Abstract
Adolescents spend a significant part of their leisure time watching TV programs and movies that portray violence. It is unknown, however, how the extent of violent media use and the severity of aggression displayed affect adolescents’ brain function. We investigated skin conductance responses, brain activation and functional brain connectivity to media violence in healthy adolescents. In an event-related functional magnetic resonance imaging experiment, subjects repeatedly viewed normed videos that displayed different degrees of aggressive behavior. We found a downward linear adaptation in skin conductance responses with increasing aggression and desensitization towards more aggressive videos. Our results further revealed adaptation in a fronto-parietal network including the left lateral orbitofrontal cortex (lOFC), right precuneus and bilateral inferior parietal lobules, again showing downward linear adaptations and desensitization towards more aggressive videos. Granger causality mapping analyses revealed attenuation in the left lOFC, indicating that activation during viewing aggressive media is driven by input from parietal regions that decreased over time, for more aggressive videos. We conclude that aggressive media activates an emotion–attention network that has the capability to blunt emotional responses through reduced attention with repeated viewing of aggressive media contents, which may restrict the linking of the consequences of aggression with an emotional response, and therefore potentially promotes aggressive attitudes and behavior.
Keywords: aggression, violence, functional magnetic resonance imaging, skin conductance response, Granger causality mapping
INTRODUCTION
Adolescents spend a significant part of their leisure time watching TV programs and movies that portray violence (Yoon and Somers, 2003). For example, it has been shown that ∼70% of 14-year-olds (and 39% of 10-year-olds) saw at least one of 51 extremely violent movies, and amongst the most popular were I Know What You Did Last Summer, Scream II and Die Hard (Sargent et al., 2002). Extensive research and media coverage have linked school shootings (Anderson et al., 2007, p. 3), real-life replications of video-game contents (Crowley, 2008) and general aggression to the exposure to extremely violent media (Anderson and Bushman, 2001, 2002). Although it has been suggested that individuals become more aggressive (Huesmann, 2007) and desensitized (Funk, 2005) to real-life violence after the repeated consumption of violent media programs (American Academy of Pediatrics, 2001), little is known about how the extent of violent media use and the severity of aggression displayed affect adolescents’ brains.
During adolescence, developmental changes occur in brain morphology and function, particularly in the prefrontal cortex (Blakemore, 2008; Giedd, 2008). One of the neural systems that undergo significant changes during adolescence (Spear, 2000) and that has been intimately linked to the processing of the incentive value of stimuli is the dopaminergic system (Schultz, 1998). Dopamine neurons in the orbitofrontal cortex (OFC) and subcortical regions encode the emotional value of primary (e.g. food, touch, smell) and secondary (e.g. money) reinforcers enabling the individual to shape goal-directed social and emotional behavior in response to changing external contingencies (Kringelbach and Rolls, 2004; Rolls and Grabenhorst, 2008). In adolescents, compared to adults and children, lateral OFC (lOFC) activation has been observed to be enhanced and reward-driven learning slowed, in the context of monetary reward value manipulations, indicating that adolescents’ lOFC development may be protracted (Galvan et al., 2006).
Whereas the medial OFC has been shown to modulate aggression by exerting inhibitory control over aggressive impulses in adults and adolescents (Damasio et al., 1994; Grafman et al., 1996; Anderson et al., 1999; Blair and Cipolotti, 2000; Pietrini et al., 2000; Strenziok et al., 2009), there is evidence that the lOFC, through its ability to adapt to external stimuli, is involved in increasing or decreasing the likelihood of aggressive behavior when aggressive cues are present (Blair, 2004). Two functional neuroimaging studies revealed that reduced activation in the lOFC is associated with exposure to aggressive media in adults. Repeated exposure to violence portrayed in movies was associated with a decrease in activation in the lOFC over the period of the experiment (Kelly et al., 2007). In the same study, reduced lOFC activation towards violent movies was associated with more reactive-affective aggression, indicating that the lOFC may be involved in increasing the likelihood of aggression through dysfunctional emotion regulation. Furthermore, during playing of a violent first-person shooter video game, the lOFC was recruited less in experienced male gamers than during non-violent virtual actions in the same game (Mathiak and Weber, 2006). This study further revealed activation changes in the rostral and dorsal anterior cingulate cortices, parietal regions including the precuneus (Prec), angular gyrus, intraparietal sulcus, and temporoparietal junction, amygdala, parahippocampus, insula, and cerebellum during violent actions, indicating the contribution of networks that regulate visual-spatial attention, sensori-motor function, and monitoring of cognitive and affective processes.
It has been proposed that repeated exposure to violent media causes emotional desensitization to subsequent aggressive stimuli (Funk et al., 2004). A neurophysiological study investigating the P300 amplitude of the event-related brain potential has shown lower cortical responses towards violent images in violent compared to non-violent video game players, indicating desensitization towards violent stimuli in individuals who have been exposed to media violence (Bartholow et al., 2006). Because the P300 amplitude has been hypothesized to reflect the activation of the aversive motivational system (Cacioppo et al., 1993), reduced brain activation during exposure to violent stimuli may indicate reduced aversive emotional responses to these stimuli. The process of emotional blunting to arousing events is also associated with a reduced sympathetic skin conductance response (SCR) to violent movies and portrayals of real life aggression in children who were previously exposed to violent media (Cline et al., 1973; Thomas et al., 1977).
The present study was designed to explore the association between violent media exposure, SCR responses, and activation changes in the lOFC in normally developing male adolescents 14–17 years of age. Adolescence is a time period that is sensitive to the adverse effects of violent media because portrayals of aggression are more appealing and pleasurable to youth, as evidenced by self-report (Benenson et al., 2007), identification with antisocial characters is more likely (Konijn et al., 2007), and parental control is low (Cheng et al., 2004), whereas the opportunities to gain access to violent media are abundant. Due to a number of interacting factors such as adrenarche, gonadarche, cortical synaptic pruning, antisocial peer pressure, family conflict and difficulties in school, particularly male adolescents have an increased risk for aggressive behavior and some develop lifelong antisocial and violent behavior patterns (Moffitt and Caspi, 2001; Kirsh, 2003; Blakemore, 2008). We used functional magnetic resonance imaging (fMRI) to investigate brain activation changes during observed aggression and Granger causality mapping (GCM) to investigate effective connectivity (connectivity network and strength of connectivity) in an media aggression network (Deshpande et al., 2008, 2009). In an event-related fMRI design, 22 healthy adolescents repeatedly viewed normed videos that displayed different degrees of realistic, age-appropriate aggression. In addition, SCRs were sampled throughout the acquisition of fMRI images to monitor autonomic changes associated with viewing aggressive videos.
Among others, two phenomena of neural activity during emotion processing in the lOFC, that are relevant in the context of the current study, have been reported: (i) a decrease in lOFC activity to stimuli that are applied repeatedly and (ii) an association between lOFC activation magnitude and stimulus magnitude (O'Doherty et al., 2001; Kringelbach et al., 2003). Based on these findings, we predicted emotional desensitization after repeated exposure to aggressive videos of varying aggression levels as evidenced by reduced SCRs and decreased activation in the lOFC over time. With increased aggression in the videos, we predicted a decline in lOFC activation based on previous neuroimaging evidence that showed decreased OFC function associated with an increase in aggression (Pietrini, 2000). If activation changes in the lOFC reflect changes in the emotional value of stimuli that motivate behavior, and if repeated exposure to aggressive media stimuli is associated with emotional desensitization to those stimuli, then repeated exposure to aggressive media should be associated with reduced activation levels in the lOFC over time. In our connectivity analysis, we predicted a central role of the lOFC in a media aggression network that subserves emotion modulation and associated activation in structures that regulate visual-spatial input, attentional demands and familiarity of recurring events.
METHODS
Subjects
Twenty-two healthy male adolescents (mean ± s.d.; years of age: 15.9 ± 1.1, range 14–17, years of education: 9.9 ± 1.1, range 8–12), who had no history of psychiatric or neurological illness, participated for financial compensation. All were native English speakers, had normal or corrected-to-normal vision, and were right-handed (Edinburgh Inventory, laterality quotient: 90.8 ± 14.2, range 50–100) (Oldfield, 1971) (see Supplementary Data, for trait aggression and exposure to violence scores). Parents gave written informed consent and adolescents gave their written assent for the procedures that were approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board.
Stimuli
From commercially available DVDs, 114 videos (each 4 s long) were retrieved that contained real scenes of aggression (e.g. fist fights, street brawls and stadium violence). In a pre-study, another group of 22 age- and education-matched males rated the videos for aggression and excitement (see Supplementary Data). Based on the ratings (mean ± s.e.m.), a total of 60 videos in three sets of 20 videos that differed in the level of aggression (F2,38 = 6924.14, P < 0.001; low: 14.91 ± 1.83; mild: 46.40 ± 2.17; moderate: 73.44 ± 1.72) but not in their levels of excitement (F2,38 = 1.24, P = 0.293; low: 50.67 ± 2.37; mild: 47.80 ± 3.23; moderate: 53.77 ± 1.67) were selected for the fMRI study.
Procedure
All subjects were seen twice for the study. During visit 1, subjects were screened for psychiatric and neurological conditions, handedness, trait aggression, and exposure to violence in the media and community. During visit 2, subjects underwent the fMRI procedures. Prior to scanning, subjects rated their emotional state using a 9-point rating scale version of the Self-Assessment Manikin (Lang et al., 1993) to assess their emotional status and were trained on the fMRI task on a separate set of stimuli.
During scanning, stimulus presentation was controlled by a computer with SuperLab Pro software (Cedrus Corporation, San Pedro, CA). Subjects were given a response pad with two buttons on which they placed their right index and middle fingers. They were asked to view and judge mute videos. At the beginning of each trial, a plus sign was presented (0.5 s) on the screen followed by a video that was shown (4 s, video viewing phase). Then, a decision screen appeared (3.5 s) to cue subjects to decide whether the video they just saw was more or less aggressive than the one that they saw in the previous trial by pressing one of two assigned response buttons (decision phase). Stimulus presentation was event-related and trials were interspersed with jittered interstimulus intervals optimized following an exponential schedule using Optseq2 software (Freesurfer, Massachusetts General Hospital, Boston, MA). Three runs of about nine minutes each were employed. During each run, all 60 videos were presented randomly among runs and subjects. Subjects were asked to make their decisions as quickly as possible and response times and decisions were recorded. Immediately after scanning, 1 day, and 2 weeks later adolescents completed an emotional assessment to assess their mood state after viewing the videos (see Supplementary Data).
Data acquisition
fMRI data
Imaging data were collected using a 3T General Electric (GE Medical Systems, Waukesha, WI) MRI scanner equipped with an eight-channel head coil. Anatomical scans were performed using a T1-weighted 3D MP-RAGE sequence (TR 9 ms, TE 4 ms, flip angle 12°, FoV 256 mm, matrix size 256 × 256, thickness 1.2 mm, in-plane resolution 0.8594 × 0.8594 mm2). Functional images were acquired using a T2*-weighted 2D gradient EPI sequence (TR 2 s, TE 23 ms, flip angle 90°, 30 slices, thickness 3 mm, in-plane resolution 3.75 × 3.75 mm2, FoV 240 mm). In each run, 320 volume images were taken parallel to the AC–PC line. The first five volumes were discarded to allow for T1 equilibration effects.
Skin conductance responses
SCRs were sampled at 80 Hz throughout MRI scanning using PSYLAB equipment (Contact Precision Instruments Inc., Boston, MA). A constant voltage of 0.5 V was applied to the middle and ring fingers of the non-dominant hand through a pair of MRI compatible surface gel cup electrodes (Ag/AgCl, 6 mm diameter; Biopac, Goleta, CA, model TSD203). Note that three subjects had to be excluded from the analysis of SCR data due to equipment failure (n = 1) and non-responding (n = 2).
Data analyses
Skin conductance responses
SCR samples were first z-transformed (Boucsein, 1992) to normalize possible baseline differences across aggression levels and subjects. Stimulus related changes were identified by extracting the maximum amplitude of the SCR during each video viewing phase. Afterwards, a 3 × 3 analysis of variance (ANOVA) on those maximum measurements was performed with Aggression (low, mild, moderate) and Time (T1, T2, T3) as within-subject factors. Because both factors had more than two levels, Mauchly’s test of sphericity was applied to determine whether the correlation between variables was the same. If the estimated chi value of Mauchly’s test was significant, then the assumption behind the normal within-subjects ANOVA was violated and Wilks’ Lambda multivariate statistics were reported. Furthermore, we computed adaptation factors (AFs) for each aggression level defined as maximum SCR measurement differences between extreme time points (T3–T1). Finally, a one-way ANOVA trend analysis on the AFs was computed with aggression (low, mild, moderate) as a within-subject factor to determine whether the subjects’ SCRs adapted in a linear fashion, from low to mild to moderate aggression levels. In those cases where AFs were positive, we defined the adaptation as sensitization, whereas negative AFs are referred to as desensitization. (See Supplementary Data for behavioral data analysis).
fMRI data
fMRI data analyses were performed using BrainVoyager QX (Brain Innovation, Maastricht, The Netherlands) and structural and functional data were preprocessed (see Supplementary Data). Functional data were co-registered with the individual’s 3D anatomical images and then reassembled into 3 × 3 × 3 mm3 isotropic voxels. A GLM corrected for first-order serial correlation was applied (Friston et al., 1999). Random-effects analyses were performed to explore brain regions that were associated with viewing aggressive videos. The GLM model consisted of four regressors: video viewing phase (n = 3: low, mildly and moderately aggressive videos) and decision phase (n = 1). Regressor time courses were adjusted for the hemodynamic response delay by convolution with a double-gamma hemodynamic response function (Buchel et al., 1998).
Multiple regression analyses were performed independently for the time course of each individual voxel. After computing parameter estimates for all predictors, using the ANCOVA random-effects analysis tool in BrainVoyager QX, a 3 × 3 ANOVA on those parameter estimates was performed with Aggression (low, mild, moderate) and Time (T1, T2, T3) as within-subject factors (see Supplementary Data for design). Activations were reported in a whole brain analysis using an FDR with a threshold of q(FDR) < 0.05 (corrected) (Genovese et al., 2002) and a cluster size threshold of 270 mm3.
To analyze the interaction effect in more detail, we derived the parameter estimates of the beforehand identified Aggression × Time interaction effect for each of the aggression and time levels. Mean values of parameter estimates were derived from each region after identifying the peaks of activation and surrounding voxels encompassing 125 mm3. Afterwards, we computed AFs for each aggression level defined as differences in parameter estimates between extreme time points (T3–T1). Furthermore, one-way ANOVA trend analyses on AFs were computed with aggression (low, mild, moderate) as a within-subject factor to determine whether subjects’ brain activations adapted in a linear fashion, from low to mild to moderate aggression levels. In those cases where AFs were positive, we defined the adaptation as sensitization, whereas negative AFs are referred to as desensitization.
Multivariate Granger causality analysis
Effective connectivity was implemented using a multivariate GCM analysis (Kaminski et al., 2001) based on a multivariate vector autoregressive (MVAR) model capable of capturing the simultaneous directional influences between multiple regions (Kus et al., 2004). For the connectivity analysis, we derived the parameter estimates from the peak voxel activations of the beforehand identified significant Aggression × Time interaction effect for each of the aggression and time levels because we were only interested in those regions that changed depending on the aggression level and repeated viewing of videos for the subsequent GCM analysis. The ROIs were constrained to the center of activation within each region and to a maximum size of 5 × 5 × 5 mm3. The entire time series of BOLD signal intensities from the selected ROIs were averaged across voxels, then normalized across runs and subjects, and finally collapsed across all runs and subjects to obtain a single time series per region (Deshpande et al., 2008, 2009).
A first order MVAR model was fit based on the time series of selected regions and a directed transfer function (DTF) matrix was obtained (Kus et al., 2004). The DTF matrix was weighted by the partial coherence between the selected regions in order to emphasize direct connections (Deshpande et al., 2008, 2009). The order of the model was determined using Akaike’s Information Criterion (Akaike, 1974). The DTF is based on the principle of Granger causality but is rendered in a multivariate framework and therefore can effectively model the inherently multivariate nature of neuronal networks (Blinowska et al., 2004). This method has been validated previously using simulations (Kus et al., 2004) and applied successfully to electrophysiological (Ding et al., 2000; Kaminski et al., 2001; Korzeniewska et al., 2003 Blinowska et al., 2004; Kus et al., 2004) and fMRI data measuring the BOLD response (Stilla et al., 2007; Deshpande et al., 2008, 2009). The DTF analysis matrix consists of a set of directional path weights describing the strength of mutual impact (in arbitrary units) from each region to each of the other regions. Surrogate null distributions were used to assess the significance of the path weights (P < 0.05) (Deshpande et al., 2008). Since the selected regions survived multiple comparison corrections in the initial fMRI analyses, no further significance correction was applied (Stilla et al., 2007).
To determine dominant directional influences between regions (i.e. whether differences between reciprocal paths were significant), differences between the reciprocal paths were calculated using surrogate data to generate a corresponding null distribution and compared it with the analogous value obtained from real fMRI data. The fractional area under the null distribution for the analogous value obtained from real fMRI data gives the probability of the null hypothesis being true, which is essentially the P-value of the significance of the dominant directional influence.
Furthermore, to determine whether regions were either predominantly driving other regions or being driven by other regions (or, alternatively, a given region may be driven as much as it is driving the other regions), we computed input-output ratios for each region (see Supplementary Data for computations).
Correlations
We correlated the AFs of SCRs, AFs of brain activation and connectivity of brain activation with self-reported measures of violent media exposure and trait aggression. On these latter measures, we asked subjects to rate how much of the media they used show fighting, crime, guns, war scenes or similar content on a scale from one to five (1 = none or very little, 5 = all or almost all), including TV programs/movies, video games, books/journals, music, and websites. Furthermore, we administrated the Aggression Questionnaire (AQ) (Buss and Warren, 2000) to assess physical aggression, verbal aggression, anger, hostility and indirect aggression. On this questionnaire, subjects were asked to rate on a 5-point rating scale (1 = not at all like me, 5 = completely like me) how they interact with other people using 34 statements.
RESULTS
Skin conductance responses
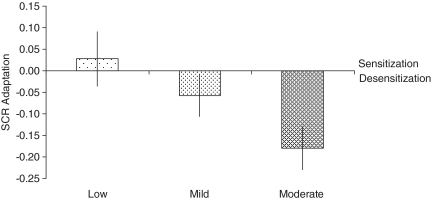
We investigated whether SCRs changed while viewing videos with different levels of aggression over time. The 3 Aggression (low, mild, moderate) × 3 Time (T1, T2, T3) ANOVA revealed no main effect of Aggression (F2,36 = 0.47, P = 0.614) but a significant main effect of Time (F2,36 = 4.38, P < 0.021), indicating that SCRs diminished over time. In addition, there was a significant Aggression × Time interaction effect, indicating that SCRs changed differently for the aggression levels over time (F4,15 = 4.84, P < 0.010; note that Wilks’ Lambda multivariate statistics were reported because of a significant chi value in the Mauchly’s test of Sphericity χ2(9) = 17.07, P < 0.048). To address this change in SCRs over time, we calculated AFs of SCRs for each aggression level and submitted them to a planned follow-up ANOVA. Results showed that SCRs adapted significantly with aggression level (F2,36 = 3.68, P < 0.036) (Figure 1). Specifically, the trend analysis to determine whether the subjects’ SCRs adapted in a linear fashion revealed a significant downward linear trend (Flin1,18 = 9.04, P < 0.008), indicating that changes in SCRs over time decreased linearly from low to mild to moderate aggression levels. For the mildly and moderately aggressive videos, SCR adaptation was negative, indicating desensitization towards these videos (see Supplementary Data and Figure S1 for behavioral results).
Fig. 1.
SCR adaptation. Adaptation factors are shown for low, mildly and moderately aggressive videos. Results revealed a linear downward trend in SCR adaptation with increasing aggression in the videos. SCR adaptation was positive (sensitization) for the low aggressive videos and negative (desensitization) for the mildly and moderately aggressive videos.
Brain activation
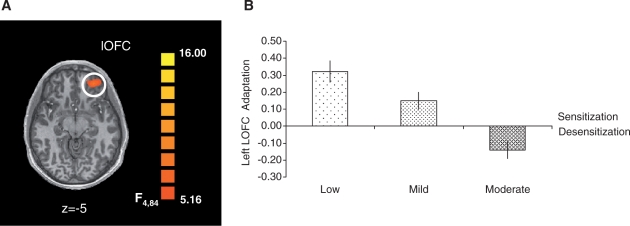
We also assessed brain activation patterns that were associated with viewing videos that displayed different levels of aggression with repeated exposure over time. The 3 Aggression (low, mild, moderate) × 3 Time (T1, T2, T3) ANOVA revealed a significant main activation effect of Aggression in the bilateral lOFC (left, BA 47; right, BA 45) in addition to a fronto-parieto-temporo-occipital network including the left inferior frontal gyrus (BA 9), rostral anterior cingulate cortex (BA 32), right posterior cingulate cortex (BA 23), bilateral middle temporal gyri (left, BA 39; right, BA 37) and bilateral middle occipital gyri (BA 19) [F2,42 = 5.80, q(FDR) < 0.05] (Figure S2, Supplementary Table S1A). Furthermore, a significant main activation effect of Time was associated with a fronto-temporo-parietal network including the bilateral middle frontal gyri (BA 10), right Prec (BA 31), right lingual gyrus (BA 18) and left middle temporal gyrus (BA 37) [F2,42 = 7.59, q(FDR) < 0.05] (Figure S3, Table S1B). Finally, there was a significant Aggression × Time interaction activation effect in the left lOFC (BA 10) (Figure 2A) in addition to the right Prec (BA 31) and bilateral inferior parietal lobules (left IPL, BA 39; right ILP, BA 7) [F4,84 = 5.16, q(FDR) < 0.05] (Supplementary Figure S4 and Table S1C).
Fig. 2.
Brain activation. LOFC activation and adaptation are displayed. (A) Left lOFC activation associated with viewing aggressive videos. We found activation changes in the left lOFC (BA 10; x, y, z coordinates, –27, 47, –5) towards repeatedly viewed videos that portray varying levels of aggression. (B) Left lOFC adaptation. Adaptation factors are shown for low, mildly and moderately aggressive videos. Results revealed a linear downward trend in lOFC adaptation with increasing aggression in the videos. Left lOFC adaptation was positive (sensitization) for the low and mildly aggressive videos and negative (desensitization) for the moderately aggressive videos.
To address the changes over time, we calculated the AFs of lOFC, Prec, left ILP and right IPL activation (parameter estimates) for each aggression level and submitted them to planned follow-up ANOVAs. Results showed that responses in all four brain regions adapted significantly with aggression level (lOFC, F2,42 = 20.22, Figure 2B; Prec, F2,42 = 25.67; left ILP, F2,42 = 12.77; right ILP, F2,42 = 14.87, Ps < 0.001, Figure S5). Specifically, linear trend analyses to determine whether subjects’ brain responses adapted in a linear fashion revealed significant downward linear trends in all four regions (lOFC, F1,21 = 40.38; Prec, F1,21 = 36.22; left ILP, F1,21 = 20.92; right ILP, F1,21 = 24.15, Ps < 0.001), indicating that brain activation changes over time decreased linearly from low to mild to moderate aggression levels. In the lOFC and right IPL, adaptation was negative for the moderately aggressive videos, indicating desensitization towards the most aggressive videos in our study.
Multivariate GCM analysis
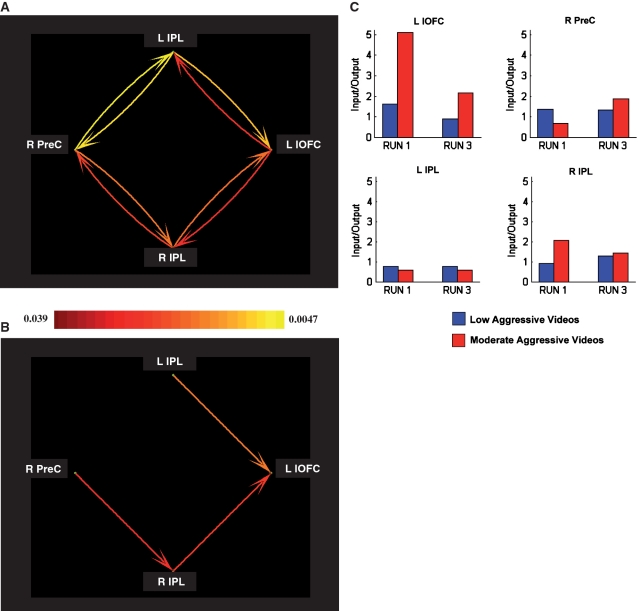
First, using GCM analyses, we identified the effective connectivity patterns among brain regions that showed an Aggression × Time interaction effect (lOFC, Prec, left IPL and right IPL) (Figure 3A, Supplementary Table S2). The Prec and lOFC were reciprocally connected with both the left and right IPL, but were not directly connected (Figure 3A).
Fig. 3.
Multivariate GCM analyses. Effective connectivity network and strengths are displayed. (A) Granger causality network for aggression. The Prec and lOFC were reciprocally connected with both the left and right IPL, but were not directly connected. A pseudo-color code was used to indicate the path weights of all connections between ROIs. (B) Granger causality network for dominant directional influences. Two dominant directional pathways were found that gave input to the lOFC, indicating that this region is mainly driven by other ROIs: inter-hemispherically, from the Prec via the right IPL (Prec → R IPL) to the lOFC (R IPL → lOFC) and intra-hemispherically, from the left IPL to the lOFC (L IPS → L lOFC). The color scales in A and B are weighted by the P-values of the corresponding paths. (C) Input–output ratio for ROI activations. Only for left lOFC, the input–output ratio decreased significantly over time for the moderately aggressive videos, but not for the low aggressive videos.
Second, the dominant directional influences for the reciprocal connections in the Granger causality network were determined. Two dominant directional pathways were found that gave input to the lOFC, indicating that in this experiment the lOFC is mainly driven by other regions: (i) inter-hemispherically, from the Prec via the right IPL (P[Prec → R IPL] = 0.032) to the lOFC (P[R IPL → lOFC] = 0.023) and (ii) intra-hemispherically, from the left IPL to the lOFC (P[L IPS → L lOFC] = 0.012) (Figure 3B).
Finally, the input-output ratios for each region were determined and their differences between low to moderately aggressive videos and changes from time T1 to T3 investigated (Figure 3C). For the lOFC, the surrogate test revealed a significant main input-output ratio effect of Aggression (P < 0.009), indicating that low aggressive videos produced a significantly lower input drive into the lOFC compared to moderately aggressive videos (the most aggressive videos in our study). Furthermore, a significant main input-output ratio effect of Time (P < 0.021) was revealed, indicating that the drive into the lOFC significantly decreased over time. Finally, the surrogate test revealed a significant interaction input-output ratio effect of Aggression (low vs moderate) × Time (T1 vs T3) (P < 0.009), indicating that the drive into the lOFC significantly decreased over time only for the moderately aggressive videos (P < 0.001) but not for the low aggressive videos (P = 0.062). The surrogate tests for the three other regions revealed no Aggression, Time, nor Aggression × Time effects.
Correlations
We found a negative correlation between the AFs of the SCRs and scores on the media violence exposure scale (r = –0.47, P < 0.05) and AFs of the SCRs and scores on the violent video games exposure scale (r = –0.46, P < 0.05), indicating that individuals with the most exposure to violent media show the lowest adaptation values (become desensitized). No correlations between SCRs and lOFC activation, including its connectivity with the parietal cortical regions, were found.
DISCUSSION
In the present study, we measured neurophysiological responses while adolescents repeatedly viewed videos that varied in the severity of aggression displayed. First, we measured SCRs to explore emotional arousal associated with viewing aggressive media as an autonomic correlate of desensitization. Second, we investigated brain activation in the media aggression network that depended on the repeated exposure to aggressive videos that differ in aggression severity. Finally, we investigated effective connectivity in the identified fronto-parietal cortical network for the interaction of repeated viewing of aggressive videos with varying levels of aggression. Our results show SCR desensitization associated with viewing aggressive videos and underline the important role of the left lOFC in the emotional representation of media-related aggression with important time and aggression level dependent inputs from the parietal cortex thought to modulate visual-spatial attention and event familiarity.
Our SCR data analysis revealed an adaptation over time, showing a linear decrease in SCRs with increasing aggression in the videos. For the mildly and moderately aggressive videos in our study, we found SCR desensitization, indicating that autonomic responses diminished over time when adolescents were exposed to more aggressive videos. Furthermore, subjects who had the most exposure to violent media in their daily life showed the greatest desensitization. Our results are in accordance with previous studies that also revealed reduced sympathetic skin conductance responses to violent movies and portrayals of real life aggression in children who were previously exposed to violent media (Cline et al., 1973; Thomas et al., 1977). Furthermore, emotional desensitization had been associated with children’s exposure to violent video games and adults’ self-reported reduced sympathy with victims in violent movie scenes (Funk, 2005; Fanti et al., 2009). It has been proposed that emotional desensitization not only reduces avoidance-related, but also increases approach-related, motivational states in situations that present with aggressive cues (Bartholow et al., 2006; Fanti et al., 2009), which may place individuals with high exposure to violent media at risk of responding aggressively in real life (Funk, 2005).
We also assessed brain responses towards the repeated exposure to videos that varied in the severity of the displayed aggression. Our interaction analysis revealed a fronto-parietal cortical network including the left lOFC, bilateral IPL, and Prec confirming findings in adult populations that linked reduced levels of lOFC activation to the viewing of aggressive movies and the active engagement in virtual aggression (Mathiak and Weber, 2006; Kelly et al., 2007). Furthermore, patients with OFC lesions are at risk for disinhibited and aggressive behavior (Damasio et al., 1994; Grafman et al., 1996; Anderson et al., 1999), fail to notice social norm violations (Stone et al., 1998; Blair and Cipolotti, 2000), and are impaired in the recognition of angry facial expressions (Hornak et al., 1996). These impairments are generally thought to reflect a broader role of the OFC in emotion regulation in specific social contexts (Adolphs, 2003). We suggest that the lOFC, besides playing a role in a variety of social-emotional behaviors, uses emotional cues (e.g. in facial expressions) to notice inappropriate behavior and make behavioral adjustments in aggression-provoking situations. Reduced activation to an aggressive stimulus after repeated exposure to this stimulus indicates that the likelihood that the lOFC connects an emotional response with an aggressive cue diminishes with an increase in the cue’s aggression severity.
There is evidence that the OFC is involved in the central control of SCRs. Results in healthy individuals revealed a coupling of lOFC activation with spontaneous fluctuations of SCR amplitudes, indicating a link between an adaptive bodily response to emotional events and the cortical representations of affective states in the lOFC (Critchley et al., 2000). A study in OFC lesion patients showed blunted SCRs in anticipation of a monetary loss in the gambling task, hindering them in using their autonomic emotional response (somatic marker) as a guide to make advantageous decisions (Bechara et al., 1997). These data and the current findings further support the OFC’s central role in emotion. The desensitization that we found in both SCRs and lOFC responses may indicate a reduced integration of generalized autonomic responses and brain activation to aggressive stimuli over time (Critchley, 2000).
Whereas there is abundant evidence for the role of the OFC in aggression, associated parietal activations have rarely been discussed. We addressed the individual contributions of the components of the fronto-parietal network in more detail by assessing the effective connectivity between the involved regions and changes in the strength of connectivity over time and with varying aggression levels in the videos. Anatomically, the anterior lOFC is connected with isocortical frontal, temporal and parietal regions (Cavada et al., 2000; Fonteijn et al., 2008), including the inferior parietal cortex. Although previous neuroimaging studies revealed evidence for dysfunctional connectivity patterns between ventral prefrontal cortex and amygdala in adult aggressive patients (Hoptman et al., 2009), connectivity in a fronto-parietal cortical network has not been shown in the context of aggressive stimuli. However, activation in orbitofrontal and parietal cortices during violent video game play as demonstrated in a media violence study in adults (Mathiak and Weber, 2006), and our own findings, indicate a role of both structures in response to aggressive environmental cues.
Evidence from both neuroimaging and neuropsychological studies suggest that the frontal and parietal cortex interact closely to direct a wide range of higher-order cognitive functions as well as sensory-motor processes (Collette et al., 1999; Culham and Kanwisher, 2001; Bush et al., 2002). Furthermore, the fronto-parietal network is understood as a core system that yields diverse mechanisms to integrate and control distributed patterns of neural activity throughout the brain (Naghavi and Nyberg, 2007). Within the parietal cortex, IPL and Prec have been associated with visuo-spatial processing (Cavanna and Trimble, 2006), episodic memory retrieval modulated by the familiarity of perceived information (Wagner et al., 2005), and processing of naturalistic video stimuli (Aalto et al., 2002).
Our results showed that lOFC responses were generally driven by the input from parietal activations rather than vice versa, but also that there was a greater influence of parietal regions on the lOFC for more aggressive videos and at initial exposure as compared to low aggressive videos and repeated exposure to the videos. This unique connectivity pattern in the lOFC indicates that only the most aggressive videos in our study (moderate) significantly modulated the input that the lOFC received from parietal regions over time, whereas lOFC input from parietal regions did not change over time for the low aggressive videos. It should be noted that none of the input-output ratios for the parietal cortical regions showed any dependence on changing aggression levels or repeated exposure over time.
But which mechanisms could explain the decrease in fronto-parietal connectivity over time for the most aggressive videos in our study? We propose that the lOFC and parietal cortex together enable the allocation of attention to emotional stimuli. Previous neuroimaging studies of the lOFC in media violence (Mathiak and Weber, 2006; Kelly et al., 2007), aggression (Damasio et al., 1994; Grafman et al., 1996; Anderson et al., 1999; Blair, 2004), and emotional responding (Hornak et al., 1996; Stone et al., 1998; Blair and Cipolotti, 2000; Adolphs, 2003) and the parietal cortex in attention to visuo-spatial input (Cavanna and Trimble, 2006) and familiarity/novelty of stimuli (Daffner et al., 2003) support this proposal. It has been suggested that a key role for the lOFC is to detect social norm violations, including aggressive behavior, and that it guides social behavior through its ability to flexibly respond to environmental changes (Berthoz et al., 2002; Blair, 2004; King et al., 2006). In this role, it may determine the emotional-motivational relevance of aggressive behavior, shown in the videos in our study, for guidance of social behavior in future situations that present with similar aggressive cues.
Activation in the IPL is frequently attributed to attentional processes (LaBar et al., 1999; Mesulam, 1999; Szczepanski et al., 2010). Furthermore, the parietal cortex, particularly the Prec, may play a role in updating one’s internal model of the environment to take into account novel events. Activation changes in this region have been shown to be reduced when familiar events are presented (Daffner et al., 2003; Wagner et al., 2005). Whereas the reduced IPL input to the lOFC over time in our study may account for an attenuation of attention to emotionally relevant videos, decreased Prec input into the lOFC through the IPL may reflect familiarity with repeatedly viewed stimuli. The decreased influence of the precuneus and IPL on lOFC activation may have reduced the emotional-motivational relevance of the video content, indicated by lOFC desensitization.
Adolescence is a time of increased psychosocial challenges including antisocial peer pressure and difficulties at school and in the family (Kirsh, 2003). Adolescents show a greater interest in and an increased incidence rate of aggressive behavior (Moffitt and Caspi, 2001; Benenson et al., 2007; Konijn et al., 2007). In addition, previous neuroimaging research has shown that monetary reward processing in the lOFC is altered in adolescents, as compared to adults and children, and large rewards are perceived most desirable, whereas small and medium rewards did not cause differential behavioral responses (Galvan et al., 2006). Altered motivational values of aggressive media, such as sensation seeking, may explain media choices that adolescents make for their everyday entertainment. A decrease in emotional responsiveness towards more aggressive media, as shown in the present study, might result in seeking a bigger variety of those media to elicit similar reward effects and may also impose an increased risk for aggressive attitudes and behavior in real life.
Many questions about central and peripheral physiological responses towards aggression, the link between both, and connectivity patterns of brain structures associated with aggression remain unanswered to date. For example, different aggression modes, such as observed vs executed aggression, may affect the direction and location of brain activation differently. In addition, aggression-related changes in other physiological parameters such as cardiac response and respiration not only showed mixed results while measured during exposure to violent media, but also may interact differently with brain responses (Bradley et al., 2001; Kuniecki et al., 2003; Gomez et al., 2005). A limitation of the current study is that it only included male subjects. The incidence rate of aggression in females, even in female teenagers that are exposed to some of the same biopsychosocial challenges as male adolescents, is low and raises the question of what brain mechanisms and autonomic differences are associated with this gender difference. This information will be invaluable for defining neurobehavioral treatment goals for aggressive individuals.
In summary, we have shown desensitization in SCRs and left lOFC activation towards repeatedly viewed videos that portray considerable aggression. We propose that exposure to aggressive media results in a blunting of emotional responses, which in turn may prevent the connection of consequences of aggression with an appropriate emotional response, and therefore may increase the likelihood that aggression is seen as acceptable behavior. It remains unknown, however, whether individuals with elevated levels of aggression may be at particular risk for altered desensitization patterns towards media violence, proviolent attitudes, and the acceptance of real-world violence as normal social behavior (Fanti et al., 2009). We also demonstrated that left lOFC activation during viewing aggressive videos is driven by input from parietal cortical regions, particularly during an initial exposure to videos with a considerable level of aggression. These findings indicate that the left lOFC uses aggression cues to determine behavior consequences of, and to make a behavioral adjustment in, an aggression-provoking situation, whereas parietal regions reflect a more general contribution to the integration and control of information.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The authors thank Dr Dimitrios Kapogiannis and Dr Edward Huey for performing the neurological examinations on our subjects. This research was funded by the intramural research program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
REFERENCES
- Aalto S, Naatanen P, Wallius E, et al. Neuroanatomical substrata of amusement and sadness: a PET activation study using film stimuli. Neuroreport. 2002;13:67–73. doi: 10.1097/00001756-200201210-00018. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- American Academy of Pediatrics Committee on Public Education. Media violence. Pediatrics. 2001;108:1222–6. doi: 10.1542/peds.108.5.1222. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Bushman BJ. Effects of violent video games on aggressive behavior, aggressive cognition, aggressive affect, physiological arousal, and prosocial behavior: a meta-analytic review of the scientific literature. Psychological Science. 2001;12:353–9. doi: 10.1111/1467-9280.00366. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Bushman BJ. Psychology. The effects of media violence on society. Science. 2002;295:2377–9. doi: 10.1126/science.1070765. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Gentile DA, Buckley KE. Violent Video Game Effects on Children and Adolescents. Theory, Research, and Public Policy. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bartholow B, Bushman B, Sestir M. Chronic violent video game exposure and desensitization to violence: behavioral and event-related brain potential data. Journal of Experimental Social Psychology. 2006;42:5329. [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Benenson JF, Carder HP, Geib-Cole SJ. The development of boys' preferential pleasure in physical aggression. Aggressive Behavior. 2007;33:1–13. doi: 10.1002/ab.20223. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy'. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Review Neuroscience. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blinowska KJ, Kus R, Kaminski M. Granger causality and information flow in multivariate processes. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics. 2004;70:050902. doi: 10.1103/PhysRevE.70.050902. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal Activity. New York, NY: Plenum Press; 1992. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–98. [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–8. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of United States of America. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Warren WL. The Aggression Questionnaire. Los Angeles, CA: Western Psychological Services; 2000. [Google Scholar]
- Cacioppo J, Crites S, Gardner G, Coles M. If attitudes affect how stimuli are processed, should they not affect the event-related brain potential? Psychological Science. 1993;4:108–12. [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10:220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cheng TL, Brenner RA, Wright JL, Sachs HC, Moyer P, Rao MR. Children's violent television viewing: are parents monitoring? Pediatrics. 2004;114:94–9. doi: 10.1542/peds.114.1.94. [DOI] [PubMed] [Google Scholar]
- Cline VB, Croft RG, Courrier S. Desensitization of children to television violence. Journal of Personality and Social Psychology. 1973;27:360–5. doi: 10.1037/h0034945. [DOI] [PubMed] [Google Scholar]
- Collette F, Salmon E, Van der Linden M, et al. Regional brain activity during tasks devoted to the central executive of working memory. Brain Research. Cognitive Brain Research. 1999;7:411–7. doi: 10.1016/s0926-6410(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley K. Video Villains Come to Life. New York, NY: New York Times; 2008. [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–63. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LF, Weitzman AM, et al. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. Journal of Cognitive Neuroscience. 2003;15:294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Hu X, Stilla R, Sathian K. Effective connectivity during haptic perception: a study using Granger causality analysis of functional magnetic resonance imaging data. Neuroimage. 2008;40:1807–14. doi: 10.1016/j.neuroimage.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Laconte S, James GA, Peltier S, Hu X. Multivariate Granger causality analysis of fMRI data. Human Brain Mapping. 2009;30:1361–73. doi: 10.1002/hbm.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Bressler SL, Yang W, Liang H. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biological Cybernetics. 2000;83:35–45. doi: 10.1007/s004229900137. [DOI] [PubMed] [Google Scholar]
- Fanti KA, Vanman E, Henrich CC, Avraamides MN. Desensitization to media violence over a short period of time. Aggressive Behavior. 2009;35:179–87. doi: 10.1002/ab.20295. [DOI] [PubMed] [Google Scholar]
- Fonteijn HM, Norris DG, Verstraten FA. Exploring the anatomical basis of effective connectivity models with DTI-based fiber tractography. International Journal Of Biomedical Imaging. 2008:423192. doi: 10.1155/2008/423192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Funk JB. Children's exposure to violent video games and desensitization to violence. Child & Adolescent Psychiatric Clinics of North America. 2005;14:387–404. doi: 10.1016/j.chc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Funk JB, Baldacci HB, Pasold T, Baumgardner J. Violence exposure in real-life, video games, television, movies, and the internet: is there desensitization? Journal of Adolescence. 2004;27:23–39. doi: 10.1016/j.adolescence.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. Journal of Adolescent Health. 2008;42:335–43. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Gomez P, Zimmermann P, Guttormsen-Schar S, Danuser B. Respiratory responses associated with affective processing of film stimuli. Biological Psychology. 2005;68:223–35. doi: 10.1016/j.biopsycho.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–8. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, D'Angelo D, Catalano D, et al. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp012. Advance online access at http://schizophreniabulletin.oxfordjournals.org/cgi/reprint/sbp012v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–61. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Huesmann LR. The impact of electronic media violence: scientific theory and research. Journal of Adolescent Health. 2007;41:S6–13. doi: 10.1016/j.jadohealth.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M, Ding M, Truccolo WA, Bressler SL. Evaluating causal relations in neural systems: granger causality, directed transfer function and statistical assessment of significance. Biological Cybernetics. 2001;85:145–57. doi: 10.1007/s004220000235. [DOI] [PubMed] [Google Scholar]
- Kelly CR, Grinband J, Hirsch J. Repeated exposure to media violence is associated with diminished response in an inhibitory frontolimbic network. PLoS ONE. 2007;2:e1268. doi: 10.1371/journal.pone.0001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Blair RJ, Mitchell DG, Dolan RJ, Burgess N. Doing the right thing: a common neural circuit for appropriate violent or compassionate behavior. Neuroimage. 2006;30:1069–76. doi: 10.1016/j.neuroimage.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Kirsh SJ. The effects of violent video games on adolescents. The overlooked influence of development. Aggression and Violent Behavior. 2003;8:377–89. [Google Scholar]
- Konijn EA, Bijvank MN, Bushman BJ. I wish I were a warrior: the role of wishful identification in the effects of violent video games on aggression in adolescent boys. Developmental Psychology. 2007;43:1038–44. doi: 10.1037/0012-1649.43.4.1038. [DOI] [PubMed] [Google Scholar]
- Korzeniewska A, Manczak M, Kaminski M, Blinowska KJ, Kasicki S. Determination of information flow direction among brain structures by a modified directed transfer function (dDTF) method. J Neurosci Methods. 2003;125:195–207. doi: 10.1016/s0165-0270(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–71. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kuniecki M, Urbanik A, Sobiecka B, Kozub J, Binder M. Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiologiae Experimentalis (Wars) 2003;63:39–48. doi: 10.55782/ane-2003-1453. [DOI] [PubMed] [Google Scholar]
- Kus R, Kaminski M, Blinowska KJ. Determination of EEG activity propagation: pair-wise versus multichannel estimate. IEEE Transactions on Biomedical Engineering. 2004;51:1501–10. doi: 10.1109/TBME.2004.827929. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Weber R. Toward brain correlates of natural behavior: fMRI during violent video games. Human Brain Mapping. 2006;27:948–56. doi: 10.1002/hbm.20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–46. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Development and Psychopathology. 2001;13:355–75. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Integrative action in the fronto-parietal network: a cure for a scattered mind. Behavioral and Brain Sciences. 2007;30:135–87. [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. American Journal of Psychiatry. 2000;157:1772–81. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Progress in Neurobiology. 2008;86:216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Sargent JD, Heatherton TF, Ahrens MB, Dalton MA, Tickle JJ, Beach ML. Adolescent exposure to extremely violent movies. The Journal of Adolescent Health. 2002;31:449–54. doi: 10.1016/s1054-139x(02)00399-3. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience Biobehavior Review. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stilla R, Deshpande G, LaConte S, Hu X, Sathian K. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. Jornal of Neuroscience. 2007;27:11091–102. doi: 10.1523/JNEUROSCI.1808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10:640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Strenziok M, Krueger F, Heinecke A, et al. Developmental effects of aggressive behavior in male adolescents assessed with structural and functional brain imaging. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsp036. online advance access at http://scan.oxfordjournals.org/content/early/2009/09/21/scan.nsp036.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci. 2010;30:148–60. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MH, Horton RW, Lippincott EC, Drabman RS. Desensitization to portrayals of real-life aggression as a function of exposure to television violence. Journal of Personality and Social Psychology. 1977;35:450–8. [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Science. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Somers CL. Aggressive content of high school students' TV viewing. Psychological Reports. 2003;93:949–53. doi: 10.2466/pr0.2003.93.3.949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.