Abstract
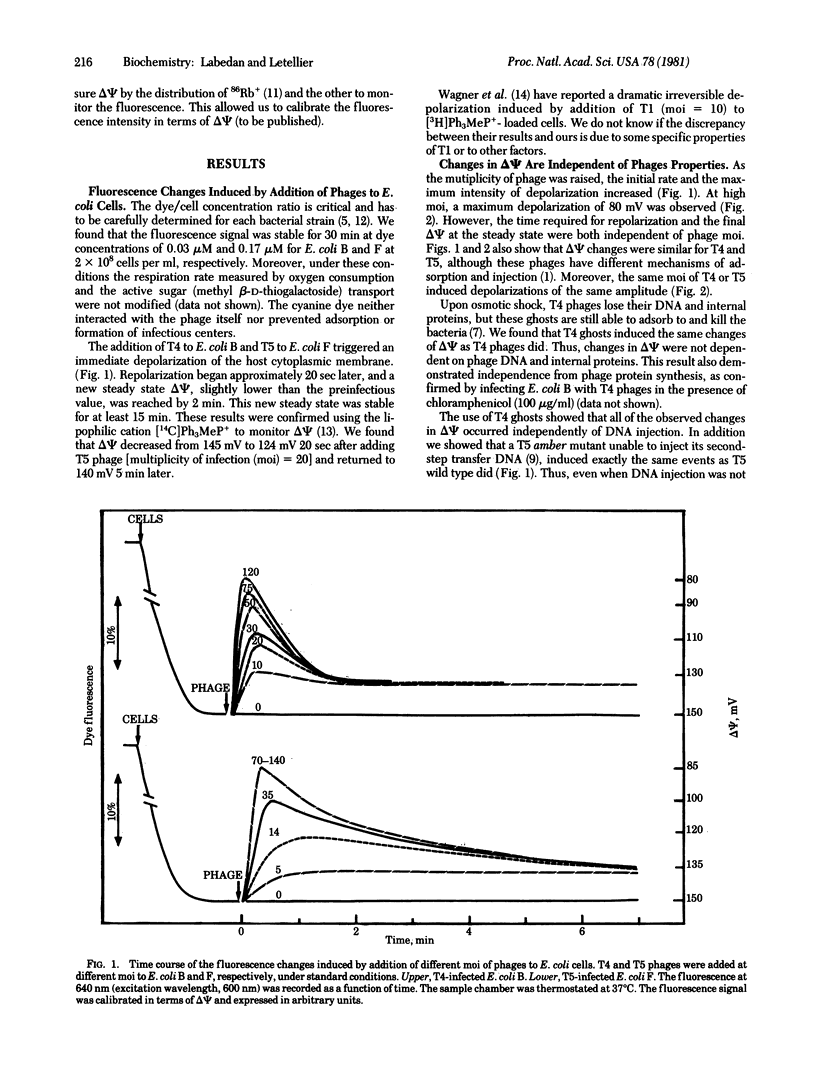
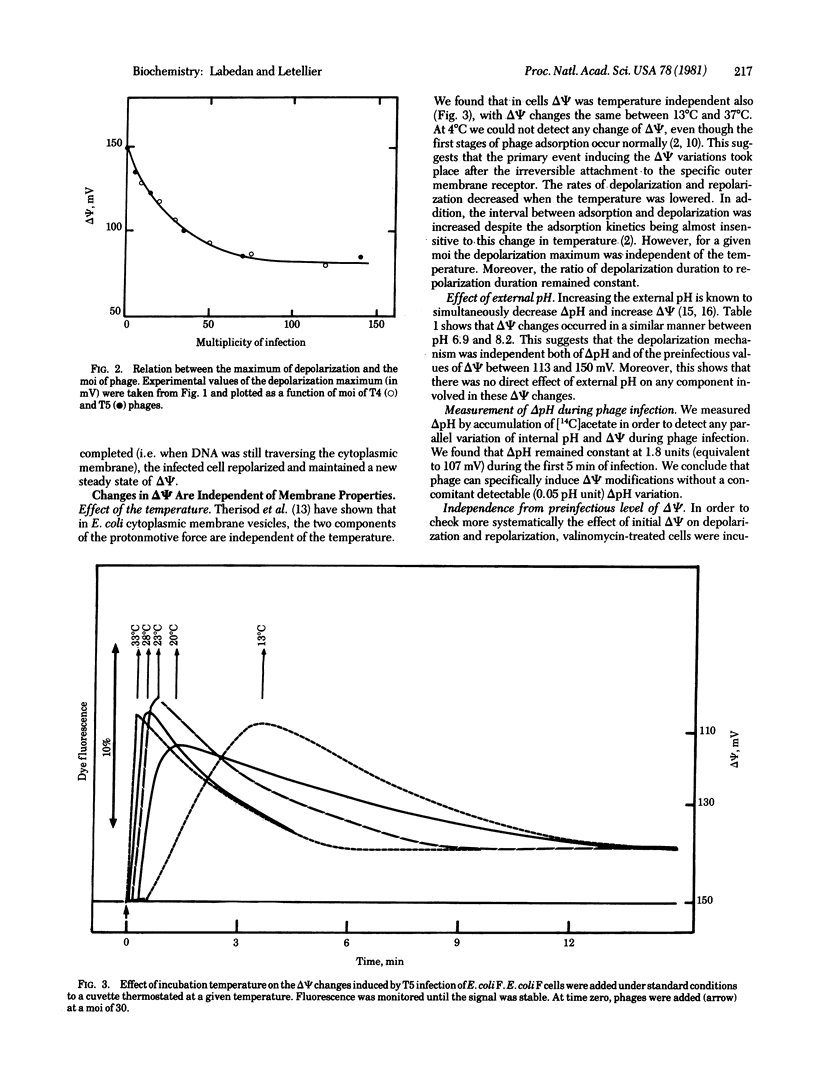
Immediately after adsorption, phages T4 and T5 induce a partial depolarization of the host cytoplasmic membrane. Infected bacteria respond to this phage-induced effect by a repolarization that leads to a new steady state of reduced membrane potential. The rate and extent of repolarization are adjusted to the intensity of depolarization, which depends on the number of adsorbed phages. Consequently, the new steady state membrane potential is attained in the same interval of time regardless of the maximum depolarization. These membrane potential changes appear to be independent of phage-specific properties (type of phage, presence of DNA and internal proteins, injection process) and of several membrane-related parameters (temperature, external pH, preinfectious level of membrane potential). We propose that phage adsorption to the outer membrane triggers the emission of a signal that is transmitted to the cytoplasmic membrane. Additivity of independent signals is possible when stimuli (phages) are added at the same time. Additional adsorption of phages has no further depolarizing effect as soon as the repolarization begins. We propose that this refractoriness to secondary depolarization nd the shut-off of the first depolarization are induced by the same chemical modification also initiated by adsorption of the first phage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Eigner J. Breakdown and exclusion of superinfecting T-even bacteriophage in Escherichia coli. J Virol. 1971 Dec;8(6):869–886. doi: 10.1128/jvi.8.6.869-886.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R., Mitchell W. J., Hamilton W. A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979 Sep 15;182(3):687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H. Biological activity of bacteriophage ghosts and "take-over" of host functions by bacteriophage. Bacteriol Rev. 1970 Sep;34(3):344–363. doi: 10.1128/br.34.3.344-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J., Duckworth D. H. Ion fluxes during T5 bacteriophage infection of Escherichia coli. Arch Biochem Biophys. 1980 May;201(2):576–585. doi: 10.1016/0003-9861(80)90547-0. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Wilson T. H. Sucrose transport by the Escherichia coli lactose carrier. J Bacteriol. 1979 Nov;140(2):395–399. doi: 10.1128/jb.140.2.395-399.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. I. Some basic kinetic features. Virology. 1960 Apr;10:501–513. doi: 10.1016/0042-6822(60)90132-x. [DOI] [PubMed] [Google Scholar]
- Labedan B., Goldberg E. B. Requirement for membrane potential in injection of phage T4 DNA. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4669–4673. doi: 10.1073/pnas.76.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Heller K. B., Jasaitis A. A., Wilson T. H., Goldberg E. B. A membrane potential threshold for phage T4 DNA injection. Biochem Biophys Res Commun. 1980 Mar 28;93(2):625–630. doi: 10.1016/0006-291x(80)91124-9. [DOI] [PubMed] [Google Scholar]
- Labedan B. In vitro study of the phage T5 DNA injection process: use of columns of Escherichia coli membranes immobilized on kieselguhr. Virology. 1978 Apr;85(2):487–493. doi: 10.1016/0042-6822(78)90455-5. [DOI] [PubMed] [Google Scholar]
- Lanni Y. Functions of two genes in the first-step-transfer DNA of bacteriophage T5. J Mol Biol. 1969 Aug 28;44(1):173–183. doi: 10.1016/0022-2836(69)90412-4. [DOI] [PubMed] [Google Scholar]
- Letellier L., Shechter E. Cyanine dye as monitor of membrane potentials in Escherichia coli cells and membrane vesicles. Eur J Biochem. 1979 Dec 17;102(2):441–447. doi: 10.1111/j.1432-1033.1979.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Cation fluxes and permeability changes accompanying bacteriophage infection of Escherichia coli. J Virol. 1968 Aug;2(8):763–771. doi: 10.1128/jvi.2.8.763-771.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therisod H., Weil R., Shechter E. Functional lac carrier protein in cytoplasmic membrane vesicles isolated from Escherichia coli: temperature and pH dependence of dansyl-galactoside binding. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4214–4218. doi: 10.1073/pnas.75.9.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M., Cornett J. B. A new gene of bacteriophage T4 determining immunity against superinfecting ghosts and phage in T4-infected Escherichia coli. Virology. 1972 Jun;48(3):777–784. doi: 10.1016/0042-6822(72)90161-4. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S. The use of cyanine dyes for the determination of membrane potentials in cells, organelles, and vesicles. Methods Enzymol. 1979;55:689–695. doi: 10.1016/0076-6879(79)55077-0. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Wang C. H., Tolles R. L. Mechanism of potential-dependent light absorption changes of lipid bilayer membranes in the presence of cyanine and oxonol dyes. J Membr Biol. 1977 May 6;33(1-2):109–140. doi: 10.1007/BF01869513. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Ponta H., Schweiger M. Development of Escherichia coli virus T1. The role of the proton-motive force. J Biol Chem. 1980 Jan 25;255(2):534–539. [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]



