Abstract
Although withdrawal severity and treatment completion are the initial focus of opioid detoxification, post-detoxification outcome better defines effective interventions. Very low dose naltrexone (VLNTX) in addition to methadone taper was recently associated with attenuated withdrawal intensity during detoxification. We describe the results of a seven-day follow-up evaluation of 96 subjects who completed inpatient detoxification consisting of the addition of VLNTX (0.125 or 0.250 mg per day) or placebo to methadone taper in a double blind, randomized investigation. Individuals receiving VLNTX during detoxification reported reduced withdrawal and drug use during the first 24 hours after discharge. VLNTX addition was also associated with higher rates of negative drug tests for opioids and cannabis and increased engagement in outpatient treatment after one week. Further studies are needed to test the utility of this approach in easing the transition from detoxification to various follow-up treatment modalities designed to address opioid dependence.
INTRODUCTION
The goal of opioid detoxification is to provide medical stabilization and a humane discontinuation of the abused drugs, but clinical objectives should have a broader perspective.1,2 Persisting withdrawal discomfort and early relapse following detoxification affect longer-term outcome, such as continued abstinence and engagement in aftercare.3,4 While substitution maintenance therapy with methadone or buprenorphine is an effective relapse prevention strategy for opioid addiction, the management of patients who do not want or must discontinue these treatments is problematic.5 Rational pharmacological approaches using opioid agonist taper or alpha-2 adrenergic agonist medications such as clonidine are relatively safe and effective detoxification interventions, but of questionable longer term value due to high relapse and poor treatment-seeking rates at follow up.6,7 Opioid antagonist medications used to facilitate withdrawal have been inconsistently effective or unsafe.8,9
Detoxification is the primary point of contact with the treatment system for almost 50% of opioid dependent patients treated each year.10 It can be an important first step within the broader therapeutic process. Thus, there is a substantial need for new approaches with potential for improving longer-term outcomes.
The detoxification process affects longer-term outcome, but it is not completely clear to what extent or by what mechanisms different methods of detoxification may directly influence such outcomes.11,12 Early drop-out or reduced treatment duration have been associated with poor outcomes at follow-up.13 On the other hand, the length of inpatient stay post-detoxification is more important in promoting longer-term abstinence than any specific detoxification procedure.14
Withdrawal discomfort after methadone discontinuation is associated with relapse and reduced rates of continuation with outpatient treatment.3,15 Up to 50% of the total patient drop-out occurs in the first week following detoxification.16 This leads frequently to a return to detoxification, a “revolving door” phenomenon that constitutes both a clinical challenge and an economic burden in the treatment of opioid dependence.17,18 Persisting withdrawal discomfort following detoxification is also an obstacle to the effective initiation of relapse prevention strategies for opioid dependence, such as antagonist treatment.19,20 Thus, the first few days subsequent to detoxification can be critical to the success of treatment.
Detoxification methods are evaluated using a wide range of criteria, making it difficult to directly compare effectiveness. Moreover, objective measures of abstinence and post-detoxification entry rates into ongoing treatment have rarely been reported,21 except in relation to opioid agonist or antagonist treatment.11,22 We describe the results of a controlled study evaluating the effect of medication on early post-detoxification discomfort, relapse rates and transition to non-pharmacological treatment.
Preclinical investigations have demonstrated that the addition of VLNTX to opioid agonist medications prevents the development of tolerance and reverses established dependence by putatively interfering with the gradual up-regulation of mu-opioid receptor function in brain areas involved with the expression of opioid effects.23 Clinical trials have confirmed that chronic VLNTX administration reduces opioid tolerance, manifested as increased opioid analgesia in chronic pain patients treated with opioid pain medications.24 In addition, VLNTX has been found ineffective in preventing relapse when administered alone and following detoxification.25,26
We have previously reported the results of a double blind, placebo-controlled, randomized study showing that the addition of very low doses of naltrexone (VLNTX) to methadone taper reduces withdrawal severity and craving during inpatient opioid detoxification.27 Opioid dependent patients receiving VLNTX adjunct in that study were treated at community programs and discharged on the day they received the last dose of methadone.
We present here short-term follow-up data on those patients, evaluating whether the addition of VLNTX to inpatient detoxification would be associated with improved post-detoxification outcomes among detoxification completers in the first week following discharge.
MATERIALS AND METHODS
Study Design
This was a one-week follow up evaluation of withdrawal intensity, drug use and engagement in treatment using a double-blind, placebo-controlled randomized, two-site clinical trial design with two adjunct oral VLNTX regimens (0.125 mg/day or 0.250 mg/day) in opioid-dependent subjects receiving daily tapering doses of methadone during inpatient opioid detoxification.
Subjects and Procedures
One-hundred and twenty opioid-dependent individuals completing a six-day detoxification treatment at two community-based programs in Chapel Hill, NC and Philadelphia, PA provided oral and written consent. The study was approved by the institutional review boards of Duke University and Thomas Jefferson University. The diagnosis of opioid dependence with physiological dependence was based on the DSM IV checklist for that disorder28 and confirmed by urine drug testing. Potential subjects had been excluded from detoxification for any of the following criteria: history of hypersensitivity to NTX, pregnancy or medical conditions that would make participation hazardous (eg, acute hepatitis, unstable cardiovascular status, liver disease, renal disease), suicide risk, DSM IV diagnosis of psychotic disorder, major depression, bipolar disorder, or current dependence on substances other than opioids, except for nicotine dependence. Data on subjects’ demographic characteristics, drug use history and social and psychological functioning were collected at admission to treatment using the Addiction Severity Index.29 Subjects received the standard methadone taper schedule that was in use at the community treatment programs: a single 30 mg dose on day one upon baseline assessment, after which methadone was tapered by five mg/day, with treatment completion and discharge on day six. In addition to this methadone taper, subjects were randomly assigned to one of three add-on medication groups: placebo, naltrexone −0.125 mg daily, or naltrexone −0.250 mg daily. Patients were asked to participate in a follow-up process including one-day and one-week evaluations following discharge. All subjects and research staff remained blind to medication assignment throughout the study. The screening process, detoxification treatment and evaluation procedures are described in detail elsewhere.27
One-Day Follow-Up
Patients were asked to return on the morning following methadone discontinuation and discharge. Severity of opioid withdrawal was assessed with vital signs and two rating scales. The Subjective Opiate Withdrawal Scale (SOWS) is a self-rating scale which evaluates the presence and intensity of 16 symptoms on five-point Likert scales.30 One item “I feel like shooting up/taking the drug right now” is also a reliable index of opioid craving.31 The Objective Opiate Withdrawal Scale (OOWS) rates the presence of 13 observable physical signs of withdrawal. It was completed by a trained staff observer during the time the subject was filling out the SOWS.30 Urine drug tests for opioids (not including methadone), cocaine, amphetamine, methamphetamine and THC (UDT-5-Panel CLIA Waived Integrated Drug Testing Cup), and breath alcohol tests (AL-6000 Alcoscan Digital Breathalyzer) were also performed.
Seven-Day Follow-Up
Patients were contacted and interviewed by telephone after one week using the Treatment Services Review (TSR)32 and the Time Line Follow Back method (TLFB),33 to gather information on attendance at drug-free outpatient treatment programs, and use of psychotropic medications, or illicit drugs, respectively. Subjects were then given an appointment to return to the hospital and provide UDT and breathalyzer test.
Compensation
Participants received $20 for completing each follow-up evaluation and an additional $20 for performing the drug screens at week one. Compensation was in the form of gift certificates.
Outcome Measures
We evaluated the following outcome measures: 1) Opioid withdrawal severity after methadone discontinuation, assessed using SOWS and OOWS scales; 2) Craving, rated using one SOWS item; 3) Illicit drug use, measured by urine drug, breath alcohol testing and TLFB one and seven days after discharge from detoxification; and 4) Engagement in aftercare at one week, as reported by TSR.
Statistical Analysis
Comparisons of the treatment groups on baseline characteristics and outcomes were done by ANOVA analyses with Bonferroni post hoc tests for continuous variables and χ2 tests for dichotomous variables. Analysis of covariance (ANCOVA) with post hoc contrast test (K Matrix) was used to compare withdrawal and craving scores (measured by SOWS and OOWS), with withdrawal measures at discharge and recent alcohol use as covariates. A logistic regression model was used to evaluate the role of VLNTX addition in predicting abstinence and engagement in outpatient treatment following detoxification. Finally, we calculated an additional measure of treatment effect, the “number needed to treat” (NNT)34 for one additional patient to better respond to pharmacologically assisted detoxification augmented with VLNTX, in comparison with methadone alone. Better response was defined by maintaining abstinence and continuing with aftercare in the first week following detoxification.
RESULTS
Subjects
Of 120 individuals completing detoxification, 96 were evaluated the following day; 61 also participated in the assessment one week later. The characteristics of the follow-up sample are summarized in Table 1. Seventy-one percent of subjects were White and 61% were male, with a mean age of 32 and 1.5 previous detoxifications. Twenty-nine individuals received placebo, 33 VLNTX 0.125 mg/day, and 34 VLNTX 0.250 mg/day during detoxification. There were no statistically significant differences in demographic and clinical measures, or in proportion of subjects lost to follow-up (not shown), by treatment condition or study site (the latter data not shown), except that self-reported frequency of recent alcohol use was significantly higher among subjects receiving NTX 0.250 mg than placebo (Table 1). There were no significant differences in characteristics between subjects lost to follow up and those who participated in the evaluations (data not shown).
TABLE 1.
Socio-demographic and substance use characteristics of 96 opioid-dependent subjects undergoing evaluation the day following discharge from detoxification
| Placebo (n = 29) | NTX 0.125 mg (n = 33) | NTX 0.250 mg (n = 34) | |
|---|---|---|---|
| Demographics % or mean (SD) | |||
| Age | 32.7 (10.6) | 31.3 (9) | 34.2 (9.3) |
| Male | 53.7% | 61.5% | 67.1% |
| African American | 24,1% | 33.3% | 29.4% |
| Years of education | 11.8 (2.2) | 11.7 (1.9) | 12 (2) |
| Married or cohabitant | 20.7% | 15.1% | 17.6% |
| Unemployed | 48.3% | 57.6% | 52.9% |
| Substance use Days of use in last month | |||
| Opioids | 19.6 (6.4) | 18.7 (6.4) | 20.9 (2.5) |
| Alcohol1 | 3.5 (6.2) | 7.8 (9.2) | 11.7 (12.2) |
| Marijuana | 7.6 (8.9) | 8.7 (10.8) | 6.3 (6.4) |
| Cocaine | 9.5 (7.5) | 7.3 (8.8) | 11 (9.2) |
| Years of use | |||
| Opioids | 7.1 (6.2) | 6.2 (5.7) | 7.8 (8.2) |
| Alcohol | 4.9 (5.8) | 6.5 (8.2) | 7.3 (9.2) |
| Marijuana | 6.8 (6.9) | 7.2 (6.1) | 9.3 (9.8) |
| Cocaine | 4.4 (7.2) | 7.1 (8.5) | 7.2 (8.2) |
| N previous detoxifications | 1.3 (2.1) | 1.4 (1.74) | 1.9 (2.8) |
| N other previous treatments | 3.5 (4.2) | 2.8 (2.8) | 3.3(2.4) |
| ASI Drug Comp Score | .285 (.18) | .310 (.16) | .299 (.16) |
| ASI Alcohol Comp Score | .067 (.19) | .142 (.25) | .143 (.24) |
| ASI Psych Comp Score | .090 (.22) | .107 (.28) | .087 (.23) |
| Admission urine tests | |||
| Positive cocaine | 55.2% | 48.5% | 50% |
| Positive THC | 51.7% | 45.5% | 53% |
| Positive amphetamine | 6.9% | 6% | 8.8% |
F = 4.42 (2,96); p = 0.005; difference between placebo and 0.250 group
All comparisons with post hoc Bonferroni test
Abbreviations: ASI = Addiction Severity Index Comp = Composite, Psych = Psychiatric, SOWS = Subjective Opiate Withdrawal Scales, OOWS = Objective Opiate Withdrawal Scales
Opioid Withdrawal and Craving
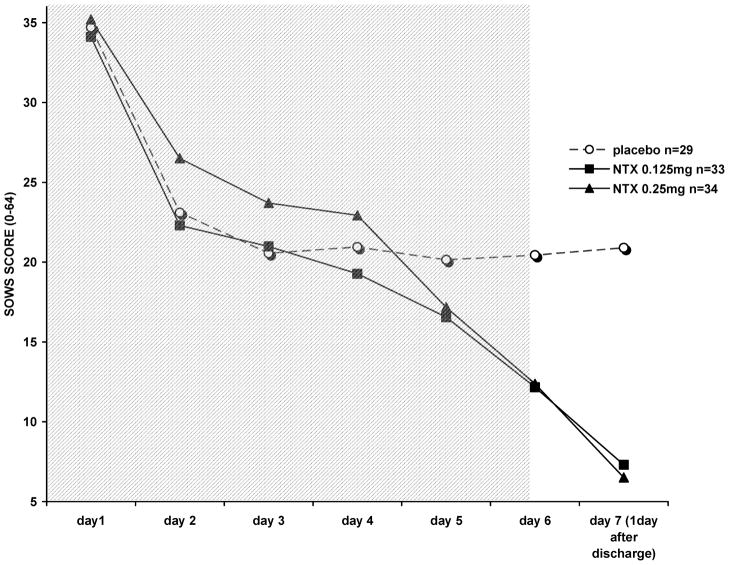
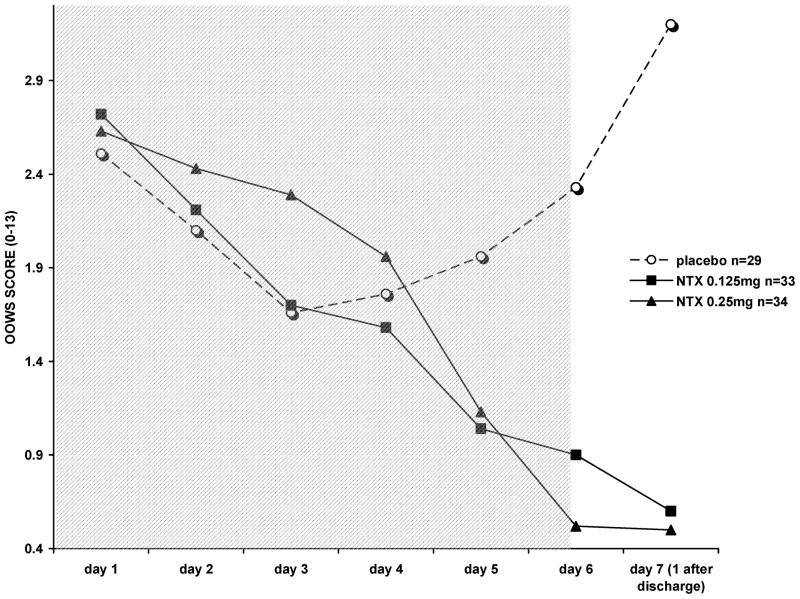
SOWS withdrawal scores were significantly lower among VLNTX-treated subjects on the day following completion of detoxification, after adjusting for discharge ratings (Figure 1; F = 174.7 (2, 94); contrast estimate between NTX and placebo—4.805 (95% CI −7.36 to −2.253); p = 0.001). Similarly, OOWS adjusted scores were lower in subjects who received VLNTX during detoxification (Figure 2; F = 169.51 (2, 96); contrast estimate between NTX and placebo—3.720 (95% CI − 4.291 to −3.148); p = 0.001).
FIGURE 1.
Subjective opioid withdrawal scores (SOWS) in 96 opioid-dependent subjects. On the day following detoxification (day seven), VLNTX-treated individuals reported significantly reduced withdrawal (ANCOVA, controlling for score on day six, F = 174.7 (2,94); p = 0.001). The shadowed area indicates inpatient detoxification.
FIGURE 2.
Objective opioid withdrawal scores (OOWS) in 96 opioid-dependent subjects. On the day following detoxification (day seven), VLNTX-treated individuals showed significantly reduced withdrawal (ANCOVA, controlling for scores on day six, F = 169.5 (2,96); p = 0.001). The shadowed area indicates inpatient detoxification.
Patients taking VLNTX reported reduced craving upon treatment discontinuation, after controlling for discharge scores (F = 38.02 (2, 94); contrast estimate between NTX and placebo—0.859 (95% CI −1.320 to −.399); p = 0.001).
There were no significant differences in withdrawal scores or craving after discharge by VLNTX condition, based on post hoc contrast. Results did not differ by study site (data not shown).
Subjects in the VLNTX groups had reported higher consumption of alcohol at admission to detoxification than did those in the placebo group (Table 1). To evaluate whether alcohol intake before detoxification could influence the degree of discomfort following discharge, we repeated the analysis, controlling for alcohol use in the month prior to treatment. Withdrawal scores and craving remained lower among VLNTX-treated subjects in both groups compared to the placebo group (SOWS, F = 29.4 (2,72), p = 0.001; OOWS, F = 17.9 (2,74), p = 0.001; craving, F = 9.9 (2,72), p = 0.002; Table 2).
TABLE 2.
Withdrawal severity and craving at discharge and after 1 day among opioid-dependent treatment completers of a six-day methadone VLNTX-based detoxification
| Measures | Placebo n = 29 |
NTX 0.125 mg n = 33 |
NTX 0.250 mg n = 34 |
F/χ2 |
|---|---|---|---|---|
| SOWS (0–64) | ||||
| Discharge | 21.4 (11.4) | 12.2 (5.8) | 12.5 (5.9) | 16.4* |
| Day one | 20.9 (11.4) | 7.3 (5.8) | 6.4 (6.9) | 174.7* |
| Day one‡ | 19 (11.2) | 8.3 (5.8) | 6.9 (8.9) | 29.4* |
| OOWS (0–13) | ||||
| Discharge | 2.8 (3.4) | 0.9 (1.4) | 0.6 (1.5) | 25.8* |
| Day one | 3.4 (1.9) | 0.6 (0.9) | 0.5 (0.1) | 169.5* |
| Day one‡ | 3 (1.0) | 0.7 (0.8) | 0.7 (1.0) | 17.9* |
| Craving (0–4) | ||||
| Discharge | 2.3 (0.9) | 0.4 (0.5) | 0.5 (0.7) | 41.4* |
| Day one | 2.3 (1.2) | 0.4 (0.7) | 0.3 (0.9) | 38* |
| Day one‡ | 2 (1.0) | 0.5 (0.6) | 0.5 (0.4) | 9.9† |
p = 0.001
p = 0.002
controllingfor alcohol use before detoxification
Abbreviations: VLNTX = very low dose naltrexone, SOWS = Subjective Opiate Withdrawal Scale, OOWS = Objective Opiate Withdrawal Scale
Drug Use
Urine drug test results and alcohol use are summarized in Table 3. Drug use was detected in 41% of the subjects the day following discharge; 27% were using opioids. One week later, the proportion of subjects positive for opioids was 29.5%; 47% of all urine drug tests were positive for drugs. Alcohol, cannabis and cocaine were used before detoxification by 45 to 55% of the subjects (Table 1). On the first day after discharge, use had declined to 28%, 23% and 12.5%, respectively. After one week, alcohol use had increased back to 41% of subjects, while cannabis and cocaine use were present in 36 and <16% of subjects, respectively.
TABLE 3.
Illicit drug use and treatment engagement following discharge among opioid-dependent treatment completers of a 6-day methadone + VLNTX-based detoxification
| % Positive samples | Placebo | NTX 0.125 mg | NTX 0.250 mg | 2# | P |
|---|---|---|---|---|---|
| Day one | n = 29 | n = 33 | n = 34 | ||
| Alcohol | 31 | 24.2 | 24 | 1.2 | 0.55 |
| Amphetamine* | 0.3 | 0.3 | 0.6 | 0.1 | 0.93 |
| Cannabis | 51.7 | 9 | 15 | 42.3 | 0.001 |
| Cocaine | 11.2 | 9 | 11.8 | 0.4 | 0.82 |
| Opioids | 32.2 | 17.1 | 8.8 | 36.1 | 0.001 |
| Any drug use | 68.9 | 27.3 | 29.4 | 26.2 | 0.001 |
| Week one | n = 16 | n = 20 | n = 25 | ||
| Alcohol | 43.7 | 40 | 40 | 0.2 | 0.89 |
| Amphetamine* | 0.6 | 0.5 | 0.8 | 0.1 | 0.96 |
| Cannabis | 62.5 | 25 | 24 | 28.4 | 0.001 |
| Cocaine | 18.7 | 10 | 20 | 3.64 | 0.16 |
| Opioids | 62.5 | 20 | 16 | 40.4 | 0.001 |
| Any drug use | 68.7 | 46 | 48 | 5.2 | 0.06 |
| % Engaged in treatment | 31.3 | 50 | 64 | 11.1 | 0.004 |
Including methamphetamine
df = 2 for all comparisons
VLNTX = very low dose naltrexone
VLNTX addition during detoxification was associated with overall reduced drug use during the 24 hours following discharge (χ2 = 26.2 (2); p = 0.001; Tables 2 and 3). Fewer VLNTX-treated subjects used opioids upon discharge, as confirmed by UDTs at one day and one week (respectively χ2 = 36.1 (2); p = 0.001; χ2 = 40.4 (2); p = 0.001; Tables 2 and 3). Reduced cannabis use was also associated with previous NTX treatment at both time points (one day: χ2 = 42.3 (2); p = 0.001; one week: χ2 = 28.4 (2); p = 0.001; Table 3). The post-detoxification decrease in self-reported drug use was similar to the results of the biological tests (data not shown). There were no significant differences between the study sites (data not shown).
Engagement in Post-Detoxification Treatment
At discharge, 85% of the subjects (102/120) expressed the intention to initiate outpatient treatment. This preference was equally distributed among treatment groups (χ2 = 0.7 (2); p = 0.501). After one week, 41% of the subjects (25/61) were attending a drug-free structured outpatient program. Subjects receiving VLNTX during detoxification had a significantly higher rate of engagement in post-detoxification treatment (χ2 = 11.1 (2); p = 0.004; Table 3), independent of study site (data not shown). No subject received psychopharmacological treatment during the follow-up period.
Detoxification Treatment and Follow-Up
Abstinence from opioids and outpatient treatment initiation at the end of the first week following detoxification completion and discharge were not significantly influenced by demographic and drug use variables (data not shown). In a logistic regression model, treatment augmentation with VLNTX at both doses increased the likelihood of abstaining from opioids and of transfer to outpatient treatment after one week, compared to methadone only (OR: 0.01–0.031, 95% CI 0.007–0.13/0.01–0.09, p = 0.001; OR: 0.09–0.048, 95% CI 0.09–0.33/0.10–0.29, p = 0.02, respectively).
One or two subjects would need to be treated with VLNTX to have one more patient remain abstinent, or engage in outpatient care, compared to the standard treatment (NNT: 1.1, 95% CI, 1–1.5, and 1.7, 95% CI, 1.4–2.3, respectively, with no differences between VLNTX doses). Individuals who received VLNTX had a 67% risk reduction of opioid relapse and a 56% increased chance of entering post-detoxification treatment in the week after discharge.
DISCUSSION
Patients randomized to VLNTX + methadone-assisted detoxification showed attenuated withdrawal, higher abstinence rates, and increased participation in aftercare following discharge from a six-day inpatient detoxification treatment regimen, compared with those who received methadone alone. These responses were not influenced by differences in socio-demographic and drug use characteristics.
Withdrawal distress usually extends beyond the conclusion of inpatient methadone detoxification and it is a frequent cause of early relapse.3 Overall, 41% of subjects in this study used drugs or alcohol in the 24 hours following detoxification completion. This is consistent with the observed early high rates of relapse among patients treated with short-taper inpatient methadone schedules.16,35,36 The degree of alcohol consumption and its increase over time across treatment conditions substantiates the existence of a risk of alcohol use among opioid dependent subjects.37 Our findings also confirm the difficulty of the inpatient-outpatient transition after detoxification, especially when methadone discontinuation is not followed by an adequate inpatient stay to minimize discomfort.14 However, the conclusion of VLNTX-enhanced treatment in our sample was followed by an overall drug use rate < 30% at one day and by opioid relapse rates of 16–20% at one week, which falls in the range of drug use observed in the initial phase of methadone and buprenorphine maintenance treatment.38
Among VLNTX-treated subjects, reduced opioid use was accompanied by lower marijuana use. This is consistent with preclinical and human experimental findings that reinforcing effects of cannabinoids are modified by opioid receptor modulation and can be blocked by lower doses of NTX, warranting further investigation.39,40 The lack of observed VLNTX benefit on alcohol use is consistent with indications that reductions in alcohol consumption may require higher naltrexone doses (around 1mg/kg).41
Early relapse is frequently associated with poor aftercare attendance.42 The rate of engagement in drug-free outpatient treatment of subjects receiving methadone taper in this study is comparable to that reported by previous investigations on initiation of treatment in the community after inpatient detoxification.16,43 However, the early commitment to treatment observed among VLNTX-treated subjects was more similar to the rate of initiation of post-detoxification treatment with buprenorphine and methadone and matched the abstinent rate findings.11 This confirms that effective outpatient treatment referral should be a primary goal of a successful detoxification.12,44
A potential limitation of this study is confounding by socio-demographic or drug use variables that might influence outcome, independent of medication. Such confounding is unlikely, because the three medication groups did not differ significantly in such characteristics, except for recent alcohol use (Table 1). This variable was controlled for statistically using covariate adjustment during data analysis. Another potential confounder is the high attrition rate observed during the study, which could have led to selection bias. Such bias is unlikely because the patients lost to follow-up did not differ significantly in socio-demographic characteristics or drug use history from those who participated in this follow-up study. Of course, it remains possible that other unassessed factors may have contributed to the observed outcome differences. In addition, the exclusion of patients with major psychiatric comorbidity may limit the external validity (generalizability) of the results.
Notwithstanding these caveats, more research is needed on effective detoxification methods that offer sustained results following treatment. The assumption that interventions and responses during detoxification may relate to post-detoxification outcomes is based on the results of studying the timing and sequence of changes in response to treatment.14,16,45,46 Our findings are limited to the phase immediately after detoxification. They are consistent with the hypothesis that VLNTX administration attenuates withdrawal severity and craving through a reduction of opioid tolerance and dependence, which would favor early recovery by preventing lapses into drug use. In particular, the finding that patients who received VLNTX during detoxification were more likely to remain opioid-free after completing detoxification suggests that early specific modifications may support longer term abstinence. For example, treatment with NTX is not easily initiated in the presence of withdrawal discomfort,20 but is facilitated when lower NTX doses are used.36 The transition to NTX treatment may be further improved by administering VLNTX during detoxification. Longer term antagonist use following detoxification is receiving renewed attention, especially now that injectable or implantable slow-release NTX formulations are available to reduce the problem of patient compliance.47
In summary, the addition of VLNTX to opioid agonist detoxification is associated with positive early post-detoxification outcomes. Further studies should test the utility of this method in helping with the induction to longer-term opioid-free treatments, such as opioid antagonist therapy and psychosocial interventions following detoxification.
Acknowledgments
Dr. Ashwin Patkar and Dr. Paolo Mannelli receive support from the following:
AstraZeneca, Bristol-Myers Squibb, Cephalon, Inc., Forest, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Lundbeck, McNeil Consumer and Specialty, Merck, Organon, Orphan Medical, Pfizer, Reckitt Benckiser and Titan.
All other authors have no financial disclosures at this time.
This research was supported by grant DA15469 from the National Institute on Drug Abuse, Bethesda, Md (Dr. Mannelli). Dr. Gorelick is supported by the Intramural Research Program, NIH, National Institute on Drug Abuse, Bethesda, Md.
The authors wish to thank Stephen P. Weinstein for helping with recruitment, Michelle Kay Anderberg and Neena Ajwani for recruitment and data collection. They also thank the personnel at Freedom House, Chapel Hill, NC and Kensington Hospital, Philadelphia, PA and all the individuals who participated in the clinical trial.
Footnotes
Portions of this study were presented at the 161st annual meeting of the American Psychiatric Association, Washington, DC, May 3–8, 2008.
This work was carried out at Duke University, Durham, NC, and Thomas Jefferson University, Philadelphia, PA. ID number of this study in clintrials.gov: NCT00135759.
References
- 1.Mattick R, Hall W. Are detoxification programmes effective? Lancet. 1996;347:97–100. doi: 10.1016/s0140-6736(96)90215-9. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor P. Methods of detoxification and their role in treating patients with opioid dependence. JAMA. 2005;294:961–963. doi: 10.1001/jama.294.8.961. [DOI] [PubMed] [Google Scholar]
- 3.Bradley BP, Phillips G, Green L, Gossop M. Circumstances surrounding the initial lapse to opiate use following detoxification. Br J Psychiatry. 1989;154:354–359. doi: 10.1192/bjp.154.3.354. [DOI] [PubMed] [Google Scholar]
- 4.Unnithan S, Gossop M, Strang J. Factors associated with relapse among opiate addicts in an out-patient detoxification programme. Br J Psychiatry. 1992;161:654–657. doi: 10.1192/bjp.161.5.654. [DOI] [PubMed] [Google Scholar]
- 5.Lowinson JH, Marion I, Joseph H, Langrod J, Salsitz EA, Payte JT. Methadone maintenance. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance abuse: a comprehensive textbook. 4. Baltimore: Lippincott, Williams & Wilkins; 2005. pp. 616–640. [Google Scholar]
- 6.Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in Geneva: follow-up at 1 and 6 months. Drug Alcohol Depend. 2000;58:85–92. doi: 10.1016/s0376-8716(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 7.Gossop M, Green L, Phillips G, Bradley B. What happens to opiate addicts immediately after treatment: a prospective study. Br Med J (Clin Res Ed) 1987;294:1377–1380. doi: 10.1136/bmj.294.6584.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins ED, Kleber HD, Whittington RA, Heitler NE. Anesthesia-assisted vs buprenorphine- or clonidine-assisted heroin detoxification and naltrexone induction: a randomized trial. JAMA. 2005;294:903–913. doi: 10.1001/jama.294.8.903. [DOI] [PubMed] [Google Scholar]
- 9.De Jong CA, Laheij RJ, Krabbe PF. General anaesthesia does not improve outcome in opioid antagonist detoxification treatment: a randomized controlled trial. Addiction. 2005;100:206–215. doi: 10.1111/j.1360-0443.2004.00959.x. [DOI] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration. Treatment Episode and Data Set Highlights-2005. Feb, 2007. [Google Scholar]
- 11.Digiusto E, Lintzeris N, Breen C, et al. Short-term outcomes of five heroin detoxification methods in the Australian NEPOD Project. Addict Behav. 2005;30:443–456. doi: 10.1016/j.addbeh.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Myrick H, Anton R, Kasser CL. Management of intoxication and withdrawal: General principles. In: Graham AW, Schultz TK, Mayo-Smith MF, editors. Principles of addiction medicine. 3. Chevy Chase: American Society of Addiction Medicine; 2003. pp. 611–618. [Google Scholar]
- 13.McLellan AT, Woody GE, Metzger D. Evaluating the effectiveness of addiction treatments: Reasonable expectations, appropriate comparisons. The Milbank Quarterly. 1996;74:51–85. [PubMed] [Google Scholar]
- 14.McCambridge J, Gossop M, Beswick T, et al. In-patient detoxification procedures, treatment retention, and post-treatment opiate use: comparison of lofexidine + naloxone, lofexidine + placebo, and methadone. Drug Alcohol Depend. 2007;88:91–95. doi: 10.1016/j.drugalcdep.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Kornør H, Waal H. From opioid maintenance to abstinence: a literature review. Drug Alcohol Rev. 2005;24:267–274. doi: 10.1080/09595230500170241. [DOI] [PubMed] [Google Scholar]
- 16.Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three-, and six-month outcomes after brief inpatient opioid detoxification. Am J Drug Alcohol Abuse. 2001;27:19–44. doi: 10.1081/ada-100103117. [DOI] [PubMed] [Google Scholar]
- 17.Fagan RW, Mauss AL. Padding the revolving door: an initial assessment of the Uniform Alcoholism and Intoxication Treatment Act in practice. Social Problems. 1978;26:232–247. [Google Scholar]
- 18.O’Toole TP, Strain EC, Wand G, McCaul ME, Barnhart M. Outpatient treatment entry and health care utilization after a combined medical/substance abuse intervention for hospitalized medical patients. J Gen Intern Med. 2002;17:334–340. doi: 10.1046/j.1525-1497.2002.10638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: a ceiling on effectiveness? Am J Drug Alcohol Abuse. 2006;32:503–517. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan MA, Garawi F, Bisaga A, et al. Management of relapse in naltrexone maintenance for heroin dependence. Drug Alcohol Depend. 2007;91:289–292. doi: 10.1016/j.drugalcdep.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gowing L, Ali R, White J. Opioid antagonists under heavy sedation or anaesthesia for opioid withdrawal. Cochrane Database Syst Rev. 2006;19:CD002022. doi: 10.1002/14651858.CD002022.pub2. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor PG, Kosten TR. Rapid and ultrarapid opioid detoxification techniques. JAMA. 1998;279:229–234. doi: 10.1001/jama.279.3.229. [DOI] [PubMed] [Google Scholar]
- 23.Wang HY, Friedman E, Olmstead MC, Burns LH. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–261. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain. 2006;7:937–946. doi: 10.1016/j.jpain.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Mannelli P, Gottheil E, Buonanno A, De Risio S. Use of very low-dose naltrexone during opiate detoxification. J Addict Dis. 2003;22:63–70. doi: 10.1300/J069v22n02_05. [DOI] [PubMed] [Google Scholar]
- 26.Rea F, Bell JR, Young MR, Mattick RP. A randomised, controlled trial of low dose naltrexone for the treatment of opioid dependence. Drug Alcohol Depend. 2004;75:79–88. doi: 10.1016/j.drugalcdep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Mannelli P, Patkar AA, Peindl K, Gorelick DA, Wu LT, Gottheil E. Very low dose naltrexone addition in opioid detoxification: A randomized, controlled trial. Addiction Biology. 2008 Aug 19; doi: 10.1111/j.1369-1600.2008.00119.x. In press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic criteria from DSM-IV-TR/American Psychiatric Association. American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- 29.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 30.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 31.Kanof PD, Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Rubinstein KJ. Clinical characteristics of naloxone-precipitated withdrawal in human opioid-dependent subjects. J Pharmacol Exp Ther. 1992;260:355–363. [PubMed] [Google Scholar]
- 32.McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien C. A new measure of substance abuse treatment. Initial studies of the treatment services review. J Nerv Ment Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 34.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. Br J Psychiatry. 1989;154:348–353. doi: 10.1192/bjp.154.3.348. [DOI] [PubMed] [Google Scholar]
- 36.Mannelli P, Patkar AA, Peindl K, Murray HW, Wu LT, Hubbard K. Effectiveness of low-dose naltrexone in the post-detoxification treatment of opioid dependence. J Clin Psychopharmacol. 2007;27:468–474. doi: 10.1097/jcp.0b013e31814e5e9d. [DOI] [PubMed] [Google Scholar]
- 37.Stenbacka M, Beck O, Leifman A, Romelsjö A, Helander A. Problem drinking in relation to treatment outcome among opiate addicts in methadone maintenance treatment. Drug Alcohol Rev. 2007;26:55–63. doi: 10.1080/09595230601036994. [DOI] [PubMed] [Google Scholar]
- 38.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 39.Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- 40.Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- 41.Modesto-Lowe V, Fritz EM. The opioidergic-alcohol link: implications for treatment. CNS Drugs. 2005;19:693–707. doi: 10.2165/00023210-200519080-00005. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann N, Miller N. Perspectives of effective treatment for alcohol and drug disorders. Psychiatr Clin North Am. 1993;16:127–140. [PubMed] [Google Scholar]
- 43.McCusker J, Goldstein R, Bigelow C, Zorn M. Psychiatric status and HIV risk reduction among residential drug abuse treatment clients. Addiction. 1995;90:1377–1387. doi: 10.1046/j.1360-0443.1995.901013779.x. [DOI] [PubMed] [Google Scholar]
- 44.Smart RG, Finley J, Funston R. The effectiveness of post-detoxication referrals: effects on later detoxication admissions, drunkenness and criminality. Drug Alcohol Depend. 1977;2:149–155. doi: 10.1016/0376-8716(77)90022-9. [DOI] [PubMed] [Google Scholar]
- 45.Strain EC, Stitzer ML, Bigelow GE. Early treatment time course of depressive symptoms in opiate addicts. J Nerv Ment Dis. 1991;179:215–221. doi: 10.1097/00005053-199104000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Tennant FS., Jr Benefits of recurrent, outpatient heroin detoxification. Int J Addict. 1985;20:1685–1691. doi: 10.3109/10826088509047256. [DOI] [PubMed] [Google Scholar]
- 47.Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: novel treatment for opioid dependence. Expert Opin Investig Drugs. 2007;16:1285–1294. doi: 10.1517/13543784.16.8.1285. [DOI] [PubMed] [Google Scholar]