearing Disorders. 1990;55:339–44. doi: 10.1044/jshd.5502.339. [DOI] [PubMed] [Google Scholar]
- Klein BP, Mervis CB. Contrasting patterns of cognitive abilities of 9- and 10-year-olds with Williams Syndrome or Down Syndrome. Developmental Neuropsychology. 1999;16:177–96. [Google Scholar]
- Korenberg JR, Chen X-N, Hirota H, et al. VI. Genome structure and cognitive map of Williams syndrome. Journal of Cognitive Neuroscience. 2000;12:89–107. doi: 10.1162/089892900562002. [DOI] [PubMed] [Google Scholar]
- Korpilahti P, Jansson-Verkasalo E, Mattila M-L, et al. Processing of affective speech prosody is impaired in Asperger syndrome. Journal of Autism and Developmental Disorders. 2007;37:1539–49. doi: 10.1007/s10803-006-0271-2. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Science. 2000;4:463–70. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–5. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–3. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Morris CA, Bertrand J, Robinson BF. Williams syndrome: findings from an integrated program of research. In: Tager-Flusberg H, editor. Neurodevelopmental Disorders. Cambridge, MA: MIT Press; 1999. pp. 65–110. [Google Scholar]
- Mills DL, Alvarez TD, St George M, Appelbaum LG, Bellugi U, Neville H. Electrophysiological studies of face processing in Williams syndrome. Journal of Cognitive Neuroscience. 2000;12(Suppl. 1):47–64. doi: 10.1162/089892900561977. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Mills DL, Bellugi U. Effects of altered auditory sensitivity and age of language acquisition on the development of language-relevant neural systems: preliminary studies of Williams Syndrome. In: Broman SH, Grafman J, editors. Atypical Cognitive Deficits in Developmental Disorders. Hillsdale, NJ: Erlbaum; 1994. pp. 68–83. [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related potentials and language comprehension. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind. Oxford: Oxford University Press; 1995. pp. 171–215. [Google Scholar]
- Reilly J, Losh M, Bellugi U, Wulfeck B. “Frog, where are you?” Narratives in children with specific language impairment, early focal brain injury and Williams syndrome. Brain & Language. 2004;88:229–47. doi: 10.1016/S0093-934X(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Riby DM, Hancock PJ. Viewing it differently: social scene perception in Williams syndrome and Autism. Neuropsychologia. 2008;46:2855–60. doi: 10.1016/j.neuropsychologia.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Riby DM, Hancock PJ. Looking at movies and cartoons: eye-tracking evidence from Williams syndrome and autism. Journal of Intellectual Disability Research. 2009;53:169–81. doi: 10.1111/j.1365-2788.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Röder B, Rösler F, Neville HJ. Event-related potentials during auditory language processing in congenitally blind and sighted people. Neuropsychologia. 2000;38(11):1482–502. doi: 10.1016/s0028-3932(00)00057-9. [DOI] [PubMed] [Google Scholar]
- Siegel DJ, Minshew NJ, Goldstein G. Wechsler IQ profiles in diagnosis of high-functioning autism. Journal of Autism and Developmental Disorders. 1996;26:389–406. doi: 10.1007/BF02172825. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–58. [PubMed] [Google Scholar]
- Tager-Flusberg H. Language impairments in children with complex neurodevelopmental disorders: the case of autism. In: Levy Y, Schaeffer JC, editors. Language Competence Across Populations: Toward a Definition of Specific Language Impairment. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 297–321. [Google Scholar]
- Tager-Flusberg H. Language and communicative deficits and their effects on learning and behavior. In: Prior M, editor. Asperger Syndrome: Behavioral and Educational Aspects. New York: Guilford Press; 2004. pp. 85–103. [Google Scholar]
- Tager-Flusberg H, Plesa Skwerer D, Joseph RM. Model syndromes for investigating social cognitive and affective neuroscience: a comparison of autism and Williams syndrome. Social, Cognitive and Affective Neuroscience. 2006;1(3):175–82. doi: 10.1093/scan/nsl035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udwin O, Yule W. Expressive language of children with Williams syndrome. American Journal of Medical Genetics. 1990;6(Suppl.):108–14. doi: 10.1002/ajmg.1320370620. [DOI] [PubMed] [Google Scholar]
- Walenski M, Tager-Flusberg H, Ullman MT. Language in autism. In: Moldin S, Rubenstein J, editors. Understanding Autism: From Basic Neuroscience to Treatment. New York: Taylor & Francis; 2006. pp. 175–203. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd edn. 1997. (WAIS-III). San Antonio, TX: Psychological Corporation. [Google Scholar]