Abstract
Self-referential evaluation of emotional stimuli has been shown to modify the way emotional stimuli are processed. This study aimed at a new approach by investigating whether self-reference alters emotion processing in the absence of explicit self-referential appraisal instructions. Event-related potentials were measured while subjects spontaneously viewed a series of emotional and neutral nouns. Nouns were preceded either by personal pronouns (‘my’) indicating self-reference or a definite article (‘the’) without self-reference. The early posterior negativity, a brain potential reflecting rapid attention capture by emotional stimuli was enhanced for unpleasant and pleasant nouns relative to neutral nouns irrespective of whether nouns were preceded by personal pronouns or articles. Later brain potentials such as the late positive potential were enhanced for unpleasant nouns only when preceded by personal pronouns. Unpleasant nouns were better remembered than pleasant or neutral nouns when paired with a personal pronoun. Correlation analysis showed that this bias in favor of self-related unpleasant concepts can be explained by participants’ depression scores. Our results demonstrate that self-reference acts as a first processing filter for emotional material to receive higher order processing after an initial rapid attention capture by emotional content has been completed. Mood-congruent processing may contribute to this effect.
Keywords: emotion, self, ERPs, negativity bias, appraisal theory
INTRODUCTION
Emotional stimuli capture processing resources and guide behavior automatically (Lang et al., 1997; Bradley and Lang, 2000; Öhman et al., 2001; Vuilleumier, 2005). Nevertheless, responses to emotional stimuli are not simply pre-determined. Emotional stimuli happen to us and ‘ourselves’, suggesting that self-reference, that is, whether a stimulus is related to us personally or not, plays a critical role in emotion processing and emotion regulation. So far, several studies have demonstrated effects of self-reference on the processing of emotional stimuli during explicit self-referential processing tasks. Most of these studies used functional imaging methods and tasks, where participants were explicitly instructed to evaluate the emotional content of trait adjectives or emotional pictures for their personal relevance (e.g. like me vs not like me). Other tasks employed in these studies asked participants to actively regulate their responses to these stimuli by reappraising their meaning with regard to the implications for themselves. Results from these studies show an increase in activity in predominantly prefrontal brain networks (Gusnard et al., 2001; Fossati et al., 2003, 2004; Moran et al., 2006) as well as changes in hemodynamic brain responses in the right and/or left amygdala (Ochsner et al., 2002, 2004; Banks et al., 2007; Yoshimura et al., 2009). Taken together these findings suggest that self-referential processing of emotional stimuli modulates activity in cortico-limbic networks, critical for the perception, experience and integration of emotional events and the generation of automatic approach and withdrawal reactions. They do not, however, provide evidence as to the temporal sequencing, i.e. the stages during emotional information processing, at which self-referential processing takes effect.
Appraisal theories of emotion (Scherer et al., 2001; Ellsworth and Scherer, 2003; Sander et al., 2005) suggest that emotional stimuli are rapidly appraised for their self-relevance. Individuals first assess the personal relevance of a stimulus, then the implications it has for their well-being, and finally how well they can cope with these implications. According to appraisal theories, the evaluation of incoming input on the basis of these relevance checks is quite automatic (Grandjean and Scherer, 2008; Grandjean et al., 2008). Chronologically, however, this is presumed to take place only after an intrinsic pleasantness check has been completed, a process that may be reflected by modulation of early event-related brain potentials (ERPs).
Methodologically, ERPs are especially suitable for the assessment of the temporal dynamics underlying the processing of emotional stimuli (Junghofer et al., 2001; Schupp et al., 2003, 2004, 2006; Kissler et al., 2007; Herbert et al., 2008). Findings from EEG–ERP studies using semantic stimuli (i.e. words) to elicit emotions suggest that emotional meaning is processed rapidly, i.e. within the first 200–300 ms after stimulus presentation (e.g. Kissler et al., 2006 for reviews). While reading, both unpleasant and pleasant emotional words enhance amplitudes of the early posterior negativity (EPN), a brain potential reflecting rapid attention capture and early conceptual processing of salient information in the visual cortex (Schupp et al., 2006; Kissler et al., 2007). Modulation of the EPN by emotional content is a robust phenomenon that has been replicated for verbal and pictorial material alike under many different processing conditions including passive viewing/silent reading (Junghofer et al., 2001; Kissler et al., 2007; Herbert et al., 2008), lexical decision (Kanske and Kotz, 2007; Schacht and Sommer, 2009) or counting of a particular stimulus class (Kissler et al., 2009). To what extent social contextual factors such as self-reference affect early conceptual processing of emotional stimuli, however, remains to be shown.
Concerning later processing stages, the N400, an index of semantic processing (Kutas and Hillyard, 1980; Kutas and Federmeier, 2000), and particularly the late positive potential (LPP), an index of sustained attention and stimulus encoding (Paller et al., 1995; Kok, 1997) have also been repeatedly shown to be modulated by the emotional content of a stimulus (Schapkin et al., 2000; Herbert et al., 2006, 2008; Kanske and Kotz, 2007; Holt et al., 2008; Hinojosa et al., 2010). Several of these studies report a processing advantage for either unpleasant (Kanske and Kotz, 2007; Holt et al., 2008; Liu et al., 2010) or specifically pleasant content (Shapkin et al., 2000; Herbert et al., 2006, 2008; Kiefer et al., 2007; Watson et al., 2007; Kissler et al., 2009). The latter finding has been observed most often in studies in which participants were asked to explicitly judge emotional trait adjectives for their emotional meaning (Herbert et al., 2006) or their self-descriptiveness (Watson et al., 2007). These studies using explicit self-referential evaluation tasks support the view that self-reference modulates emotional stimulus processing at a higher order, semantic processing stage. As proposed by appraisal theory, this process occurs chronologically after an initial intrinsic pleasantness check, probably reflected by emotion-driven EPN effects, has been terminated.
The current study aimed at a new approach by investigating if these effects of self-reference on emotional word processing also occur during an implicit self-referential processing task and, if so, at what stage in the emotional processing stream these effects occur. More specifically, we measured ERPs in healthy participants, while they silently read a series of emotional and neutral nouns, that were preceded either by personal pronouns (‘my’) indicating self-reference (self-condition) or a definitive article (`the’) without self-reference (control-condition). Linguistically, personal pronouns are agents or markers of possession indicating to whom a respective content, represented by a noun, belongs. Articles, on the other hand, convey no semantic or self-related information. Such an approach allows for the examination of the effects of self-reference on emotional stimulus processing in a more implicit manner than has been done in previous studies, which tended to only focus on emotion effects driven by explicit self-evaluations.
In summary, we aimed at testing the following hypotheses: Enhancement of early brain potentials like the EPN is expected to arise in the ERP waveforms whenever a word with emotional as compared with neutral significance is processed (Kissler et al., 2007; Herbert et al., 2008). If, as suggested by previous research and appraisal theory, self-reference impacts on emotion processing particularly during later stages of sustained stimulus encoding, emotional modulation of early brain potentials like the EPN should occur, regardless of the word’s self-reference. In contrast, emotional modulation of later ERP potentials such as the LPP should vary as a function of the word’s self-reference. In this context, a processing bias toward self-descriptive traits and states with pleasant content has been observed in healthy individuals (Herbert et al., 2006, 2008; Watson et al., 2007). Sustained processing of words of specifically pleasant content may be an accidental finding, restricted to the type of task (explicit evaluation) and/or material used (i.e. adjectives). The current study was designed to investigate if self-reference biases the processing of emotional words when other stimuli than trait adjectives (i.e. nouns) are used and self-reference is manipulated directly via an experimental manipulation and not by explicit self-referential appraisal instructions.
MATERIALS AND METHODS
Participants
Participants were 15 undergraduate Psychology students of the University of Würzburg (7 males, 8 females, mean age: 21 years), who according to the Edinburgh Handedness Inventory (Oldfield, 1971) were all right handed and native speakers of German. Only participants, who reported to be in good health (i.e. no current or history of drug abuse, chronic physical conditions, neurological diseases, mental ill-health) and with normal sense of hearing and normal or corrected to normal vision were recruited. All participants scored normally on self-report measures of mood (M = 4.4; s.d. = 3.7) (BDI, Hautzinger et al., 1994), and also state (M = 41.13; s.d. = 3.13) and trait anxiety (M = 46.8; s.d. = 6.34) (STAI, Laux et al., 1981). Participants gave written informed consent prior to the experiment and received course credit in return for participation. The experiment was conducted in accordance with the Declaration of Helsinki.
Stimulus material
Experimental stimuli were 40 pleasant, 40 unpleasant and 40 neutral nouns. Nouns were taken from a corpus of words, previously collected by our own research group,1 which provided for every word both arousal and valence ratings from 45 adult native German speakers who had comparable backgrounds and ages to participants in the current study. Nouns were selected such that pleasant and unpleasant nouns did not differ significantly in emotional arousal, but were both significantly more emotionally arousing than neutral nouns. Mean valence differed appropriately (pleasant > neutral > unpleasant). Nouns, unpleasant, pleasant and neutral did not differ significantly in non-emotional attributes such as concreteness, word length or word frequency. Pleasant, unpleasant and neutral nouns comprised on average six characters and according to the CELEX data base (Baayen et al., 1995) were frequently used in German. Mean valence, arousal and concreteness scores as well as word length and word frequency counts of the words are presented in Table 1.
Table 1.
Stimulus material characteristics (nouns—normative ratings)
| Normative ratings | |||
|---|---|---|---|
| Pleasant | Unpleasant | Neutral | |
| Valence | 7.12 (0.15) | 2.49 (0.87) | 5.16 (0.71) |
| Arousal | 5.20 (0.15) | 5.43 (0.18) | 2.41 (0.14) |
| Concreteness | 4.15 (0.21) | 4.52 (0.12) | 4.05 (0.29) |
| Word length | 6.53 (0.33) | 6.85 (0.38) | 6.61 (0.35) |
| Word frequency | 139.79 (24.31) | 132.15 (24.8) | 91.22 (15.47) |
Mean valence, arousal and concreteness scores as well as word length (number of letters) and word frequency counts (words per million) of pleasant, unpleasant and neutral nouns.
Note: For valence, arousal and concreteness, ratings range from 1 (extremely negative valence, extremely low arousal or concreteness) to 9 (extremely positive valence, extremely high arousal or concreteness). Standard errors are in parentheses. Word frequency counts for written language are based on the standardized word-database CELEX (Baayen et al., 1995).
Experimental design
Words were presented in separate sessions (`Self’ vs ‘Control’). In the ‘Self’ condition nouns were preceded by the personal pronoun ‘my’, in the ‘Control’ condition by the respective article ‘the’. Articles instead of pronouns (i.e. `his/hers’) were chosen as control stimuli as not to confound self-reference with social reference (his/hers).
In the ‘Self’ and the `Control’ conditions, each stimulus (personal pronouns, articles and nouns) was shown for 600 ms. Stimuli (pronoun–noun and article–noun pairs) were presented randomly in every session and followed by an inter-stimulus interval in which a fixation cross was shown for about 1200 ms. The session order (‘Self’ vs ‘Control’) was counterbalanced across participants. Half of the participants started with the ‘Self’ condition, the other half with the ‘Control’ condition. Participants were instructed that a set of pronoun–noun or article–noun pairs would be presented, which they should read silently and attend to for the entire viewing period. Participants were told that the personal pronoun ‘my’ indicates self-reference, but they were not informed that stimuli differed in emotional content nor were they explicitly instructed to engage in further self-referential or emotional processing of the words. In cognitive terms, this is an implicit processing task. In a third session half of the previously presented nouns (20 nouns per word category) were presented together with the personal pronoun ‘my’ and the other half with the respective articles ‘the’. This condition was always presented as the last session and aimed to investigate the effects of self-reference on participants’ later memory recall of the presented emotional words, which was assessed via an unexpected free recall test after the experimental recording sessions. In this memory test, participants were instructed to write down as many of the pronoun–noun and article–noun pairs of the last session as they remembered and then to rate them for perceived valence and arousal on the Self-Assessment Manikin (SAM), an established non-verbal pictorial technique used to assess emotional valence and arousal ratings (Bradley and Lang, 1994).
Experimental runs were generated and controlled by ‘Presentation’ software (Neurobehavioral Systems, Inc.).
Physiological data collection and reduction
Electroencephalographic recordings
Upon arrival, participants were familiarized with the laboratory setting. They were seated in an electrically shielded, sound attenuated room. The electroencephalogram (EEG) was recorded from 28 electrode channels using an EasyCap system and NEUROSCAN BrainAmp amplifier. EEG electrodes were connected to ground and referenced to the Vertex electrode (Cz). Impedance was kept below 5 kΩ for all electrodes and raw EEG signals were recorded continuously with bandpass from DC to 500 Hz. Off-line, raw EEG signals were digitally re-referenced to an average reference, filtered from 0.01 to 30 Hz and corrected for eye- (Gratton et al., 1983) and movement artifacts. Off-line analyses were performed using the BrainVision Analyzer software (BrainProductsGmBH). Artifact-free EEG data were segmented separately for each recording session from 200 ms before pronoun or article onset, until 600 ms after noun offset. The 200 ms interval before onset of the pronouns or articles was used for baseline correction.
ERPs elicited during noun reading were determined for each participant, stimulus condition (‘Self’, `Control’) and word category (pleasant, unpleasant and neutral) in five discrete time windows from 50 to 120 ms (P1), 120 to 200 ms (N1), 200 to 300 ms (EPN), 300 to 450 ms (N400) and 450 to 600 ms (LPP) after noun-onset. Early brain potentials (P1, N1 and EPN) were analyzed at left and right parieto-occipital electrodes (P7, O1, P8, O2). The N400 and the LPP were analyzed at a group of centro-parietal electrodes including Cz, C3, C4, CP6, CP5, CPz, P3, P4 and Pz, respectively. Electrode groups and time intervals were selected in line with previous emotional word processing studies (Herbert et al., 2006, 2008; Kissler et al., 2007, 2009) on the basis of ERP grand average waveforms reflecting the topography and temporal dynamics of each of the five brain potentials. ERP components (P1, N1, EPN, N400, LPP) were analyzed as the averaged activity (µV) within the respective time interval at each electrode channel of interest.
Statistical data analysis
ERPs elicited during reading of emotional or neutral nouns were statistically analyzed with repeated measures analysis of variance (ANOVA), each containing the factor ‘Condition’ (‘Self’ vs ‘Control’), `Valence’ (pleasant, unpleasant and neutral) and ‘Location’ (electrode position within electrode cluster) as within-subject factors. Main effects of the factor `Location’ are reported only if at the same time interaction effects between `Location’ and ‘Valence’ and/or `Condition’ were observed.
In case of violation of the assumption of sphericity, degrees of freedom were adjusted according to Greenhouse and Geisser (1959). Uncorrected F-values are reported together with the adjusted Greenhouse–Geisser probability levels. Significant main effects and interaction effects were tested with follow-up planned comparison tests and P-values were corrected using the Bonferroni adjustment.
Memory performance and subjective ratings
Participants’ free recall memory performance (number of correctly remembered words) and ratings were analyzed with separate 2 × 3 ANOVAs, each including the factors `Condition’ (‘Self’ vs `Control’) and ‘Category’ (pleasant, unpleasant and neutral) as within-subject factors.
RESULTS
ERPs—Nouns
Early cortical processing effects—P1, N1, EPN
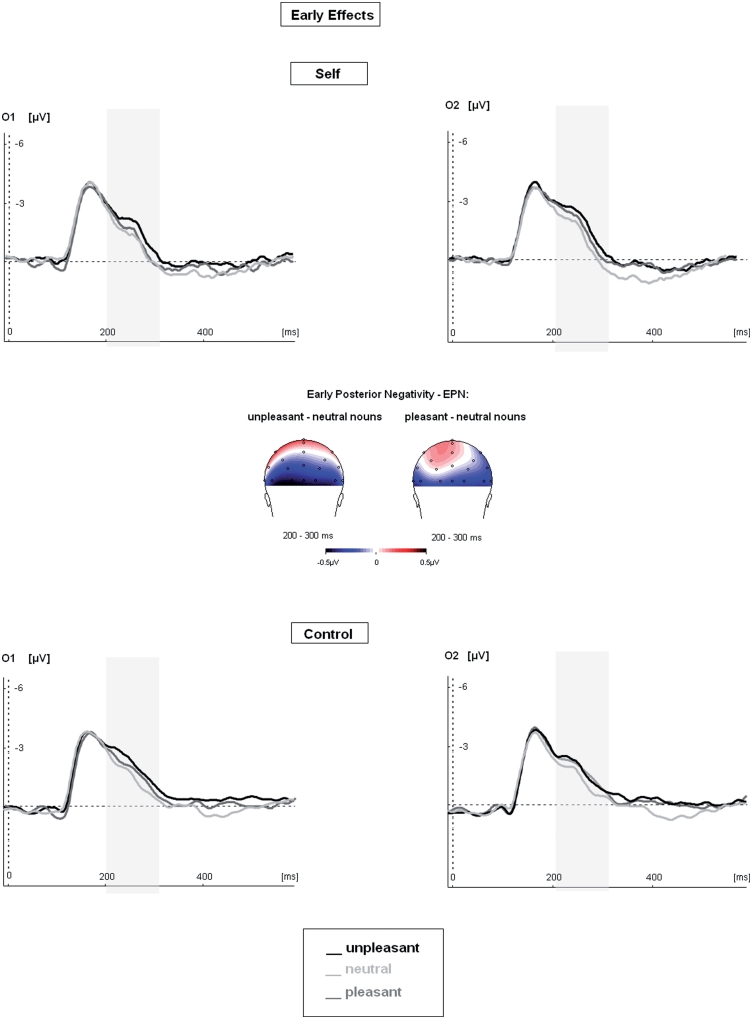
Cortical processing of emotional and neutral nouns differed significantly in the EPN time window from 200 to 300 ms after noun presentation (see Figure 1). Significant main effects of the factor `Valence’ indicated that reading of unpleasant and pleasant nouns elicited significantly larger amplitudes of the EPN than reading neutral nouns at left and right parieto-occipital electrodes [‘Valence’: F(2,28) = 10.53, P < 0.01; unpleasant–neutral: F(1,14) = 27.80, P < 0.01; pleasant– neutral: F(1,14) = 4.47, P = 0.05]. The interaction between the main factors `Valence’ and `Condition’ did not show any significant effect (P > 0.3). Significant earlier ERP differences (P1, N1) between nouns preceded by pronouns and articles were not observed. Both the P1 and the N1 did not show any significant main effects of the factors ‘Condition’ or ‘Valence’, or any interactions thereof (all P > 0.1).
Fig. 1.
Modulation of early ERP components during reading of emotional and neutral nouns when preceded by personal pronouns (‘Self’) or articles (`Control’). The EPN showed a main effect of stimulus valence, i.e. the EPN was enhanced for emotional nouns as compared with neutral nouns across conditions (‘Self’ and ‘Control’). For illustration the EPN effects (gray bars) are shown at the occipital electrodes O1 and O2, separately for each condition. Topographic maps of the difference potentials of the EPN, subtracting neutral from unpleasant and neutral from pleasant nouns are shown collapsed across the condition (‘Self’ and ‘Control’).
Late cortical processing effects—N400 and LPP
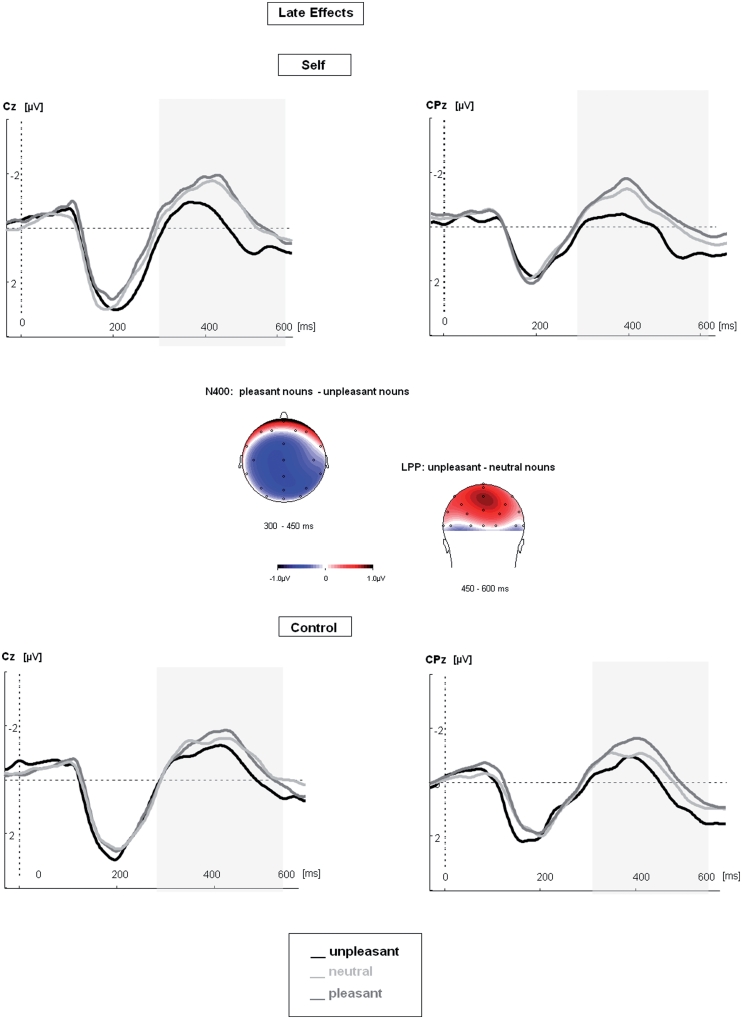
In the N400 time window there was a significant interaction effect of the main factors ‘Valence × Condition’ [F(2,28) = 7.4, P < 0.05]. Post hoc tests revealed significantly reduced amplitudes during processing of unpleasant as compared with both pleasant and neutral nouns if nouns were preceded by pronouns [unpleasant– neutral: F(1,14) = 8.18, P < 0.05; unpleasant–pleasant: F(1,14) = 13.7, P < 0.05]. Significant interactions with the factor ‘Condition’ showed that these effects were most pronounced at central and centro-parietal electrodes, respectively [‘Valence × Condition × Location’: F(16,224) = 2.40, P < 0.05].
The LPP also showed a significant interaction effect of the factors ‘Valence × Condition’ [‘Valence × Condition’: F(2,28) = 4.6, P < 0.05]. Only in the ‘Self’ condition did post hoc tests reveal larger amplitudes of the LPP for unpleasant as compared with neutral or pleasant nouns [unpleasant–neutral: F(1,14) = 5.19, P < 0.05; unpleasant–pleasant: F(1,14) = 5.32, P < 0.05]. As for the N400, effects were most pronounced at central and centro-parietal electrodes [‘Valence × Condition × Location’: F(16,224) = 2.13, P < 0.01].
The N400 and LPP effects, therefore, suggest that specifically unpleasant concepts benefited from higher order processing if preceded by personal pronouns. The results are shown in Figure 2.
Fig. 2.
Modulation of late ERP components during reading of emotional and neutral nouns when preceded by personal pronouns (‘Self”) or articles (‘Control’). Late ERPs in the N400-LPP time windows showed larger effects for unpleasant than pleasant or neutral nouns only in the ‘Self’ condition. Effects are illustrated at central and centro-parietal electrodes, separately for each condition. Topographic maps show the difference potentials in the N400 and the LPP time windows, subtracting pleasant from unpleasant and unpleasant from neutral nouns in the ‘Self’ condition.
Behavioral data
Subjective ratings
Unpleasant nouns were rated as more unpleasant and pleasant nouns as more pleasant than neutral nouns. Furthermore, pleasant and unpleasant nouns were rated as more emotionally arousing than neutral nouns, irrespective of whether nouns were paired with articles or pronouns (see Table 2). However, unpleasant nouns were rated as significantly more unpleasant when paired with personal pronouns than with articles [‘Category x Condition’: F(2,28) = 5.09, P < 0.01; post hoc: unpleasant pronoun–noun pairs–unpleasant article-noun pairs: F(1,14) = 21.8, P < 0.01]. Participants’ rating data are summarized in Table 2.
Table 2.
Rating data of pronoun–noun and article–noun pairs (Participants)
| Ratings—participants | |||
|---|---|---|---|
| Pleasant | Unpleasant | Neutral | |
| Pronoun–noun pairs | |||
| Valence | 6.96 (0.16) | 2.10 (0.09) | 5.10 (0.18) |
| Arousal | 5.46 (0.31) | 6.22 (0.32) | 3.14 (0.29) |
| Article–noun pairs | |||
| Valence | 6.72 (0.17) | 2.65 (0.12) | 4.99 (0.12) |
| Arousal | 5.27 (0.34) | 5.96 (0.37) | 2.91 (0.30) |
Mean valence, arousal and concreteness scores of pleasant, unpleasant and neutral pronoun–noun and article–noun pairs according to post-experimental ratings of the participants.
Note: Valence, arousal and concreteness ratings range from 1 (extremely negative valence, extremely low arousal or concreteness) to 9 (extremely positive valence, extremely high arousal or concreteness). Standard errors are in parentheses.
Memory performance
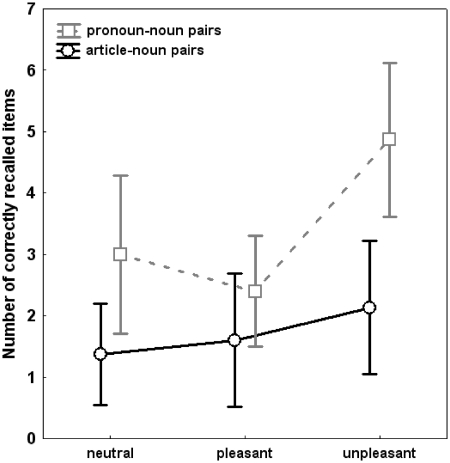
Emotional nouns were better remembered than neutral nouns [‘Category’: F(2,28) = 13.2, P < 0.01]. Significant effects of the main factor ‘Condition’ [F(1,14) = 27.1, P < 0.01], as well as the interaction of the factors ‘Category × Condition’ [F(2,28) = 7.7, P < 0.05], indicated that participants remembered nouns that were paired with personal pronouns better than nouns that were paired with an article. This was particularly the case regarding unpleasant nouns [pronoun–noun pairs: unpleasant–pleasant: F(1,14) = 19.3, P < 0.01; unpleasant–neutral: F(1,14) = 34.5, P < 0.001; unpleasant pronoun-noun pairs–unpleasant article–noun pairs: F(1,14) = 29.2, P < 0.001]. Participants’ free recall memory performance is displayed in Figure 3.
Fig. 3.
Free recall memory performance. Memory for correctly remembered emotional and neutral pronoun–noun and article–noun pairs as obtained from the free recall test after spontaneous processing of pleasant, unpleasant and neutral pronoun–noun and article–noun pairs.
DISCUSSION
This ERP study investigated whether implicit self-reference, as indicated by the personal pronoun ‘my’ modulates the processing of emotionally positive, negative and neutral nouns during a silent reading task. Results showed enhanced processing of pleasant and unpleasant nouns at the early processing stages associated with automatic attention capture (EPN). At later processing stages cortical processing was facilitated for unpleasant nouns only when nouns were preceded by personal pronouns instead of articles. Our results are the first to show an effect of self-reference on the processing of emotional stimuli in designs without explicit instructions for self-referential processing.
Reading personal pronouns did not affect early visual processing of subsequently presented emotional and neutral nouns. The P1 and the N1 were also unaffected by the emotional content of the nouns in either condition. In contrast, the EPN was larger for pleasant and unpleasant as compared with neutral nouns; modulation of the EPN by emotional content was observed irrespective of whether emotional nouns were preceded by articles or self-related pronouns. Emotional modulation of the EPN is a robust phenomenon, which is largely unaffected by factors such as task demands (e.g. Kissler et al., 2009; Herbert et al., 2008; Hinojosa et al., 2010). The current results corroborate these findings and extend them to the domain of social contextual factors such as self-reference. Moreover, the current EPN results support the view raised by appraisal theory that interactions between self-reference and emotional content occur after an initial intrinsic pleasantness check or rapid attention capture by emotional content as is reflected by the EPN.
In line with this suggestion as well as previous ERP studies using explicit self-referential evaluations tasks (e.g. Watson et al., 2007), self-reference modulated emotional processing at a higher order, cortical processing level: the N400 and the LPP were modulated by the emotional content of the words only when related to the self, i.e. if preceded by personal pronouns. Both these components have been shown to reflect semantic processing or to index processes of sustained attention, stimulus encoding (Paller et al., 1995; Kok, 1997), or cognitive re-appraisal (Hajcak and Nieuwenhuis, 2006; Hajcak et al., 2006; Moser et al., 2006). Appraisal of emotional stimuli is thought to be an essential feature of normal emotion processing, triggered automatically and implicitly whenever an emotional stimuli of subjective relevance is presented (Scherer, 2001). Accordingly, such implicit appraisals should be more pronounced and facilitated when triggered by personal pronouns as compared with articles. In line with this argument, larger amplitudes of the LPP were observed only during the ‘Self’ condition.
As displayed in Figure 3 the N400 and the LPP were partly overlapping in terms of topography. Therefore, it is possible that the reported attenuation of the N400 amplitude for self-related unpleasant nouns. Therefore, in the present study, already reflects the onset of the LPP indicating sustained attention to and deeper encoding of unpleasant words when related to the self. Deeper encoding of self-related, particularly unpleasant nouns was further substantiated by participants’ free recall memory performance.
This negativity bias toward self-related unpleasant concepts contrasts with previous findings that report a self-positivity bias. A processing bias that favors pleasant traits has been replicated many times, in particular in studies that used emotional trait adjectives as stimuli (Herbert et al., 2006, 2008, Kiefer et al., 2007) and explicit self-referential processing tasks (Watson et al., 2007). It is currently assumed that this positivity bias occurs because healthy individuals evaluate positive personality traits as more self-descriptive (Tagami, 2002; Herbert et al., 2008) and as more congruent with their self-concept than negative traits (Mezulis et al., 2004; Pahl and Eiser, 2005). Thus, adjectives may induce self-referential processing more easily than nouns, resulting in facilitated responses to pleasant concepts. Nevertheless, the present findings demonstrating a self negativity bias are not necessarily at odds with these previous findings because of important differences in the stimulus material presented: the current study used nouns instead of adjectives, and unpleasant and pleasant nouns were both related to the self. Our results, therefore, suggest that if stimuli other than trait adjectives are used and both unpleasant and pleasant stimuli are equally related to the self, participants appear to encode self-related unpleasant words more deeply than self-related pleasant ones. Under such conditions, self-related unpleasant stimuli may be more relevant and important to individuals than self-related pleasant ones, presumably due to their greater embodiment and challenge to one`s self-concept (e.g. Miall, 1986; Baumeister et al., 2001; Jing-Schmidt, 2007). In further support of this suggestion, we found that unpleasant nouns were rated as more unpleasant if paired with personal pronouns than with articles.
It could be argued that mood biases processing toward self-related pleasant or unpleasant events (Diener and Diener, 1996; Gotlib and Neubauer, 2000; Deldin et al., 2001; Kiefer et al., 2007). To follow up on this possibility, participants’ memory data and ERP responses were correlated with state and trait anxiety scores as well as depression scores. Results showed no significant associations with state or trait anxiety (all P > 0.3). However, for self-related unpleasant nouns memory performance was positively correlated with participants’ depression scores (r = 0.47, P = 0.04). The LPP also showed a correlation with participants’ depression scores at the midline sensor CPz, but also only for self-related unpleasant nouns (r = 0.48, P = 0.035). Given that individuals with high scores on the BDI are characterized by negative self-schema and ruminating negative thoughts about their self, these findings are in line with Beck’s theory (1976) and suggest that the larger processing effects for self-related unpleasant than pleasant events may in part be driven by individual differences in mood. Nevertheless, these correlation results should be considered preliminary, since we did not explicitly manipulate participants’ mood prior to the study nor did we preselect participants according to their self-reported mood and examine the role of positive and negative effect on this bias. Future studies using this paradigm may show whether mood-congruent processing effects can be replicated in larger samples of healthy subjects scoring high or low on positive and negative affect.
While preferential processing of emotional and self-related material is by now relatively well documented in the literature, it is hotly debated as to what extent these two types of preferential processing interact and if so, at which processing stages this interaction occurs. By means of ERP methods, we were able to selectively track how participants establish self-reference during silent reading of personal pronouns and how this affected the processing of stimuli containing emotional content at an electrophysiological level when no explicit instruction for self-related or emotional appraisal is given. Our data extends the findings of previous research demonstrating the effects of self-reference on emotion processing in explicit self-referential processing tasks. In particular, our data supports the general assumption from appraisal theory that self-reference acts as a processing filter for emotional material to receive higher order processing. Theoretically, this appears plausible, because if the meaning of a stimulus is irrelevant, there is no need to engage in complex information processing or ruminative thoughts about how to cope with its implications.
Further studies should aim to validate the current findings of an implicit self-referential processing effect, especially toward unpleasant material, in larger samples of healthy participants. An extension of our paradigm to clinical samples may help to better understand the mechanisms underlying the interplay between the self and emotions in various disorders, such as depression, autism, sociopathy and schizophrenia, which are characterized by deficits in either or both domains.
Conflict of Interest
None declared.
Acknowledgments
We thank Claus Vögele for proof-reading of the manuscript. Research was supported by the German Research Foundation (DFG).
Footnotes
1The complete list of the words used in this study (original and translation) together with valence and arousal ratings is available from the authors upon request.
REFERENCES
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavlavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–70. [Google Scholar]
- Beck AT. Cognitive Therapy and the Emotional Disorders. New York: New American Library; 1976. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of Behavioral Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: behaviour, feeling, and physiology. In: Lane RD, Nadel L, editors. Cognitive Neuroscience of Emotion. Oxford New York: Oxford University Press; 2000. pp. 49–59. [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX Lexical Database (CD-ROM) Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1995. [Google Scholar]
- Diener E, Diener C. Most people are happy. Psychological Science. 1996;7:181–5. [Google Scholar]
- Deldin PJ, Keller J, Gergen JA, Miller GA. Cognitive bias and emotion in neuropsychological models of depression. Cognition and Emotion. 2001;15:787–802. [Google Scholar]
- Ellsworth PC, Scherer KR. Appraisal processes in emotion. In: Davidson RJ, Goldsmith HH, Scherer KR, editors. Handbook of the Affective Sciences. New York: Oxford University Press; 2003. pp. 572–95. [Google Scholar]
- Esslen M, Metzler S, Pascual-Marqui R, Jancke L. Pre-reflective and reflective self-reference: a spatiotemporal EEG analysis. Neuroimage. 2008;42:437–49. doi: 10.1016/j.neuroimage.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, et al. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Neubauer DL. Information processing approaches to the study of cognitive biases in depression. In: Johnson SL, Hayes AM, editors. Stress, Coping and Depression. Mahwah, NJ: Erlbaum; 2000. pp. 117–43. [Google Scholar]
- Grandjean D, Sander D, Scherer KR. Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Consciousness and Cognition. 2008;17:484–95. doi: 10.1016/j.concog.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Grandjean D, Scherer KR. Unpacking the cognitive architecture of emotion processes. Emotion. 2008;8:341–51. doi: 10.1037/1528-3542.8.3.341. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:4259–264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–22. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective and Behavioral Neuroscience. 2006;6:291–7. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Herbert C, Kissler J, Junghofer M, Peyk P, Rockstroh B. Processing of emotional adjectives: evidence from startle EMG and ERPs. Psychophysiology. 2006;43:197–206. doi: 10.1111/j.1469-8986.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Herbert C, Junghofer M, Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology. 2008;45:487–98. doi: 10.1111/j.1469-8986.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- Hinojosa JA, Carretie L, Valcarcel MA, Mendez-Bertolo C, Pozo MA. Electrophysiological differences in the processing of affective information in words and pictures. Cognitive Affective and Behavioral Neuroscience. 2009;9:173–89. doi: 10.3758/CABN.9.2.173. [DOI] [PubMed] [Google Scholar]
- Hinojosa JA, Méndez-Bértolo C, Pozo MA. Looking at emotional words is not the same as reading emotional words: Behavioral and neural correlates. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.00982.x. May 2009 [Epub] [DOI] [PubMed] [Google Scholar]
- Holt DJ, Lynn SK, Kuperberg GR. Neurophysiological correlates of comprehending emotional meaning in context. Journal of Cognitive Neuroscience. 2009;21:2245–62. doi: 10.1162/jocn.2008.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghofer M, Sabatinelli D, Bradley MM, Schupp HT, Elbert TR, Lang PJ. Fleeting images: rapid affect discrimination in the visual cortex. Neuroreport. 2006;17:225–29. doi: 10.1097/01.wnr.0000198437.59883.bb. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Research. 2007;1148:138–48. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Jing-Schmidt Z. Negativity bias in language: A cognitive-affective model of emmotive intesifiers. Cognitive Linguistics. 2007;18:417–43. [Google Scholar]
- Junghofer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38:175–8. [PubMed] [Google Scholar]
- Kiefer M, Schuch S, Schenck W, Fiedler K. Mood states modulate activity in semantic brain areas during emotional word encoding. Cerebral Cortex. 2007;17:1516–30. doi: 10.1093/cercor/bhl062. [DOI] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Peyk P, Junghofer M. Buzzwords: early cortical responses to emotional words during reading. Psychological Science. 2007;18:475–80. doi: 10.1111/j.1467-9280.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- Kissler J, Herbert C, Winkler I, Junghofer M. Emotion and attention in visual word processing: an ERP study. Biological Psychology. 2009;80:75–83. doi: 10.1016/j.biopsycho.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biological Psychology. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading between the Lines: event-related brain potentials during Natural Sentence Processing. Brain and Language. 1980;11:354–73. doi: 10.1016/0093-934x(80)90133-9. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. The lateral distribution of event-related potentials during sentence processing. Neuropsychologia. 1982;20(5):579–90. doi: 10.1016/0028-3932(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and Emotion: Sensory and Motivational Processes. Mahwah, NJ: Erlbaum; 1997. pp. 97–135. [Google Scholar]
- Liu B, Jin Z, Wang Z, Hu Y. The interaction between pictures and words: evidence from positivity offset and negativity bias. Experimental Brain Research. 2010;201:141–53. doi: 10.1007/s00221-009-2018-8. [DOI] [PubMed] [Google Scholar]
- Mezulis AH, Abramson LY, Hyde JS, Hankin BL. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychological Bulletin. 2004;130:711–47. doi: 10.1037/0033-2909.130.5.711. [DOI] [PubMed] [Google Scholar]
- Miall DS. Emotion and the self: the context for remembering. British Journal of Psychology. 1986;77:389–97. [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–6. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K N, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–78. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Pahl S, Eiser JR. Valence, comparison focus and self-positivity biases: does it matter whether people judge positive or negative traits? Experimental Psychology. 2005;52:303–10. doi: 10.1027/1618-3169.52.4.303. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Scherer KR. A systems approach to appraisal mechanisms in emotion. Neural Networks. 2005;18:317–52. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Schacht A, Sommer W. Time course and task dependence of emotion effects in word processing. Cognitive Affective and Behavioral Neuroscience. 2009;9:28–43. doi: 10.3758/CABN.9.1.28. [DOI] [PubMed] [Google Scholar]
- Schapkin SA, Gusev AN, Kuhl J. Categorization of unilaterally presented emotional words: an ERP analysis. Acta Neurobiologiae Experimentalis (Wars) 2000;60:17–28. doi: 10.55782/ane-2000-1321. [DOI] [PubMed] [Google Scholar]
- Scherer KR, Schorr A, Johnstone T. Appraisal Processes in Emotion: Theory, Methods, Research. New York: Oxford University Press; 2001. [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychological Science. 2003;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology. 2004;41:441–9. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghofer M. Emotion and attention: event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Tagami K. Negative bias on self-referent processing in depression: focused on mood congruent effects. Shinrigaku Kenkyu. 2002;73:412–8. doi: 10.4992/jjpsy.73.412. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Watson LA, Dritschel B, Obonsawin MC, Jentzsch I. Seeing yourself in a positive light: brain correlates of the self-positivity bias. Brain Research. 2007;1152:106–11. doi: 10.1016/j.brainres.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain and Cognition. 2009;69:218–25. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]