Abstract
Findings from twin studies yield heritability estimates of 0.50 for prosocial behaviours like empathy, cooperativeness and altruism. First molecular genetic studies underline the influence of polymorphisms located on genes coding for the receptors of the neuropeptides, oxytocin and vasopressin. However, the proportion of variance explained by these gene loci is rather low indicating that additional genetic variants must be involved. Pharmacological studies show that the dopaminergic system interacts with oxytocin and vasopressin. The present experimental study tests a dopaminergic candidate polymorphism for altruistic behaviour, the functional COMT Val158Met SNP. N = 101 healthy Caucasian subjects participated in the study. Altruism was assessed by the amount of money donated to a poor child in a developing country, after having earned money by participating in two straining computer experiments. Construct validity of the experimental data was given: the highest correlation between the amount of donations and personality was observed for cooperativeness (r = 0.32, P ≤ 0.001). Carriers of at least one Val allele donated about twice as much money as compared with those participants without a Val allele (P = 0.01). Cooperativeness and the Val allele of COMT additively explained 14.6% of the variance in donation behaviour. Results indicate that the Val allele representing strong catabolism of dopamine is related to altruism.
Keywords: altruism, COMT Val158Met, dopamine, prosocial behaviour, genetics
INTRODUCTION
Social neuroscience is a rather new scientific discipline examining the biological basis of behaviour in social contexts. It mainly has its origins in social psychology and sociology, but instead of establishing algorithms valid for each individual on how behaviour is modulated by group dynamics and social contexts, social neuroscience tries to identify biological factors accounting for individual differences in social behaviour. The origin of all behaviour (including social behaviour) can be linked to cognitive, motivational and affective processes located in the brain. These processes are influenced by biological and environmental factors, which in turn interact with each other. Besides social cognition, interpersonal exchange and group interactions, prosocial behaviour––including empathy, cooperativeness and altruism––is a core research field in this area.
Altruism is defined as selfless concern for the welfare of others. However, there is a great debate in the literature if true altruism really exists (Fehr and Fischbacher, 2003). Pure altruism is giving without regard to reward or the benefits of recognition and need. People who doubt the existence of pure altruism argue that helping others is intrinsically rewarding for altruistic persons and therefore they are exercising their personal interest to benefit their own selves rather than others. In other words, helping others makes them feel good. This line of argumentation overcomes the seemingly incompatibility with economic concepts like the homo economicus postulating that humans are selfish rational beings motivated through self-interest (Ng and Tseng, 2008). However, it is widely acknowledged that there exist dramatic individual differences in the proclivity for altruistic behaviour. The crucial question arises if altruism represents a trait with a strong genetic impact or if it is a learned behaviour influenced by upbringing, education and other environmental factors like, e.g. religiosity. Findings from twin studies yield mean heritability estimates of about 0.50 for prosocial behaviours like empathy, cooperativeness and altruism indicating that nature and nurture have an equal impact on prosocial behaviour. These behavioural genetic studies mostly rely on self-report data: a twin study by Rushton et al. (1986) of 563 pairs of monozygous (MZ) and dizygous (DZ) twins, using an altruism and an emotional empathy scale, reported that 50% of the variance in altruism and empathy was due to genes and the other 50% to environmental factors. Noteworthy, the total environmental variance came from non-shared environmental sources and not from shared ones. Another study by Matthews et al. (1981) found 72% heritability for a self-report adjective checklist measure of empathy in 114 MZ and 116 DZ middle-aged male twins. In an additional twin study of 322 pairs of twins, Rushton (2004) replicated the strong genetic effects on prosocial behaviour. They found that heritability estimates of 0.40 for females and of 0.50 for males for social responsibility. In contrast to the study of 1986, shared environmental factors accounted for about 23% of the variance, whereas in the previous studies the environmental effects were exclusively due to non-shared environmental effects. Findings show that prosocial behaviours have a strong genetic influence and find support from a recent longitudinal study in 409 pairs of young twins that were investigated between 14 and 36 months of age (Knafo et al., 2008). Although, no genetic effects were observable at the age of 14 months, heritability estimates for empathy, which is a prerequisite for altruism, increased with the age. At the age of 24 and 36 months, genetics accounted for 34–47% of the variance in a global empathy factor. Shared environmental effects decreased from 0.69 at 14 months to 0.00 at 36 months, whereas non-shared environment accounted for 31–53% of the variance across ages. Even though these data were obtained in early infancy, the results are comparable with those of Rushton et al. (1986) based on the data of the adults.
There is also evidence for a genetic influence on reciprocating behaviour measured by trust and ultimatum games (Wallace et al., 2007; Cesarini et al., 2008). The latter two studies stem from the field of neuroeconomics and made use of experimental instead of self-report data. The paradigms used have high ecological validity because participants’ choices were related to real monetary loss and gain. Heritability estimates for trust range between 10% and 20%, and >40% of subjects’ rejection behaviour in the ultimatum game (rejection of unfair offers in a bargain situation that ends in personal costs) is explained by additive genetic variance.
However, not all studies in the literature are supportive for the claim that prosocial behaviour is highly heritable. Krueger et al. (2001) reported no genetic effect at all for altruism in a study on 170 pairs of MZ and 106 pairs of DZ males, although Krueger applied only a slightly modified version of the Self-Report-Altruism Scale used in the study by Rushton et al. (1986). Furthermore, Bouchard and Loehlin (2001) failed to find any evidence of genetic influence on self-assessed altruism. However in sum, the balance of evidence suggests a genetic effect on prosocial behaviours, especially on altruism.
Whereas quantitative genetics try to prove and estimate heritability of a given phenotype, molecular genetics, the second branch of behavioural genetics, is indebted to identify those genes that build the basis of heritability. Candidate genes for this endeavour stem from animal as well as human studies highlighting the prominent role of the nonapeptides, oxytocin and vasopressin for prosocial behaviours like attachment and pair bonding (for a review see Ebstein et al., 2010; Insel, 2010). Prosocial behaviours include a broad class of phenotypes that include those sorts of behaviours that are characterized by a positive view on man, helping, trusting and caring. A prerequisite for prosocial behaviour to occur is the ability to have empathy. Due to the fact that genetic association studies on prosocial behaviours are scarce, we try to give examples of the first pioneer studies in this field. First genetic association studies have successfully linked polymorphisms of the oxytocin receptor gene (OXTR) and the vasopressin 1a receptor gene (AVPR1A) to prosocial behaviours (Prichard et al., 2007; Israel et al., 2008, 2009; Lerer et al., 2008; Meyer-Lindenberg et al., 2008; Levin et al., 2009). However, the proportion of variance explained by these gene loci is rather low indicating the involvement of additional genetic variants in the expression of prosocial behaviour.
The dopaminergic system is another target for the investigation of the genetic basis of prosocial behaviours because dopamine has been related to parenting behaviour (Lee et al., 2008; van IJzendoorn et al., 2008), affective modulation of emotional stimuli (Montag et al., 2008) and personality traits of positive emotionality (Reuter and Hennig, 2005; Reuter et al., 2006). There is also evidence that vasopressin interacts with dopamine in the genesis of prosocial behaviour: meadow voles characterized by promiscuity in contrast to the monogamous prairie voles, also show monogamous behaviour after injection of an AVPR1a vector into the pallidum. However, administration of a dopamine antagonist before the injection of the AVPR1a vector prevents this shift from promiscuous to monogamous behaviour in these animals (Lim et al., 2004). In the same line, facilitation of partner preference formation in voles by the activation of oxytocin receptors is not effective when dopamine D2 receptors are blocked (Liu and Wang, 2003). Therefore, it is plausible that dopaminergic gene variants have also an influence on other prosocial behaviours besides pair bonding. In the context of the dopaminergic neurotransmission, especially the COMT Val158Met polymorphism is an interesting candidate polymorphism because this gene locus has turned out to be functional. Catechol-O-methyltransferase is an enzyme which plays a crucial role in the metabolism of catecholamines by inactivating them in the synaptic cleft, mostly in the prefrontal cortex. A single nucleotide polymorphism (SNP), a G→A transition in codon 158 of the COMT gene located at the q11 band of human chromosome 22 (rs4680), results in 3- to 4-fold reduction in COMT enzyme activity by coding for the synthesis of the amino acid methionine (MET) instead of valine (VAL). Carriers of the Val/Val genotype have highest, carriers of the Met/Met genotype lowest and heterozygotes (Val/Met genotype) have intermediate levels of COMT activity (Lachman et al., 1996).
The aim of the present study was to extend current knowledge of the molecular genetic basis of prosocial behaviours by investigating the potential role of the COMT Val158Met polymorphism for altruism. This was done in an experimental approach by studying human donation behaviour under conditions of high ecological validity.
METHODS
Participants
N = 101 healthy Caucasian students of German origin with no present or former ICD-10 diagnosis of psychopathology (26 males: age: mean = 23.88, s.d. = 4.60; 74 females: age: mean = 22.42, s.d. = 4.56) studying at the University of Bonn, Germany, participated in the study.
Participants gave written consent and were debriefed after the study was completed. They were given the opportunity to get excluded from the study if desired. The study was approved by the ethics committee of the German Psychologist Association and was conducted in accordance to the ethical standards of the Declaration of Helsinki.
Personality assessment
Cloninger’s ‘Temperament and Character Inventory (TCI)’ was administered in order to assess personality (Cloninger et al., 1993). The TCI consists of 240 dichotomous variables and measures the four temperaments ‘novelty seeking’, ‘harm avoidance’, ‘reward dependence’ and ‘persistence’ and the three characters ‘cooperativeness’, ‘self-directedness’ and ‘self-transcendence’. The rationale for using trait measures of personality were as follows: due to the fact that most genetic studies on prosocial behaviour were based on self-report data, it was intended to obtain validation data for questionnaire data by means of our experimental measure of altruism. If the magnitude of donations represents altruism, it is expected that we also find positive correlations with the cooperativeness scale of the TCI (measuring prosocial behaviour) and should observe non-significant correlations to the other TCI personality dimensions.
Genetic analyses
DNA was extracted from buccal cells. Automated purification of genomic DNA was conducted by means of the MagNA Pure® LC system using a commercial extraction kit (MagNA Pure LC DNA isolation kit; Roche Diagnostics, Mannheim, Germany). Genotyping of the COMT Val158Met polymorphism (rs4680) was performed by real time PCR using fluorescence melting curve detection analysis by means of the Light Cycler System (Roche Diagnostics, Mannheim, Germany). By means of the melting curve analyses, SNPs can be detected without conducting gel electrophoresis or ensuing sequencing after amplification. The primers and hybridization probes (TIB MOLBIOL, Berlin, Germany) and the PCR protocol for rs4680 are as follows:
forward primer: 5′-GGGCCTACTGTGGCTACTCA-3′;
reverse primer: 5′-GGCCCTTTTTCCAGGTCTG-3′;
anchor hybridization probe: 5′-LCRed640-TGTGCATGCCTGACCCGTTGTCA-phosphate-3′ and
sensor hybridization probe: 5′-ATTTCGCTGGCATGAAGGACAAG -fluorescein-3′.
Further details of the PCR protocol are described elsewhere (Reuter et al., 2006).
Experimental data
The total study consisted of three parts: first, subjects were paid 5 € for participating in a working memory experiment (n-back task, Weinberger et al., 1996). Next, they had the chance to increase their endowment in a gambling experiment (Iowa Gambling Task, IGT, Bechara et al., 2000). Finally, in the third and essential part of the study, participants had the choice to either keep all their money for themselves or to donate the money in part or in total to a poor child in a developing country. Participants were shown a picture of a cute little girl, Lina from Peru, and a bracelet that was knitted by her. The stimulus material was taken from advertisement material of a charity organization. Donations were made optional and in pretended anonymity: After the study was completed, the experimenter announced the sum of the endowment to the participant and left him/her alone in the laboratory. The student could take the endowment from a money tray including 20 pieces of each possible Euro coin. The participant was free to give as much money as he/she wanted from his/her endowment into a piggy bank. The amount of money that was already in the savings box was known to the experimenter but participants were unaware of this. By that, the experimenter was able to reconstruct the amount of donated money after the participant had left the laboratory. The duration of the total study was about 30 min. After the completion of the study, we donated all the money to the charity organization from which we took the advertisement material.
The reason for conducting a demanding n-back task at the beginning was that participants should get the feeling that they had worked hard for their money, i.e. that they do not donate additional money that they got out of the blue. The additional administration of the IGT increased the variance in participants’ endowment and helped to answer the question if the amount of the participants’ endowment has an influence on the amount of their donations.
Statistical analyses
One-factorial ANOVA models were calculated to test the influence of the COMT Val158Met polymorphism on altruism. On the genotype levels the independent factor had three levels (Val/Val, Val/Met and Met/Met) and on the Val allele level there were two levels Val+ (genotypes Val/Val and Val/Met) and Val– (genotype Met/Met). Altruism as dependent variable was defined by the amount of money donated, first as raw data (amount of donated money) and second as the percentage of donated money, i.e. the percentage of each participant’s endowment that was donated to the little girl from a developing country. The later variable controls for differences in the endowment which are likely to influence the magnitude of the donation. Bivariate Pearson correlations between all personality variables and the two dependent variables ‘amount of money donated and percentage of money donated’ were calculated. In order to assess the cumulative predictive power of COMT Val158Met and personality, a hierarchical linear regression model with percentage of donated money as criterion was conducted.
RESULTS
Genotyping
The genotype frequencies of COMT Val158Met were as follows: Val/Val: n = 24, Val/Met: n = 49, Met/Met; n = 28 and did not deviate from the Hardy–Weinberg equilibrium (χ2 = 0.08, df = 1, n.s.). There were no differences in genotype distributions between both gender groups (χ2 = 1.38, df = 2, P = 0.501).
Altruism
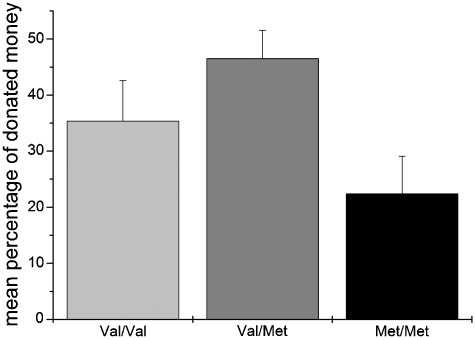
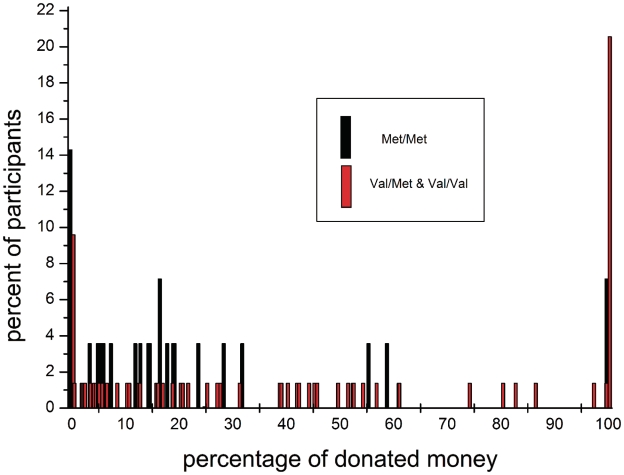
The average endowment at the end of the experiment was 4.77 € (s.d. = 0.75). The three COMT Val158Met genotype groups did not differ significantly with respect to their endowment [F(2,98) = 0.45, P = 0.640] indicating no effect of the COMT SNP on the amount of money won in the Iowa Gambling task. However, results showed that the COMT Val158Met polymorphism was significantly related to the percentage of donated money [F(2,98) = 4.18, P = 0.018; see Figure 1] and showed a trend towards significance with respect to the total amount of donation [F(2,98) = 2.84, P = 0.063]. When analysing the results on the allele level by grouping subjects into Val+ (genotypes Val/Val and Val/Met) and Val– subjects (genotype Met/Met) it turned out that the effects became more robust [percentage of donated money: F(1,99) = 6.72, P = 0.011; total amount of donation: F(1,99) = 4.96, P = 0.028]. Carriers of the Val+ group donated about half of their money (43%) for little Lina whereas the donation of the Val– group (22%) was about half as high as in the Val+ group. In order to illustrate this effect, the distribution of the percentage of donated money dependent on the allele group (Val– and Val+) was portrayed in Figure 2. It becomes apparent that >20% of the Val+ carriers donated their total endowment and that only a small percentage of Val– carriers are located in the right half of the distribution (high donations). There was no effect of gender on donation amounts [percentage of donated money: F(1,99) = 2.53, P = 0.115; total amount of donation: F(1,99) = 1.91, P = 0.170].
Fig. 1.
Percentage of participants’ endowment donated for a little girl in a developing country dependent on the COMT Val158Met polymorphism (rs4680). Results of the ANOVA: depicted are means and standard errors of means (s.e.m.)
Fig. 2.
Frequency distribution of the percentage of participants’ endowment donated for a little girl in a developing country dependent on the Val allele of the COMT Val158Met polymorphism (rs4680).
Personality and altruism
None of the three temperaments of the TCI were significantly correlated with the percentage of donated money. However, the two character dimensions self-directedness and cooperativeness showed significant positive correlations (r = 0.22; P = 0.028 and r = 0.32; P = 0.001, respectively) with donation behaviour. After Bonferroni correction for multiple testing only the correlation with cooperativeness remained significant.
Prediction of altruism by personality and COMT Val158Met
In order to test if the two predictors, cooperativeness and COMT Val158Met, explain additive or shared proportions of variance in donation behaviour a hierarchical multiple regression model was calculated. In the first block of the regression model the Val allele was added. The gene locus explained 5.8% of the variance in donation behaviour (F = 5.93, P = 0.017). In a second block, the personality variable cooperativeness was added increasing the explained variance significantly by 8.9% (total R2 = 0.147; change in F = 9.98, P = 0.002). Adding the interaction term Val allele by cooperativeness in a third block into the regression model did not increase the explained variance significantly (incremental explained variance 0.06 %; F = 0.69, P = 0.410). It has to be mentioned that the COMT Val158Met SNP was not related to cooperativeness [F(2,98) = 0.16, P = 0.851).
Controlling for confounding variables
The performance in the n-back task was on no level correlated with donation behaviour (1-back: r = 0.135, P = 0.182; 2-back: r = 0.151, P = 0.135; 3-back: r = 0.179, P = 0.074; 4-back: r = 169, P = 0.093; total n-back performance: r = 0.187, P = 0.062). Although the association between executive control function (n-back) and altruism is only a small trend, the direction of the trend is plausible: Val+ carriers show more altruism and many studies have shown that Val allele carriers exhibit better working memory performance (Weinberger et al., 1996). Therefore, the positive relation between altruism and n-back performance seems to be moderated by the Val allele.
Also the correlation between the net-score in the IGT was not significantly correlated with altruism (r = 0.164, P = 0.101). There were also no significant effects of genotype or gender on IGT net-score or the performance in the n-back task (all P-values in the ANOVAs > 0.4).
DISCUSSION
Prosocial behaviour is one of the prerequisites for the growth and prosperity of societies and is observable in many species besides primates (Zak and Knack, 2001). Evolutionary theories have been shown to be useful to explain non-selfish behaviours by introducing the terms ‘inclusive fitness’ and ‘reciprocal altruism’ (Hamilton, 1964; Trivers, 1971). It is known that there is great variability between and within societies in prosocial behaviour (Henrich et al., 2005). Especially, the latter one has been of scientific interest in humans because shared cultural background variables like norms and ethics of a given society cannot be the reason for such variability. Twin studies have disentangled genetic and environmental influences on prosocial behaviours indicating that ∼50% of the variance in altruism can be accounted by genetic effects (Rushton et al., 1986; Rushton, 2004). However, these heritability estimates were based on self-report data. The ecological validity of self-report data in science has often been questioned (Brewer, 2000). Experimental settings, where decision making has direct costs or benefits for the participants are likely to be superior in this respect. Neuroeconomics often makes use of monetary rewards to increase the ecological validity of human decision making, because money is the most potent generalized secondary reinforcer available. Despite the ongoing debate on how social decision making and altruism as a specific form of prosocial behaviour is assessed adequately, there are no studies available investigating the molecular genetic basis of altruism (although molecular genetic studies on prosocial behaviour assessed by economic games are reported in the literature).
Therefore, the aim of the present study was to identify those gene loci that contribute to the heritability of altruism. Starting point were existing studies that demonstrated the influence of polymorphisms of the OXTR and the AVPR1A on social behaviours (Prichard et al., 2007; Israel et al., 2008, 2009; Lerer et al., 2008; Meyer-Lindenberg et al., 2008; Levin et al., 2009). As known from other traits, many genes, additively or in interaction, contribute to the expression of complex phenotypes. Pharmacological studies have shown that the dopaminergic system interacts with the ‘prosocial’ hormones oxytocin and vasopressin (for a review see Skuse and Gallagher, 2008; Moos and Richard, 1982). The striatum is involved in reward-related learning (Delgado, 2007) and both ventral and dorsal striatum contain vasopressin and oxytocin receptors, in addition to DA receptors. Oxytocin interacts with dopaminergic circuits in the nucleus accumbens shell (NAS) and in the ventral tegmental area (VTA). Most prominent evidence for the interplay of dopamine with the neuropeptides oxytocin and vasopressin comes from animal studies showing that administration of dopamine antagonists can influence pair-bond formation in voles that was beforehand influenced by centrally acting genetic neuropeptide vectors (Liu and Wang, 2003; Lim et al., 2004). Also, in studies in humans, a neural network has been identified underlying social behaviour including social cognition. The normal functioning of that network engages the neuropeptides oxytocin and vasopressin with activity of dopaminergic receptors in the striatum and the orbitofrontal cortex (Kringelbach, 2005; Delgado, 2007). For example, oxytocin promotes interpersonal trust by inhibiting defensive behaviours and by linking this inhibition with the activation of dopaminergic reward circuits, enhancing the value of social encounters (Campbell, 2008).
Given this interaction between neuropeptides and dopamine it is not surprising that also polymorphisms related to the dopaminergic system, especially the COMT Val158Met SNP, have been demonstrated to be related to prosocial behaviour like extraversion and positive emotionality (Reuter and Hennig, 2005; Reuter et al., 2006).
By means of an experimental approach human donation behaviour was assessed as a proxy for altruism. Participants who had previously worked hard for a monetary reward could decide to donate money for a poor little child in a developing country or to keep the endowment for themselves. It turned out that individual differences in altruism could be explained by the COMT Val158Met SNP. Carriers of at least one Val allele (Val+ group) donated about twice as much money than those who were homozygous for the Met allele (Val– group). This finding is in line with previous studies relating the Met allele to negative emotionality (Goldman et al., 2005; Reuter and Hennig, 2005). Persons with habitually more negative affect (Met allele carriers or worriers in terms of Goldman’s ‘warrior/worrier’ model) are putatively less likely to show prosocial behaviour because of being too much occupied with their own problems.
External validity for the experimental paradigm assessing altruism comes from self-report personality questionnaire data measuring the basic temperament and character dimensions of the TCI. Cooperativeness was the only personality trait that showed substantial correlations to donation behaviour. Most interestingly, both predictors, the Val allele of COMT Val158Met and cooperativeness, could explain together ∼15% of the variance in the percentage of donated money.
Unfortunately the study did not test for interaction effects between COMT Val158Met and polymorphisms on the OXTR and AVPR1a receptors. In this first attempt, we deliberately did not test multiple SNPs together to warrant the theory driven approach of the study. Testing multiple gene loci simultaneously harbours the risk of multiple testing and would give the study an exploratory approach. Furthermore, an even larger sample size is needed to test for such an epistasis effect. However, after successful replication of the present findings the interaction of COMT Val158Met and polymorphisms on the ‘prosocial’ genes OXTR and AVPR1a has to be tested. Further future directions in altruism research are the investigation of the interaction of the dopaminergic system and the nonapeptides oxytocin and vasopressin by means of fMRI or pharmacological challenge tests.
A further shortcoming of the study is that we did not control for the economic background of our participants. Although the variance in the financial situation of students is rather low it cannot be excluded that this might be a confounding influence on donation behaviour. However, this would imply that the financial background of the participants would also co-vary with COMT Val158Met.
It has to be pointed out that the nature of the study is explorative. Normally sample sizes in case–control studies are much larger. However, experimental studies investigating so called endophenotypes of broader traits tend to have more power and therefore could rely on smaller sample sizes. But this argument does not argue against the need for an independent replication study.
In sum, the present study demonstrates that the dopaminergic system influences altruism in an ecological valid experimental paradigm.
Conflict of Interest
None declared.
REFERENCES
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, Jr, Loehlin JC. Genes, evolution, and personality. Behavior Genetics. 2001;31:243–73. doi: 10.1023/a:1012294324713. [DOI] [PubMed] [Google Scholar]
- Brewer M. Research design and issues of validity. In: Reis H, Judd C, editors. Handbook of Research Methods in Social and Personality Psychology. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological Psychology. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Cesarini D, Dawes CT, Fowler JH, Johannesson M, Lichtenstein P, Wallace B. Heritability of cooperative behavior in the trust game. Proceedings of the National Academy of Sciences USA. 2008;105:3721–26. doi: 10.1073/pnas.0710069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–90. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65:831–44. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425:785–91. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nature Reviews. 2005;6:521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour I and II. Journal of Theoretical Biology. 1964;7:1–16, 17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- Henrich J, Boyd R, Bowles S, et al. “Economic man” in cross-cultural perspective: behavioral experiments in 15 small-scale societies. Behavioral and Brain Sciences. 2005;28:795–815. doi: 10.1017/S0140525X05000142. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, et al. Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: from autism to altruism with some notes in between. Progress in Brain Research. 2008;170:435–49. doi: 10.1016/S0079-6123(08)00434-2. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS ONE. 2009;4:e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, Zahn-Waxler C, Van Hulle C, Robinson J L, Rhee SH. The developmental origins of a disposition toward empathy: genetic and environmental contributions. Emotion. 2008;8:737–52. doi: 10.1037/a0014179. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, McGue M. Altruism and antisocial behavior: independent tendencies, unique personality correlates, distinct etiologies. Psychological Science. 2001;12:397–402. doi: 10.1111/1467-9280.00373. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lee SS, Chronis-Tuscano A, Keenan K, et al. Association of maternal dopamine transporter genotype with negative parenting: evidence for gene x environment interaction with child disruptive behavior. Molecular Psychiatry. 2008;15:548–58. doi: 10.1038/mp.2008.102. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Molecular Psychiatry. 2008;13:980–8. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Levin R, Heresco-Levy U, Bachner-Melman R, Israel S, Shalev I, Ebstein RP. Association between arginine vasopressin 1a receptor (AVPR1a) promoter region polymorphisms and prepulse inhibition. Psychoneuroendocrinology. 2009;34:901–8. doi: 10.1016/j.psyneuen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–7. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–44. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Batson CD, Horn J, Rosenman RH. ‘‘Principles in his nature which interest him in the fortune of others … ’’: the heritability of empathic concern for others. Journal of Personality. 1981;49:237–47. [Google Scholar]
- Meyer-Lindenberg A. Impact of prosocial neuropeptides on human brain function. Progress in Brain Research. 2008;170:463–70. doi: 10.1016/S0079-6123(08)00436-6. [DOI] [PubMed] [Google Scholar]
- Moos F, Richard P. Excitatory effect of dopamine on oxytocin and vasopressin reflex releases in the rat. Brain Research. 1982;241:249–60. doi: 10.1016/0006-8993(82)91061-7. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, et al. COMT genetic variation impacts fear processing: psychophysiological evidence. Behavioral Neuroscience. 2008;122:901–9. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Ng ICL, Tseng L-M. Learning to be sociable: the evolution of homo economicus. American Journal of Economics and Sociology. 2008;67:265–86. [Google Scholar]
- Prichard ZM, Mackinnon AJ, Jorm AF, Easteal S. AVPR1A and OXTR polymorphisms are associated with sexual and reproductive behavioral phenotypes in humans. Mutation in brief no. 981. Human Mutation. 2007;28:1150. doi: 10.1002/humu.9510. [DOI] [PubMed] [Google Scholar]
- Reuter M, Hennig J. Association of the functional COMT VAL158MET polymorphism with the personality trait of extraversion. NeuroReport. 2005;16:1135–8. doi: 10.1097/00001756-200507130-00020. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmitz A, Corr P, Hennig J. Molecular genetics support Gray’s personality theory: the interaction of COMT and DRD2 polymorphisms predicts the behavioral approach system. International Journal of Neuropsychopharmacology. 2006;9:155–66. doi: 10.1017/S1461145705005419. [DOI] [PubMed] [Google Scholar]
- Rushton JP. Genetic and environmental contributions to pro-social attitudes: a twin study of social responsibility. Proceedings of the Royal Society London. 2004;271:2583–5. doi: 10.1098/rspb.2004.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton JP, Fulker DW, Neale MC, Nias DKB, Eysenck HJ. Altruism and aggression: the heritability of individual differences. Journal of Personality and Social Psychology. 1986;50:1192–8. doi: 10.1037//0022-3514.50.6.1192. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic–neuropeptide interactions in the social brain. Trends in Cognitive Sciences. 2008;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Trivers RL. The evolution of reciprocal altruism. Quarterly Review of Biology. 1971;46:35–57. [Google Scholar]
- van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Mesman J. Dopamine system genes associated with parenting in the context of daily hassles. Genes Brain and Behavior. 2008;7:403–10. doi: 10.1111/j.1601-183X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Wallace B, Cesarini D, Lichtenstein P, Johannesson M. Heritability of ultimatum responder behavior. Proceedings of the National Academy of Sciences USA. 2007;104:15631–4. doi: 10.1073/pnas.0706642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Mattay V, Callicott J, et al. fMRI applications in schizophrenia research. Neuroimage. 1996;4:118–26. doi: 10.1006/nimg.1996.0062. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Knack S. Trust and growth. Economic Journal. 2001;111:295–321. [Google Scholar]