Abstract
Medial frontal scalp-recorded negativity occurring ∼200–300 ms post-stimulus [known as feedback-related negativity (FRN)] is attenuated following unpredicted reward and potentiated following unpredicted non-reward. This encourages the view that FRN may partly reflect dopaminergic ‘reward–prediction–error’ signalling. We examined the influence of a putatively dopamine-based personality trait, extraversion (N = 30), and a dopamine-related gene polymorphism, DRD2/ANKK1 (N = 24), on FRN during an associative reward-learning paradigm. FRN was most negative following unpredicted non-reward and least-negative following unpredicted reward. A difference wave contrasting these conditions was significantly more pronounced for extraverted participants than for introverts, with a similar but non-significant trend for participants carrying at least one copy of the A1 allele of the DRD2/ANKK1 gene compared with those without the allele. Extraversion was also significantly higher in A1 allele carriers. Results have broad relevance to neuroscience and personality research concerning reward processing and dopamine function.
Keywords: feedback-related negativity, reward–prediction–error, extraversion, dopamine, DRD2/ANKK1
INTRODUCTION
The observation that rewards alter behaviour is now widely explained in terms of the mesencephalic dopamine (DA) system and its role in behavioural adaptation (Robbins and Everitt, 1996; McClure et al., 2004; Schultz, 2007). Animal studies show that DA projections from the ventral tegmental area (VTA) to the nucleus accumbens and anterior-cingulated cortex (ACC) play a central role in mediating the effects of reward on approach behaviour and learning (Schultz, 1998; Paus, 2001). Sites of DA release are ideally located for transmitting reinforcement signals to corticostriatal synapses, which show long-term potentiation or depression (i.e. connections are strengthened or weakened) during reinforcement learning (Wickens and Kotter, 1995). Phasic DA activity increases following unpredicted rewards and decreases following unpredicted non-rewards (Schultz, 1998, 2007); following the same pattern as the ‘teacher signal’ proposed in classic and contemporary models of reinforcement learning (Sutton and Barto, 1998). As such, many have suggested that provision of this signal is one of the major roles of DA neurons (Waelti et al., 2001). According to this view, the function of DA in approach behaviour and learning appears to be the communication of ‘reward–prediction–error’ (RPE), indicating that events are better (or worse) than expected.
DA-signalling of RPE may modulate event-related potentials occurring ∼200–300 ms after motivationally salient stimuli. A negative deflection during this time window appears over medial-frontal areas after feedback is delivered, which has been referred to as feedback-related negativity (FRN).1 Although originally studied as a response to error feedback, it is also elicited by positive feedback, resulting in a similar but less negative waveform (Boksem et al., 2006; Potts et al., 2006; Eppinger et al., 2008; Santesso et al., 2008). Functional imaging suggests that FRN originates from the ACC (Holroyd et al., 2004), one of the major termini of mesencephalic DA projections conveying the RPE signal. In accord with this, Holroyd and colleagues have argued that the FRN is modulated by phasic DA activity in response unpredicted reward or unpredicted non-reward, a proposal that rests on computational models, empirical data and biological plausibility (Holroyd and Coles, 2002; Nieuwenhuis et al., 2004; Holroyd and Krigolson, 2007). Supportive evidence includes data from a S1–S2 paradigm contrasting predicted vs unpredicted reward and predicted vs unpredicted non-reward (Potts et al., 2006). A significant 2 × 2 interaction revealed that FRN amplitude was most negative following unpredicted non-reward and least negative following unpredicted reward. This pattern mirrors the amplitude of phasic-DA release (increase following unpredicted reward and decrease following unpredicted non-reward) during single-cell recordings (Schultz, 1998). Subsequent research shows, furthermore, that difference waves contrasting unpredicted reward vs unpredicted non-reward—arguably an index of overall RPE magnitude—increase as predictability decreases (Eppinger et al., 2008).
As in other areas of basic neuroscience (Kosslyn et al., 2002), there has been increasing interest in individual differences that characterize DA signalling and may support the validity of markers such as FRN. For instance, genetic markers of DA function are associated with individual differences in both reinforcement-learning (Klein et al., 2007) and FRN amplitude (Frank et al., 2007). Similarly, when individuals are assigned to groups based upon reinforcement-learning performance (i.e. learners vs non-learners), corresponding differences in FRN amplitude are observed (Frank et al., 2007; Santesso et al., 2008). Interestingly, Cohen (2007) found that a computational reinforcement-learning model showed improved fit to behavioural and neuroimaging data collected during a reinforcement-learning task when individual differences in the RPE parameter were incorporated. Cohen concluded that identification of relevant individual differences is therefore critical for full understanding of these processes.
For some time, personality neuroscientists (Depue and Collins, 1999; Pickering and Gray, 1999) have suggested that variation in DA functioning may contribute to variation in a major dimension of temperament (see Pickering and Smillie, 2008, for a recent review). Some of these have focussed on the Extraversion–Introversion continuum (henceforth, extraversion), a trait that appears in all major models of personality and is characterized by positive affectivity, behavioural approach and agency (Wilt and Revelle, 2009). Others have focussed instead on various conceptualizations of impulsiveness, a complex cluster of traits reflecting disinhibited or poorly regulated responding (Arche and Santisteban, 2006). Both Extraversion and Impulsivity-related personality traits have been found to predict putatively DA-mediated behaviour, such as individual differences in reinforcement and feedback learning (Pickering, 2004; Smillie et al., 2007). In addition, such personality traits have been associated with various genotypic and endophenotypic indices of DA function, including functional neuroimaging, responses to pharmacologic DA-challenge tests and DA-related genetic polymorphisms (Depue and Collins, 1999; Reuter et al., 2002; Cohen et al., 2005; Wacker et al., 2006; Smillie et al., 2010). Using the S1–S2 paradigm described above (which is also employed in the present article), Martin and Potts (2004) demonstrated that FRN was most negative following unpredicted non-reward and least negative following unpredicted reward, but only for individuals scoring above the median on trait impulsivity. We are unaware of any data that have examined this putative marker of RPE signalling in relation to Extraversion, which some have argued may best capture personality-related differences in DA function (Rammsayer, 1998; Depue and Collins, 1999; Pickering and Smillie, 2008).
In this study, we used the difference wave contrasting FRN after an unpredicted reward minus FRN after an unpredicted non-reward as an index of overall RPE. We predicted that this would be more pronounced for those scoring high (+ 1 s.d.) vs low (−1 s.d.) on a measure of trait Extraversion. It was also possible to explore relationships among RPE, Extraversion and a DA-relevant genetic polymorphism, as the majority of participants in the present experiment had participated in a previous gene-association study reported elsewhere (Smillie et al., 2010). The Taq1A polymorphism of the DRD2 gene (which in fact lies within the encoding region of the adjacent ANKK1 gene; Fossella et al., 2006) has been associated with a one-third reduction in D2-receptor-binding sites in carriers of the less frequently occurring A1 allele (Ritchie and Noble, 2003). We found that participants with the A1 allele had significantly higher scores on trait Extraversion (Smillie et al., 2010). Such individuals may be characterized by relatively higher DA activity (as a result of receptor down-regulation) and thus may show more pronounced RPE signalling. Converging associations among an electrophysiological index of RPE, trait differences in Extraversion, and genotypic differences in DRD2/ANKK1 variation, would be suggestive of core underlying DA processes.
METHODS
Participants
Thirty right-handed participants (M age = 23.39, s.d. = 5.06; 14 females), most of whom were students of Goldsmiths, University of London, UK, participated in this experiment in exchange for cash (£15). Twenty-four participants reported ethnicity as White/European, three as Asian and one as Black (the remaining two participants declined to indicate their ethnicity). Participants were recruited via a psychology-research participation scheme, in which first-year students can sign up for experiments (via a dedicated intranet noticeboard), typically in exchange for course credit (the present study was advertised as ‘cash only’). Recruitment was also facilitated by advertisements placed on noticeboards around the university and on the Goldsmiths student intranet.
In order to participate, individuals were first required to complete the Revised Eysenck Personality Questionnaire (EPQ-R; Eysenck and Eysenck, 1991), which includes one of the most well-validated and widely used measures of Extraversion. This scale consists of 25 questions concerned with behavioural activation/approach and agency (e.g. ‘Can you easily get some life into a rather dull party?’) to which participants can respond ‘yes’ or ‘no’. A total score is calculated by summing all responses (high score = high Extraversion). Only participants with scores exceeding 1 s.d. above or below the published means for extraversion were invited to complete the experiment. In our sample, scores for participants in the high-extraversion group (M = 21.09; s.d. = 1.16) were significantly higher than for those in the low-extraversion group (M = 6.15; s.d. = 2.97), F(1,28) = 18.18, P < 0.001. Scores on other personality dimensions in the EPQ-R did not vary significantly across groups, and neither did age nor gender (all P’s > 0.05).
Genotyping
Buccal-swab DNA samples were available for 24 of the 30 participants, who had participated in a larger study (N = 224) described previously (Smillie et al., 2010). While this is a small sample size, it is similar to that of other recent genomic imaging studies (Cohen et al., 2005; Canli, 2006). Genotypes were identified via an allelic-discrimination assay based on fluorogenic 5′-nuclease activity: TaqMan Single Nucleotide Polymorphism (SNP) Genotyping Assay (Applied Biosystems INC). Primers and probes specific to the DRD2/ANKK Taq1A polymorphism were designed and assays were performed according to the manufacturer’s instructions. As the distribution of the DRD2/ANKK Taq1A polymorphism is severely skewed (owing to the low frequency of the A1 allele), we followed the convention of dividing participants into A1+/A1– groups (N = 8 and 16, respectively). Genotype frequencies were in Hardy–Weinberg equilibrium, χ2(1) = 0.044, P > 0.05 (A2A2 = 16; A2A1 = 7; A1A1 = 1) and were unrelated to age, F(1,22) = 1.74, P > 0.05, gender, χ2(1) = 0.505, P > 0.05, and ethnicity, χ2(2) = 0.905, P > 0.05.
Experimental design, task and procedure
The experiment used a passive S1–S2 randomized-block design with two within-subjects factors reflecting differences in trial type: reward vs non-reward and predicted vs unpredicted. Participants were told that the task was similar to a ‘fruit machine’ (‘slot machine’ in American English), but that they did not need to do anything except attend closely to each trial. S1 and S2 were images of either a gold bar or a lemon. Each trial sequence consisted of a fixation point (300 ms); S1 (500 ms); fixation point (300 ms); S2 (500 ms); fixation point (300 ms); trial and cumulative earnings (600 ms), as described in Potts et al. (2006). On 80% of the trials for which S1 was a gold bar, S2 was also a gold bar and a reward (£0.50) was earned (predicted reward; 192 trials). On the remaining 20% of trials, S2 was a lemon and no reward was earned (unpredicted non-reward; 48 trials). Conversely, on 80% of the trials for which S1 was a lemon, S2 was also a lemon and no reward was earned (predicted non-reward; 192 trials). On the remaining 20% of trials, S2 was a gold bar, and a reward (£0.50) was earned (unpredicted reward; 48 trials). Thirty practice trials were provided for familiarization of the trial sequence, and in total there were eight blocks of 60 experimental trials, separated by rest breaks (total experimental trials = 480). To minimize EEG artefacts due to blinking, we also displayed the message ‘blink now’ as part of an irregular 2000–3600 ms inter-trial interval and instructed participants to restrict blinking to this interval. Participants were told they would be paid the sum won during the highest earning of the eight blocks (this was fixed at £15 for all participants).
EEG recording and analysis
Continuous EEG was acquired from 64 active channels placed according to the extended 10–20 system using Easycap® electrode caps. Four additional channels were recorded to detect eye movements [electrooculogram (EOG)]; vertical EOG was recorded from the supra-orbit and sub-orbit of the right eye, while horizontal EOG was recorded from the external canthi of each eye. Electrode impedances were under 5 kΩ and impedances for homologous electrode sites were kept within 1 kΩ. EEG was amplified using a BioSemiActiveTwo® amplifier. To ensure high-quality recordings the experimenter continuously monitored EEG during the experiment, while participant vigilance and head movement was monitored via a closed circuit video camera. Data were sampled at 512 Hz and filtered offline using a 0.1–100 Hz bandpass filter. An average reference was applied and data segmented into 500 ms epochs beginning 100 ms before S2 and finishing 400 ms after S2.
Artefacts were detected according to a maximum/ minimum voltage criterion (±70 μV on target frontal channels and EOG channels) and then kept/rejected after visual inspection for eye/muscle movements or other artefacts. After artefact rejection, there were a minimum (maximum) of 30 (48) usable segments per person for each of the less frequently occurring trial-types (M for unpredicted reward = 41.47, M for unpredicted non reward = 42.30) and 106 (192) usable segments for each of the more frequently occurring trial-types (all M > 120). The number of artefacts per trial type did differ significantly neither between the two personality groups nor the two genotypic groups (all F ’s < 1, ns). In addition, appropriate blinking following the ‘blink now’ message occurred during almost all of the 479 inter-trial intervals (M = 477, s.d. = 8.35) and did not vary between personality groups nor genotypic groups (all F ’s < 1, ns).
FRN was averaged over six medial-frontal channels (F1, F2, Fz, FC1, FC2 and FCz), and a grand average was calculated for each individual, for each of the four conditions. The internal consistency of these composite scores was very high, as indicated by Cronbach’s alpha values (α > 0.90). Results were also compared with those based only on Fz, possibly the most commonly reported medial-frontal channel. The mean amplitude of the ERP for a time window spanning 200–300 ms post-S2 was then exported for analysis.
RESULTS
Manipulation check
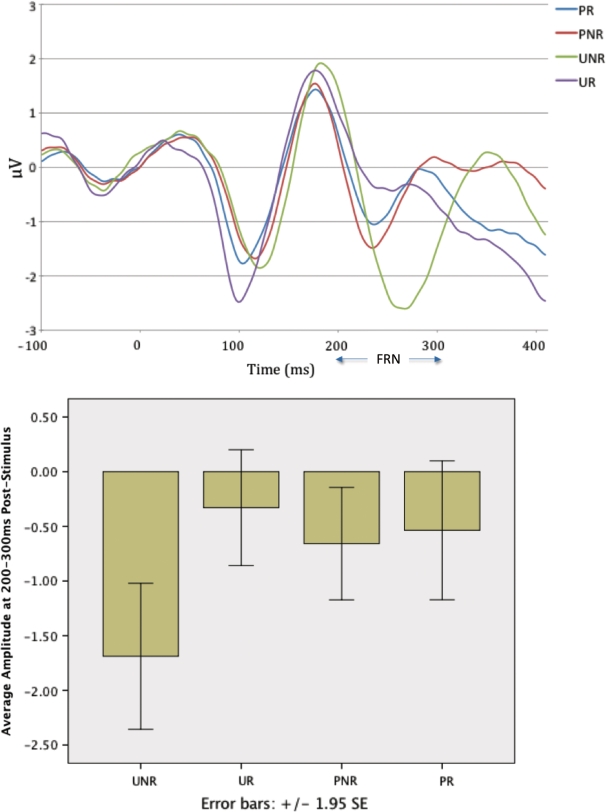
A 2 (unpredicted trial, predicted trial) × 2 (reward trial, non-reward trial) ANOVA was conducted to ensure that variation in FRN following the four experimental trial types was driven, as expected, largely by ERPs to unpredicted trials. Variation in the FRN waveform over the four conditions closely replicated findings by Potts et al. (2006). Specifically, ERP averaged over medial-prefrontal channels was more negative after non-reward than reward, F(1,29) = 20.97, P < 0.001 and marginally but significantly more negative for unpredicted than for predicted trials, F(1,29) = 4.64, P < 0.05. However, both main effects were qualified by a significant reinforcement × prediction interaction, F(1,29) = 14.47, P < 0.001. Negativity was greater for an unpredicted non-reward than for an unpredicted reward, F(1,29) = 29.51, P < 0.001 and did not differ significantly for predicted rewards and non rewards, F(1,29) = 1.60, P > 0.05. Substantively, identical results were obtained when analyses were based only on Fz rather than a 6-channel composite. This pattern is depicted in Figure 1 and, like previous findings using this paradigm, is suggestively similar to phasic activity recorded from DA cells during reinforcement learning (Schultz, 1998, Figure 1).
Fig. 1.
Waveforms and mean amplitude of FRN for unpredicted non-reward (UNR), unpredicted reward (UR), predicted non-reward (PNR) and predicted reward (PR).
Main analysis
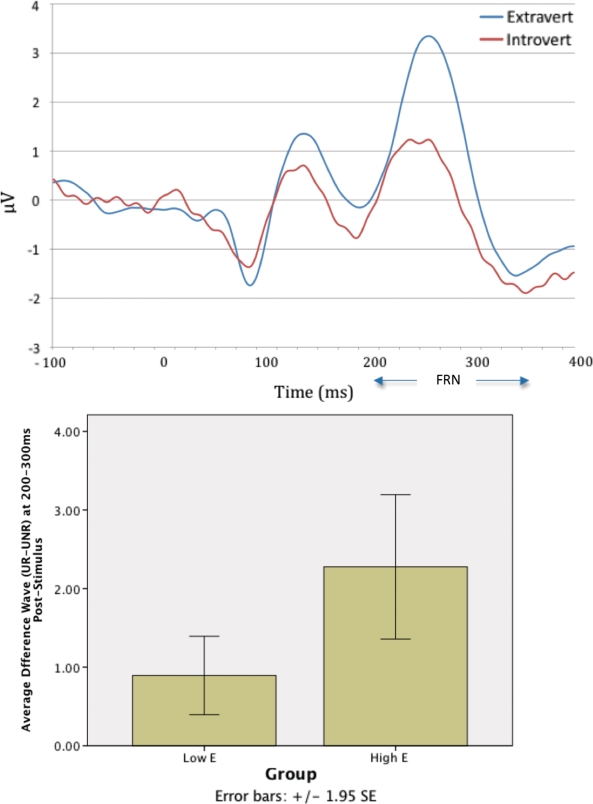
Main analyses sought to determine whether differences in FRN following unpredicted reward vs unpredicted non-reward—the specific trial types which should elicit phasic DA responses—varied across Extraversion groups and DRD2/ANKK1 alleles. A 2 (unpredicted reward, unpredicted non-reward) × 2 (high-extraversion, low-extraversion) ANOVA was conducted to determine the influence of Extraversion on RPE. Again, FRN was more negative for unpredicted non-reward than for unpredicted reward, F(1,28) = 35.32, P < 0.001, however this was qualified by a significant 2-way interaction F(1,28) = 6.71, P < 0.01. FRN for the high-extravert group was more negative following unpredicted non-reward and less negative following unpredicted reward (M = −2.33, s.d. = 2.09 and M = −0.05, s.d. = 1.81), compared with the low-extravert group (M = −1.25, s.d. = 1.32 and M = −0.33, s.d. = 1.33), although neither between-group comparison was statistically significant (all P ’s > 0.05). The difference in amplitude for unpredicted reward vs unpredicted non-reward (i.e. the magnitude of overall RPE) was very robust for those in the high-extraversion group, F(1,14) = 36.40, P < 0.001 and considerably weaker, although still significant, for those in the low-extraversion group, F(1,14) = 5.62, P < 0.05. As can be seen in Figure 2, the difference wave reflecting overall RPE (mean amplitude for unpredicted reward minus mean amplitude for unpredicted non-reward) in the high-extraversion group (M = 2.27, s.d. = 1.81) was more than twice as large as in the low-extraversion group (M = 0.89, s.d. = 0.99). Finally, the correlation between raw Extraversion scores and this index of RPE was indicative of a moderate effect size, r(28) = 0.46, P < 0.05. Again, near identical results were obtained when analyses were based only on Fz.
Fig. 2.
Waveforms and mean amplitude of RPE (unpredicted reward minus unpredicted non-reward) for Extraverted and Introverted individuals.
A second 2 (unpredicted reward, unpredicted non-reward) × 2 (A1+, A1−) ANOVA was conducted to determine the influence of genotype on RPE. Again, FRN was more negative for unpredicted non-reward than for unpredicted reward, F(1,22) = 26.91, P < 0.001. Contrary to predictions, the interaction between genotype and RPE fell short of formal significance, F(1,22) = 2.22, P = 0.15. Results based only on Fz were substantively identical, although here the genotype by RPE interaction was slightly closer to formal significance, F(1,22) = 2.86, P = 0.10. Nevertheless, the RPE difference wave contrasting unpredicted reward with unpredicted non-reward was almost twice as large in the A1+ group (M = 2.17, s.d. = 1.80) as in the A1− group (M = 1.20, s.d. = 1.28), and the point-biserial correlation between genotype and RPE indicated a moderate effect size, r = 0.30 (for Fz, r = 0.34). Furthermore, consistent with Smillie et al.’s (2010) findings, A1 allele frequency varied significantly with high/low Extraversion groups, χ2(1) = 5.04, P < 0.05. Specifically, of the 12 low-extravert participants for whom genetic data were available, only one was an A1 carrier. Conversely, in the same number of high-extravert participants, six were A1 carriers. Additionally, the point-biserial correlation between polymorphic group and raw Extraversion score was strong and significant, r(22) = 0.51, P < 0.05.
To explore these data further, we conducted a final 2 (unpredicted reward, unpredicted non-reward) × 2 (high-extraversion, low-extraversion) ANOVA, this time including genotypic group as a covariate. Logically, if Extraversion predicts variation in RPE signalling because of its partial basis in DA function, then this relationship should be weaker if the variance it shares with a genetic index of DA function is partialled out. Results support this reasoning: within the subset of participants for whom genetic data was available, FRN was again more negative for unpredicted non-reward relative to unpredicted reward, F(1,22) = 27.02, P < 0.001, and this effect was qualified by a marginally significant interaction with Extraversion group, F(1,22) = 3.92, P = 0.06. However, when DRD2 group was first included as a covariate, this effect was weakened considerably, F(1,21) = 2.01, P = 0.17. Therefore, after controlling for variation in a genetic marker of DA function, differences in Extraverted personality no longer significantly predict variations in an electrophysiological index of RPE signalling.
DISCUSSION
Findings from this experiment are broadly consistent with Holroyd and Coles’ (2002) proposal that FRN is modulated by RPE signalling. First, FRN was most negative following unpredicted non-reward (when DA neuronal firing is inhibited below baseline levels), and least negative following unpredicted reward (when DA neurons show a phasic burst of firing). This replicates previous work by Potts et al. (2006), and is also the first independent replication of this effect using the same paradigm. Second, this putative index of RPE varied with trait Extraversion, which a significant body of theory and research suggests has a partial basis in DA function (Depue and Collins, 1999; Wacker et al., 2006; Hooker et al., 2008). Specifically, for high-extraverts relative to low-extraverts, FRN was more negative following unpredicted non-reward and less negative following unpredicted reward. The difference wave contrasting these trial types was more than twice as large for Extraverts as for Introverts. Although to our knowledge this is the first time that the FRN component has been associated with Extraversion, our results mirror findings presented by Martin and Potts (2004) in relation to trait impulsiveness. Third, in a subset of our participants who had also participated in a separate gene-association study, Extraversion scores were significantly higher for participants carrying at least one copy of the A1 allele of the Taq1A polymorphism of the DRD2/ANKK1 gene. This demonstrates that a recent finding reported by Smillie et al. (2010) is recoverable in a small subset of the original data. Contrary to expectations, the relationship between this polymorphism and FRN, though moderate, was statistically non-significant.
Support for Holroyd and Coles’ (2002) suggestion that FRN may partly reflect DA signalling has already been gleaned from computational modelling and high-resolution source analysis. The present data offers further support from the perspective of individual differences—in particular, individual differences in extraverted personality. Various groups of researchers have converged upon the hypothesis that Extraversion partially reflects variation in DA function (Rammsayer, 1998; Depue and Collins, 1999; Pickering and Smillie, 2008) and there are now several compelling studies supporting this model. For instance, Reuter et al. (2002) found that Extraverts have stronger hormone responses to DA agonists and antagonists. Similarly, Cohen et al. (2005) found that Extraversion predicts increased activity in DA-rich areas of the brain in response to financial rewards. More recently, Smillie et al. (2010) found that Extraversion covaried significantly with the DRD2/ANKK1Taq1A polymorphism; an association that was confirmed in the present study. In light of such data, the present finding that variation in FRN was also associated with Extraversion offers further suggestion that this component is modulated by DA function. This also arguably goes beyond one other study relating impulsive personality to FRN using a RPE paradigm (Martin and Potts, 2004). Although impulsivity is often viewed as a potential trait manifestation of DA, it has been equally, if not more strongly, related to the behavioural regulatory functions of serotonin (5-HT; Carver, 2005; Crockett and Robbins, 2010).
Results concerning inter-individual variation in the DRD2/ANKK1 Taq1A polymorphism were less encouraging than the personality data. The lack of a formally significant effect on our RPE index, coupled with the fact that genetic data from this study was incomplete and conveniently obtained (i.e. 24 of our 30 subjects simply happened to have participated in a previous gene association study), suggests that any conclusions drawn from these data should be very tentative. Nevertheless, the genetic data did contribute some valuable information to this investigation. Specifically, the finding that A1-allele carriers reported significantly higher scores on Extraversion supports a key assumption of this study; that variation in Extraverted personality is partly reflective of variation in DA functioning. It also confirms that the recently reported relationship between Extraversion and the DRD2 Taq1A polymorphism (Smillie et al., 2010) is recoverable within a small sub-sample of the original data. Furthermore, supplementary analysis showed that Extraversion was no longer a significant predictor of RPE after controlling for genotypic group. This potentially indicates that the variance Extraversion shares with the DRD2 gene is responsible for its association with variation in RPE. It should also be noted that the non-significant association of the DRD2 gene with our index of RPE is likely to have resulted from low power. The observed effect is medium in size and in the direction anticipated, and therefore at the very least encourages the inclusion of genotypic data in further replications and extensions of this research.
There is a growing appreciation that individual differences constructs can be combined with basic experimental paradigms to help ‘reveal the structure of psychological function’ (Kosslyn et al., 2002). An issue that should always be borne in mind, however, is the threat that lurking variables pose to non-random between-subject designs (e.g. group assignment based upon person characteristics). For instance, in addition to variation in DA function, differences in vigilance and attentiveness provide another reason that RPE signalling might vary between participants. As such, if it happened that subjects in the low-Extravert group were simply less attentive than subjects in the high-Extravert group, personality-related differences in attentiveness would be a viable alternative explanation of our findings. Though possible, this state of affairs seems very unlikely for two reasons. First, ancillary analyses indicate that both participant groups were equally diligent in terms of not blinking during experimental trials, and correctly blinking during the inter-trial interval (i.e. when they were explicitly instructed to ‘blink now’). This basic analysis suggests that all participants were equally vigilant and attentive throughout the task. Furthermore, much of what is known about Extraversion/Introversion from cognitive experiments suggests that Introverts are more vigilant and attentive than Extraverts, not the other way around (Matthews and Gilliland, 1999). Nevertheless, future investigations of individual differences in RPE might benefit from additional control procedures, such as the introduction of a behavioural component to the paradigm to confirm that all participants had successfully learned key task contingencies.
Results from this experiment build upon a substantial and growing literature which integrates individual differences in reward processing at multiple levels of analysis; genotype, endophenotype and phenotype. Despite mixed support for our predictions, the potential picture is one of converging relationships between well-known genotypic and phenotypic markers of DA function with variation in FRN, supporting the view that it may partly reflect RPE signalling at an endophenotypic level. A challenge for all studies that traverse multiple levels of analysis is explaining how those levels are functionally interconnected. The present research is no exception, although the following account is plausible: trait Extraversion is characterized by behavioural approach and agency, processes that have a functional basis in the dopaminergic ‘reward system’. Signals of RPE carried along these pathways arrive at the ACC (among other locations), to which FRN has been localized (Holroyd et al., 2004). This neurotransmission is in turn influenced by DA receptor availability, which varies markedly with the DRD2/ANKK1 polymorphism (Ritchie and Noble, 2003). This interpretation of our data almost certainly underestimates the complexity of the neural and psychological systems involved. Nevertheless, it provides a framework to facilitate further multi-modal investigations of individual differences in reward processing.
Conflict of Interest
None declared.
Acknowledgments
L.D.S. acknowledges financial support from the British Academy (PDF/2006/291) and the University of London (R/CRF/B).
Footnotes
1Related or identical negative components in this time period have been referred to as Anterior P2 (P2a; Potts et al., 2006) and feedback-error-related negativity (f-ERN, related to the earlier response-locked component error-related negativity or ERN; Eppinger et al., 2008).
REFERENCES
- Arche E, Santisteban C. Impulsivity: A review. Psichothema. 2006;18:213–20. [PubMed] [Google Scholar]
- Boksem MAS, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Canli T. Genomic imaging of extraversion. In: Canli T, editor. Biology of Personality and Individual Differences. London, UK: The Guildford Press; 2006. pp. 93–115. [Google Scholar]
- Carver CS. Impulse and constraint: perspectives from personality psychology, convergence with theory in other areas, and potential for integration. Personality and Social Psychology Review. 2005;9:312–33. doi: 10.1207/s15327957pspr0904_2. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Individual differences and the neural representations of reward expectation and reward prediction error. Social Cognitive and Affective Neuroscience. 2007;2:20–30. doi: 10.1093/scan/nsl021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics reflect reactivity of neural reward circuitry. Cognitive Brain Research. 2005;25:851–61. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Robbins TW. Role of central serotonin in impulsivity and compulsivity: Comparative studies in experimental animals and humans. In: Muller C, Jacobs B, editors. Handbook of Behavioral Neurobiology of Serotonin. London, UK: Academic Press; 2010. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioural and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mock B, Mecklinger A. Better or worse than expected Aging, learning, and the ERN. Neuropsychologia. 2008;46:521–39. doi: 10.1016/j.neuropsychologia.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. The Eysenck Personality Questionnaire-Revised. Sevenoaks, UK: Hodder & Stoughton; 1991. [Google Scholar]
- Fossella J, Green AE, Fan J. Evaluation of a structural polymorphism in the ankyrin repeat and kinase domain containing 1 (ANKK1) gene and the activation of executive attention networks. Cognitive Affective and Behavioural Neuroscience. 2006;6:71–8. doi: 10.3758/cabn.6.1.71. [DOI] [PubMed] [Google Scholar]
- Frank MJ, D’Lauro C, Curran T. Cross-task individual differences in error processing: neural, electrophysiological, and genetic components. Cognitive Affective and Behavioural Neuroscience. 2007;7:297–308. doi: 10.3758/cabn.7.4.297. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Krigolson OE. Reward prediction error signals associated with a modified time estimation task. Psychophysiology. 2007;44:913–7. doi: 10.1111/j.1469-8986.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, et al. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience. 2004;7:1–2. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Miyakawa A, Knight RT, D’Esposito M. The influence of personality on neural mechanisms of observational fear and reward learning. Neuropsychologia. 2008;46:2709–24. doi: 10.1016/j.neuropsychologia.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;7:1642–5. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Cacioppo JT, Davidson RJ, et al. Bridging psychology and biology. The analysis of individuals in groups. American Psychologist. 2002;57:341–51. [PubMed] [Google Scholar]
- Martin LE, Potts GF. Reward sensitivity in impulsivity. Cognitive Neuroscience and Neuropsychology. 2004;15:1519–22. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- Matthews G, Gilliland K. The personality theories of H.J. Eysenck and J.A. Gray: a comparative review. Personality and Individual Differences. 1999;26:583–626. [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neuroscience and Biobehavioural Reviews. 2004;28:441–8. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nature Neuroscience Reviews. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pickering AD. The neuropsychology of impulsive antisocial sensation seeking personality traits: from dopamine to hippocampal function? In: Stelmack RM, editor. On the Psychobiology of Personality: Essays in Honour of Marvin Zuckerman. London, UK: Elsevier; 2004. pp. 453–76. [Google Scholar]
- Pickering AD, Gray JA. The neuroscience of personality. In: Pervin L, John O, editors. Handbook of Personality. 2nd edn. New York, NY: Guilford Press; 1999. pp. 277–99. [Google Scholar]
- Pickering AD, Smillie LD. The behavioural activation system: Challenges and opportunities. In: Corr PJ, editor. The Reinforcement Sensitivity Theory of Personality. Cambridge, UK: Cambridge University Press; 2008. pp. 120–54. [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: the medial frontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18:1112–9. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Extraversion and dopamine: individual differences in response to changes in dopaminergic activity as a possible biological basis of extraversion. European Psychologist. 1998;3:37–50. [Google Scholar]
- Reuter M, Netter P, Toll C, Hennig J. Dopamine agonist and antagonist responders as related to types of nicotine craving and facets of extraversion. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26:845–53. doi: 10.1016/s0278-5846(01)00329-3. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochemical Research. 2003;28:73–8. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology. 1996;6:228–36. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Dillon DG, Birk JL, et al. Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008;42:807–16. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioural dopamine signals. Trends in Neurosciences. 2007;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Smillie LD, Cooper A, Proitsi P, Powell J, Pickering AD. Variation in DRD2 dopamine gene predicts extraverted personality. Neuroscience Letters. 2010;468:234–7. doi: 10.1016/j.neulet.2009.10.095. [DOI] [PubMed] [Google Scholar]
- Smillie LD, Dalgleish LI, Jackson CJ. Distinguishing between learning and motivation in behavioural tests of the Reinforcement Sensitivity Theory of personality. Personality and Social Psychology Bulletin. 2007;33:476–89. doi: 10.1177/0146167206296951. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: The MIT Press; 1998. [Google Scholar]
- Wacker J, Chavanon ML, Stemmler G. Investigating the dopaminergic basis ofextraversion in humans: a multilevel approach. Journal of Personality and Social Psychology. 2006;91:171–87. doi: 10.1037/0022-3514.91.1.171. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–8. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wickens J, Kotter R. Cellular models of reinforcement. In: Houk JC, Davis JL, Beiser DG, editors. Models of Information Processing in the Basal Ganglia. London, UK: The MIT Press; 1995. pp. 189–214. [Google Scholar]
- Wilt J, Revelle W. Extraversion. In: Leary M, Hoyle R, editors. Handbook of Individual Differences in Social Behaviour. London, UK: The Guilford Press; 2009. pp. 27–45. [Google Scholar]