Abstract
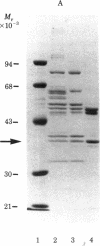
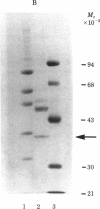
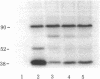
We have purified the translation restoring factor (RF) and the eukaryotic initiation factor 2 (eIF-2) stimulating protein (ESP) to near homogeneity from the postribosomal supernatant and the ribosomal salt wash, respectively, of rabbit reticulocyte lysate. They were isolated in the form of eIF-2 complexes, apparently in a 1:1 ratio. Their virtually identical NaDodSO4/polyacrylamide gel electrophoretic patterns show, in addition to the eIF-2 alpha (38,000), beta (52,000), and gamma (54,000) bands, peptide bands at approximately 80, 65, 57, 40, and 32 kilodaltons. The apparent Mr of either complex is about 450,000, whereas that of free translation restoring factor (RF) is approximately 25,000. At 0.5 mM Mg2+, both ESP and RF stimulate ternary complex (eIF-2.GTP.Met-tRNAi) formation catalytically with unphosphorylated eIF-2. Phosphorylation of the eIF-2 alpha subunit by preincubation with eIF-2 alpha kinase and ATP, which virtually blocks eIF-2-ESP interaction, results in only partial blocking of the interaction with RF. This may explain the translation restoring activity of RF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz H., Goumans H., Haubrich-Morree T., Voorma H. O., Benne R. Purification and characterization of a protein factor that reverses the inhibition of protein synthesis by the heme-regulated translational inhibitor in rabbit reticulocyte lysates. Eur J Biochem. 1979 Aug 1;98(2):513–520. doi: 10.1111/j.1432-1033.1979.tb13212.x. [DOI] [PubMed] [Google Scholar]
- Benne R., Wong C., Luedi M., Hershey J. W. Purification and characterization of initiation factor IF-E2 from rabbit reticulocytes. J Biol Chem. 1976 Dec 10;251(23):7675–7681. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Das A., Ralston R. O., Grace M., Roy R., Ghosh-Dastidar P., Das H. K., Yaghmai B., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes: mechanism of protein synthesis inhibition by heme-regulated inhibitor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5076–5079. doi: 10.1073/pnas.76.10.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Leroux A., London I. M. Site-specific phosphorylation of the alpha subunit of eukaryotic initiation factor eIF-2 by the heme-regulated and double-stranded RNA-activated eIF-2 alpha kinases from rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1286–1290. doi: 10.1073/pnas.77.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosfeld H., Ochoa S. Purification and properties of the double-stranded RNA-activated eukaryotic initiation factor 3 kinase from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6526–6530. doi: 10.1073/pnas.77.11.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. Control of protein synthesis by hemin. Isolation and characterization of a supernatant factor from rabbit reticulocyte lysate. Biochim Biophys Acta. 1976 Nov 1;447(4):445–459. doi: 10.1016/0005-2787(76)90082-4. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Ochoa S., de Haro C. Regulation of protein synthesis in eukaryotes. Annu Rev Biochem. 1979;48:549–580. doi: 10.1146/annurev.bi.48.070179.003001. [DOI] [PubMed] [Google Scholar]
- Ralston R. O., Das A., Grace M., Das H., Gupta N. K. Protein synthesis in rabbit reticulocytes: characteristics of a postribosomal supernatant factor that reverses inhibition of protein synthesis in heme-deficient lysates and inhibition of ternary complex (Met-tRNAfMet.eIF-2.GTP) formation by heme-regulated inhibitor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5490–5494. doi: 10.1073/pnas.76.11.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: additional initiation factor required for formation of ternary complex (eIF-2.GTP.Met-tRNAf) and demonstration of inhibitory effect of heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1079–1083. doi: 10.1073/pnas.76.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and characterization of heme-reversible translational inhibitor. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3654–3658. doi: 10.1073/pnas.75.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Datta A., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1978 Jan;75(1):243–247. doi: 10.1073/pnas.75.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1741–1745. doi: 10.1073/pnas.76.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor: stimulating protein acts at level of binary complex formation. Proc Natl Acad Sci U S A. 1979 May;76(5):2163–2164. doi: 10.1073/pnas.76.5.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis: studies with factors from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2713–2716. doi: 10.1073/pnas.75.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]