Abstract
The stem cells (SCs) of the corneal epithelium located in the limbal basal layer are the ultimate source to maintain corneal epithelial homeostasis. Like other adult tissue-specfic SCs, self renewal and fate decision of limbal SCs are regulated by a specialized in vivo microenvironment, termed “niche”. Loss of limbal SCs or dysfunction of the limbal niche renders corneas with a unique clinical disease labeled limbal stem cell deficiency (LSCD). Besides transplantation of autologous or allogeneic limbal SCs or amniotic membrane, a new strategy of treating LSCD is to transplant a bio-engineered graft by expanding limbal SCs ex vivo. Herein, we conduct a critical appraisal of six protocols that have successfully been practiced in treating human patients with LSCD, and identify issues whether niche regulation has been disrupted or maintained during isolation and expansion. Consequently, we propose a future direction that may circumvent the potential pitfalls existing in these conventional protocols by preserving the interaction between limbal SCs and their native niche cells during isolation and expansion. Such an approach may one day help realize considerable promise held by adult SCs in treating a number of diseases.
Keywords: Epithelium, ex vivo expansion, limbal stem cell deficiency, limbus, ocular surface, reconstruction, stem cells
INTRODUCTION
Stem cells (SCs) with extensive proliferative potential and the ability to give rise to one or more differentiated cell types are common in early mammalian embryos. By adulthood, such SCs are dispersed and kept in a unique anatomic location of each self-renewing tissue where they continue to perform remarkable and relentless self renewal to replenish the SC population lost during progeny production. Although SCs hold considerable promise for the treatment of a number of diseases, the collection of sufficient numbers of adult tissue-specific SCs and the control of their fate decision are two major obstacles to overcome.
It becomes increasingly clear that self renewal and fate decision of adult SCs are regulated by a specialized in vivo microenvironment, termed “niche” (reviewed in [1,2]). Regulation of SCs in their native niche is conceivably mediated by a subset of neighboring cells (including its progeny), extracellular matrix (ECM), and factors sequestered therein. Therefore, one critical step in overcoming the aforementioned obstacles is to recapitulate the in vivo niche via ex vivo expansion of SCs in an in vitro environment.
Using the human corneal epithelium as a model, we will critically appraise all published protocols used for ex vivo expansion of human limbal epithelial progenitor cells including SCs. By analyzing whether their experimental variables have recapitulated in vivo niche regulation, we will identify several potential pitfalls of each protocol that may diminish the potency of bio-engineered grafts suitable for an FDA-regulated clinical trial. In the end, we will provide a forward-looking view on whether a more effective protocol can be developed by focusing on the issue of niche regulation especially by maintaining the natural close contact between SCs and their in vivo niche cells (NCs) during isolation and subsequent ex vivo expansion.
THE LIMBUS AS A UNIQUE MODEL FOR STUDYING ADULT EPITHELIAL STEM CELLS AND THEIR NICHES
Among all adult epithelial tissues, the model of the corneal epithelium is most unique in having its SCs located at the basal epithelial layer of the limbus (between the cornea and the conjunctiva), while its transient amplifying cells (TACs), i.e., the immediate progeny of SC, are located in both limbal and corneal basal epithelia [3] (also reviewed in [4a]).
When compared to the differentiated corneal epithelium, the SC-containing limbal basal layer is known to have the smallest cell size [4b], positive expression of cytokeratin (CK) 19 [5] and CK15 [6], and a high proliferative potential in different cultures [7–10]. Limbal epithelial progenitor cells are more resistant to the inhibition by tumor-promoting phorbol esters [8], and are devoid of the expression of such cornea-specific differentiation markers as CK 3 [3] and CK12 [11,12] and gap junction-mediated connexin 43 [13]. Importantly, when limbal SCs are identified by label-retaining studies in rabbits [14], not all limbal basal epithelial cells are SCs, suggesting that SCs are intermixed with their transit amplifying cells (TACs) in the limbal basal layer [4a]. The notion that not all limbal basal epithelial cells are SCs is further suggested by heterogeneous expression of vimentin [5,15], p63 [16], especially its ΔNp63α isoform [17], ABCG-2 [18–20], integrin α9 [21,22], and N-cadherin [23] in cross-sections of the limbal basal epithelium. Vimentin-expressing epithelial cells in the limbal region are thought to be a transit of limbal SCs to corneal differentiation [24]. It remains unclear whether any of these proteins can be regarded as bona fide bio-markers for identifying limbal SCs.
The easy access of limbal SCs owing to their unique anatomic location is more advantageous than other epithelial tissues [25] such as the bulge of the epidermis [26,27], the crypt of the intestine [28], and the intraheptic biliary tree of the liver [29]. Anatomically, the limbal epithelium contains melanin pigments [30] and is highly organized to form “limbal palisades of Vogt”, where the epithelial sheet folds to increase the surface area (Fig. 1) [31]. The limbal stroma is highly vascularized and innervated [32], and is a loose connective tissue containing limbal fibroblasts. Serial histological sectioning revealed a unique epithelial crypt-like structure containing smallest basal epithelial cells [33–35], suggesting that genuine limbal SCs might lie “deeper” into the limbal stroma than expected. Furthermore, the limbus has been found to have unique ultrastructural features [36] where unique extracellular matrix components such as laminin γ3, SPARC, and tenascin-C are found [24]. The unique role of human limbal stroma in serving as a SC niche is supported by its control of epithelial plasticity in rabbits [37], and by a recent study showing transdifferentiation of hair follicle SCs into corneal epithelial-like cells [38] when tissue recombinant experiments are performed. It remains unknown how cellular and extracellular components uniquely present in the limbal stroma might constitute the limbal niche, and whether conventional methods based on trypsin/EDTA or Dispase digestion can isolate limbal SCs together with their native NCs (reviewed in [39]).
Fig. (1). The Limbal Palisades of Vogt.
Palisades of Vogt (arrow) are readily recognized in the human limbus (A). Such a unique pigmented structure can be identified on the flat mount preparation of dispase-isolated human limbal epithelial sheets (B, Bar represents 500 µm in A and B). Schematic drawing of the limbal epithelium and the limbal niche shows the cellular components of SC, TAC, PMC (post-mitotic cells), TDC (terminally differentiated cells), M (melanocytes), LC (Langerhan’s cells) and MC (presumed NC) as well as BM (basement membrane), Bo (Bowman’s layer), N (nerves) and BV (blood vessels) (both modified from [39] with permission).
CORNEAL DISEASES WITH LIMBAL STEM CELL DEFICIENCY (LSCD)
Ultimately, limbal epithelial SCs are responsible for the homeostasis of the corneal epithelium, a rapid self-renewing tissue (for reviews see [4a,40–42]). The importance of limbal SCs in achieving this role can be appreciated by knowing what happens when they are deficient. In rabbits, we have reported that total regeneration occurs each time when a large corneal epithelial defect is created in corneas with healthy limbal SCs. However, if limbal SCs are partially [43,44] or totally [45,46] damaged, such wounding results in abnormal corneal wound healing. Limbal deficient corneas manifest conjunctival epithelial ingrowth (i.e., conjunctivalization), vascularization, chronic inflammation, and scarring, which collectively are indicators of limbal (SC) deficiency (LSCD) (reviewed in [4a,40,47]). LSCD can be found in a number of human corneal diseases cytologically defined by evidence of conjunctivalization on the corneal surface [48] (see Table 1).
Table 1.
Human Corneal Diseases Characterized by Limbal Deficiency
I. Destructive Loss of Limbal Stem Cell Population
|
II. Dysfunction of Limbal Stromal Microenvironment
|
Patients inflicted with LSCD often suffer from a severe loss of vision, light sensitivity, and potential bacterial infections and are thus poor candidates for conventional corneal transplantation because only short-lived corneal TACs are transplanted and conjunctivalization may still recur. Furthermore, preexisting corneal vascularization and inflammation in LSCD increase the risk of allograft rejection. As shown in Table 1, these LSCD diseases can further be subdivided into two major categories. Category I diseases are characterized by having a clear extrinsic cause that destroys the limbal SC population. Category II diseases with diverse intrinsic causes do not have such an extrinsic destructive cause but their limbal stromal niche is altered by chronic inflammation. That is why we have speculated that the effective measure in restoring the normal phenotype in corneas with LSCD should include strategies to replenish the missing limbal SCs as well as to restore the healthy state of the limbal stromal niche (reviewed in [4a]).
CORNEAL SURFACE RECONSTRUCTION BY LIMBAL STEM CELL TRANSPLANTATION AND AMNIOTIC MEMBRANE TRANSPLANTATION
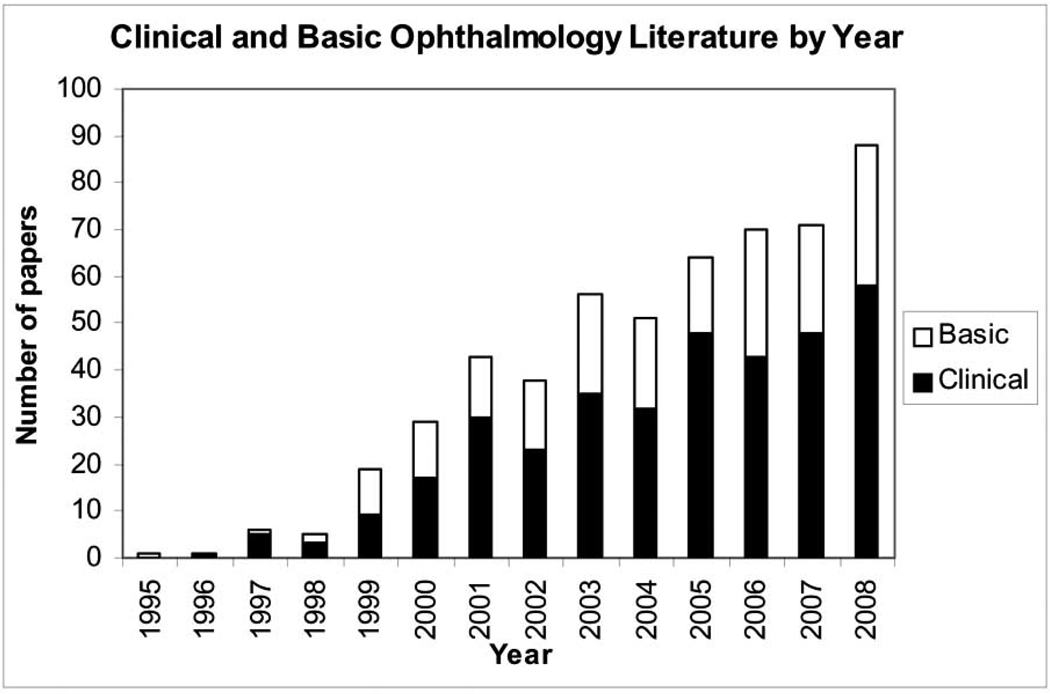
Indeed the first strategy to treat corneas with unilateral LSCD is to transplant autologous limbal SCs from the fellow eye via a surgical procedure termed Conjunctival Limbal Autograft, first introduced by Kenyon and Tseng in 1989 [49]. Subsequently, Tsai and Tseng in 1994 [50] noted that transplantation of allogeneic limbal SCs from cadaveric donors is effective in restoring corneas with bilateral LSCD (for historical development and classification of various transplantations see reviews in [51,52]). The second strategy to treat corneas with LSCD is to transplant amniotic membrane (AM) to restore the damaged limbal stroma, first introduced by Kim and Tseng in 1995 [53]. Since then, the popularity of using amniotic membrane transplantation (AMT) for corneal and conjunctival surface reconstruction has escalated (Fig. 2). A number of clinical studies have shown that the AM-covered ocular surface rapidly heals with reduced inflammation and scarring in the stroma (reviewed in [54–58]). Transplantation of autologous or allogeneic limbal SCs and AMT have been approved by Medicare as standard surgical procedures since January 2004 in the United States of America.
Fig. (2). Clinical and Basic Ophthalmology Literature Regarding to AMT by Year.
Over the past decade, there has been a surge of interest in amniotic membrane transplantation (AMT) for ocular surface reconstruction. PubMed search of papers published in Ophthalmology per year are plotted from the PI’s pioneering paper in 1995 [53]). These studies collectively showed that AMT is effective in facilitating epithelial wound healing and reducing stromal inflammation, scarring and unwanted new blood vessel formation.
During the course of clinical investigation, we and others have learned that AMT alone is sufficient to restore corneas with partial (i.e., less than 360° involvement) LSCD [59–61]. Furthermore, AMT is effective in promoting the success of transplanting autologous [62,63] and allogeneic [64] limbal SCs for treating total LSCD. These clinical data collectively indicate that restoration of the limbal stroma by AM is as important and beneficial as transplanting limbal SCs. They also suggest that AM helps expand residual or transplanted limbal SCs in vivo. We have thus proposed that AM is an ideal substrate to help ex vivo expansion of limbal SCs in culture (reviewed in [65]).
CONVENTIONAL EX VIVO EXPANSION PROTOCOLS OF LIMBAL SCs ON AMNIOTIC MEMBRANE
Because of the concern of removing the limbus from a healthy eye, a new surgical approach was introduced by Pellegrini et al in 1997 [66] to transplant ex vivo expanded human limbal epithelium for treating human patients with LSCD (also reviewed in [67], see Protocol 1, Table 2). Presumably because AMT can help expand human limbal SCs in vivo, several groups have developed a total of five protocols for expanding limbal SCs using AM as a substrate in culture also successfully to treat human patients with LSCD (Protocol 2 to 6, Table 2). Although there are other basic and pre-clinical studies dealing with ex vivo expansion of limbal epithelial progenitor cells, we limited our evaluation only to these six protocols because they all have successfully been used in treating human patients with LSCD.
Table 2.
Ex Vivo Expansion Protocols of Autologous Human Limbal Epithelial Cells Practiced Successfully in Human Patients
| Protocols | 1 Pellegrini 1997 [66] |
2 Schwab 2000 [89] |
3 Tsai 2000 [90] |
4 Nakamura 2004 [91] and 2006 [92] |
5 Sangwan 2003 [93] |
6 Shimazaki 2002 [94] and Kawashima 2007 [95] |
|
|---|---|---|---|---|---|---|---|
| Epithelial Isolation from Limbal Biopsy | Trypsin/EDTA | Trypsin/EDTA | Brief Dispase | Cut into small pieces or Dispase and Trypsin/EDTA | Cut into Small pieces | Cut into Small pieces | |
| 3T3 Coculturing for Pre-amplification | Yes | Yes | No | No | No | No | |
| Substrate | Fibrin | dAM by Trypsin/EDTA | iAM | dAM by EDTA and scraping | dAM by Trypsin/EDTA and scraping | dAM by NH4OH and scraping | |
| Medium | Base Medium | D or K | D/F(1:1) | K | M/F(1:1) | D/F(1:1) | |
| Serum | 10% FBS then serum-free | 5% FBS | 5% FBS or HS | 10% FBS, or HS | 5% FBS or HS | ||
| EGF | + | + | + | NA | + | + | |
| Hc | + | + | + | NA | + | − | |
| ITS | − | + | NA | + | − | ||
| CTX | + | + | NA | + | + | ||
| DMSO | − | + | NA | − | − | ||
| Co-culturing with 3T3 on Plastic | No | No | No | Yes | No | Yes | |
| Air-lifting | No | No | No | Yes | No | Yes | |
| Days to Achieve a Transplantable Size | 14–21 | NA | 14 – 21 | at least 14 | 14 – 21 | 14 | |
[Not]: Abbreviation used: CTX: Cholera Toxin; D: DMEM; D/F: DMEM/F12; dAM: denuded AM; EGF: Epithelial Growth Factor; FBS: fetal bovine serum; Hc: Hydrocortisone; HS: Human Serum; iAM: intact AM; ITS: Insulin Transferrin Selenium; K: KGM; M: MEM.
In short, the new surgical approach starts with a small biopsy performed at the limbal region of a healthy eye. Afterwards, these six protocols differ from one another in a number of areas before engineering an epithelium with a transplantable size. These differences can grossly be categorized according to the following 4 aspects: (1) whether limbal epithelial cells are isolated from the biopsy tissue and/or rendered into single cells, (2) whether murine 3T3 fibroblasts are used as a feeder layer, (3) whether and how AM is prepared and used as a carrier, and (4) whether air-lifting is used to promote epithelial stratification (Table 2). These six protocols use similar media containing serum, EGF and cholera toxin to support growth of limbal epithelial SCs. To meet the regulatory requirements, fetal bovine serum has successfully been interchanged with the patient’s own serum in Protocols 4 to 6. At the present time, no study has systemically compared all manufacturing variables used by these six protocols. Hence it remains unclear which variable is crucial for achieving effective ex vivo expansion of human limbal epithelial SCs. As a result, one may question which protocol can be considered the most optimal one to adopt for an FDA-regulated clinical trial.
Herein, we would like to appraise these six protocols from the viewpoint whether these manufacturing variables disrupt the native limbal niche, and if so what measures have been taken to restore what is lost regarding the niche regulation. We identify the experimental maneuver that might not pass the regulatory requirement. Furthermore, we examine whether some maneuvers may potentially be harmful to limbal epithelial SCs during isolation and expansion based on recent research results.
1. Separation of Limbal Epithelial Sheets from the Limbal Stroma
Although poorly defined, it is plausible that limbal SCs are regulated by their native niche in the normal in vivo environment. Therefore, the first issue one may face is whether it is necessary to separate the limbal epithelium from the underlying limbal stroma in the limbal biopsy specimen. All except for Protocols 5 and 6 separate limbal epithelial sheets from the underlying stroma, and/or render them into single cells by enzymatic digestion. Protocols 1 and 2 use trypsin/EDTA. Protocol 3 subjects the limbal biopsy sample to a brief digestion with Dispase but does not remove the epithelium from the limbal stroma. It has been reported that Dispase digestion results in the isolation of intact and viable human limbal epithelial sheets [68]. Protocol 4 uses Dispase digestion followed by a brief treatment of trypsin/EDTA to yield single cells. Our recent study showed that the proliferative capacity, clonogenicity, and p63-positive progenitors are better preserved in such Dispase-isolated sheets than in single cells obtained by subsequent Trypsin/EDTA treatment [69]. Therefore, human limbal epithelial sheets may lose their proliferative capacity and increase their tendency to differentiate when their intercellular junctions are disrupted by Trypsin/EDTA into single cells. As a result, one may question the validity of rendering epithelial sheets into single cells used in Protocols 1, 2 and 4. Although epithelial sheets isolated by Dispase preserves a higher p63-enriched proliferative capacity [69], it remains unknown whether Dispase removes the entire limbal epithelial SCs, niche cells or both. This concern is raised because limbal SCs lie deeper in the stroma than expected [33–35]. Assuming that limbal SCs are indeed isolated, it is still not clear whether NCs in the stroma are also included during Dispase isolation. Even if we assume Dispase-isolated human limbal epithelial sheets contain both SCs and NCs, it remains unknown whether they can be better used for ex vivo expansion in the future without being rendered into single cells.
Although physical contact between limbal SCs and NCs is not disrupted in Protocol 3, in which a brief Dispase digestion is used, and in Protocols 5 and 6, in which biopsy samples are mechanically minced into smaller pieces without removing the remaining limbal stroma, one major concern for these protocols is whether these limbal SCs opt to migrate out from the limbal explants onto the substrate. We have noted that limbal basal epithelial cells can also undergo intrastromal invasion when epithelial sheets are not separated from the underlying limbal stroma [70]. As a result, the growth potential and clonogenicity of the epithelial progenitor cells on the substrate decline over time [70]. This new finding raises a serious concern in these protocols that there might be a gradual loss of limbal SCs, and justifies a continued pursuit of a better method of separating limbal epithelial SCs from the underlying stroma.
2. Co-culturing with Feeder Layers
In as much as it remains uncertain whether the success of the aforementioned isolation might be hampered by disruption of intercellular interaction/support between SCs and NCs, evidence suggests that restoration of such support is crucial for ex vivo expansion of limbal epithelial SCs. In fact, many types of adult somatic SCs have a limited proliferative capacity when detached from their in vivo niche. To circumvent this problem, one common approach to ex vivo expansion resorts to co-culturing on a feeder layer made primarily of growth-arrest mesenchymal cells as a surrogate niche. For many types of epithelial progenitor cells, ex vivo expansion resorts to co-culturing on β-irradiated or mitomycin C-treated murine 3T3 fibroblast feeder layers, a technique first pioneered by Rheinwald and Green in 1975 [71]. Thus, Protocols 1 and 2 immediately seed isolated single cells on murine mitotic-arrested 3T3 fibroblast feeder layers. Although the exact mechanism remains to be elusive, it is generally believed that epithelial-mesenchymal interaction from the use of feeder layers restores the clonogenicity of epithelial progenitor cells. Therefore, one might suspect that feeder layers function like surrogate NCs in Protocols 1 and 2.
In contrast to the direct contact with 3T3 fibroblast feeder layers used in Protocols 1 and 2, Protocols 4 and 6 seed either single cells or small pieces of limbal explants on epithelially-denuded AM and then co-culture them with 3T3 fibroblasts, which are seeded on the plastic surface without a direct contact with human limbal epithelial cells. Even without a direct contact, co-cultured 3T3 fibroblasts are still effective in delaying epithelial differentiation by limbal epithelial cells seeded on denuded AM [72]. A duplex of 3T3 fibroblast feeder layers has been used to promote CK15-expressing corneal epithelial cells [73]. These results suggest that soluble factors derived from 3T3 fibroblasts might be involved in promoting niche regulation of limbal SCs.
Even if we assume that murine 3T3 fibroblasts may serve as surrogate NCs, its use for engineering surgical graft for human transplantation does post a great regulatory concern. The FDA has issued strict guideline against the use of xenogenic cells for fear of transmitting as yet unknown murine diseases to humans.
Even if single progenitor cells can potentially be “revived” by immediate seeding with 3T3 fibroblast feeder layers, the importance of controlling the time lapsed between isolation of single cells and subsequently co-culturing cannot be overlooked. This concern is particularly relevant when single cells obtained by treatment with trypsin/EDTA are used to enrich SC population by fluorescent-activated cell sorting (FACS). One technique of FACS uses the unique property of effluxing Hoechst 33342 dye [74,75] to isolate the side population (SP) of adult somatic SCs, which preferentially express Bcrp1/ABCG2, a member of ATP-binding cassette transporters (also reviewed in [76]). Using this method, SP cells have been isolated from human [18–20], rat [77], and rabbit [20,78,79] limbal tissues. However, SP cells generate fewer clones than non-SP cells when freshly isolated limbal epithelial cells of both human [20] and rabbit [20,78,79] are cultured on 3T3 fibroblast feeder layers. One may wonder if prolonged separation from the native niche during manipulation by FACS severely hinders the clonogenicity of limbal epithelial progenitor cells.
3. Use of AM as a Substrate
Except for Protocol 1, in which fibrin gel is used as a substrate, all other protocols use either intact AM (iAM in Protocol 3) or epithelially-denuded AM (dAM) following treatment by EDTA (Protocol 4), NH4OH (Protocol 5) or trypsin/EDTA (Protocols 2 and 4). The rationale for using either iAM or dAM is not clearly stated when they were first practiced. We have conducted a series of experiments to compare the efficacy between iAM and dAM, and our engraftment studies in nude mice have disclosed that iAM, but not dAM, preserves a limbal epithelial phenotype after ex vivo expansion [80]. Others have subsequently substantiated the notion that iAM can, but dAM cannot, preserve the status of limbal epithelial progenitor cells regarding the maintenance of slow-cycling properties, the lack of connexin-mediated intercellular junction, and the positive expression of p63 and CK19 [81–83]. These data collectively support the necessity of including 3T3 fibroblasts as a feeder layer when dAM is used as a substrate in Protocols 4 and 6.
To circumvent the regulatory concern of using murine cells as feeder layers, we discovered that feeder layers made of mitomycin C-arrested human amniotic epithelial cells are superior to those made of 3T3 fibroblasts in supporting expansion of human limbal epithelial SCs according to the expression of putative SC markers and the promotion of clonal growth [84]. Intriguingly, limbal epithelia cells supported by feeder layers made of human amniotic epithelial cells also exhibit plasticity in adopting neural differentiation [84]. Recently, human mesenchymal stem cell-derived feeder layers have been found to promote expression of CK3, CK15, p63α, and ABCG2 of cultured human limbal epithelial cells [85]. These results collectively explain why iAM used in Protocol 3 can circumvent the need of using 3T3 fibroblast feeder layers because limbal epithelial cells migrating from the explant are immediately in contact with devitalized human amniotic epithelial cells retained in iAM. Furthermore, if a surrogate feeder layer has to be included for ex vivo expansion of human limbal SCs, it may be substituted by the aforementioned human equivalents in the future.
4. Promotion of Epithelial Stratification by Air-Lifting
If, however, the limbal epithelium is not separated from the underlying stroma as shown in Protocols 3, 5 and 6, another potential drawback is the gradual loss of the limbal SC population because invading limbal basal epithelial cells also undergo epithelial-mesenchymal transition into fibroblasts in the limbal stroma [86,87]. Interestingly, using rabbit limbal explants, we have noted that air-lifting, i.e., exposing epithelial cultures to the air-medium interface, further promotes intrastromal invasion and epithelial mesenchymal invasion by limbal basal epithelial progenitor cells [86]. This experimental maneuver of air-lifting is known to promote epithelial stratification [37,86,88]. However, we recently reported that stratification promoted by air-lifting is coupled with squamous metaplasia in human limbal explants [88], which may reduce the efficacy of being used as a graft. Therefore, it remains to be determined whether airlifting used by Protocols 4 and 6 to promoted epithelial stratification is necessary for engineering limbal epithelial sheets before human transplantation.
EXPANSION PROTOCOL BY PRESERVING PHYSICAL CONTACT BETWEEN LIMBAL SCs AND NCs
The aforementioned appraisal let us conclude that there are potential pitfalls in each of the six protocols currently used for manufacturing human limbal epithelial graft for human transplantation. For Protocols 1, 2 and 4, in which human limbal epithelial sheets are isolated from the limbal stroma and then rendered into single cells, the success of ex vivo expansion relies on the verification that limbal SCs are indeed removed from the limbal stroma, and whether their progenitor status is successfully maintained by surrogate 3T3 fibroblasts. As stated above, meeting FDA requirements is difficult with the use of murine 3T3 fibroblasts. Future studies are needed to see if human equivalent feeder layers based on human amniotic epithelial cells or human mesenchymal stem cells can be a more effective substitute. For Protocols 3, 5 and 6, in which human limbal epithelial cells are left in contact with the underlying limbal stroma, the success of ex vivo expansion relies on the verification that limbal SCs indeed migrate out of the explant, and if so whether the progenitor status of migrating limbal epithelial cells is preserved by devitalized amniotic epithelial cells (Protocol 3) or by 3T3 fibroblast feeder layers (Protocol 6).
Taken together, we believe that the efficacy of the above 6 reported ex vivo expansion protocols can be improved by addressing the issues raised above. We suspect that the native limbal niche environment is more supportive of SC expansion than surrogate feeder layers. Therefore, it is our belief that ex vivo expansion of limbal SCs can further be optimized by developing a protocol focusing on the preservation of the close contact between limbal SCs and their NCs during isolation and expansion. The development of such a protocol relies on the realization that limbal SCs are closely associated with their NCs in the limbal niche, and the success of identifying and isolating NCs. To prove the hypothesis that the close contact between limbal SCs and their native NCs is more supportive than that with surrogated feeder layers, one will first require the successful identification and isolation of limbal NCs. Furthermore, a new protocol can be devised by preserving the close contact between limbal SCs and their native NCs during isolation and expansion. Such a protocol may meet the requirements for initiating an FDA-approved Phase I clinical trial so as to determine the safety and efficacy of this new bio-engineered corneal surface tissue in treating patients inflicted with total LSCD.
PROPRIETARY INTERESTS
SCGT and S-Y Chen, but not others, have filed a patent on the method and clinical uses of ex vivo expansion of epithelial progenitor cells.
ACKNOWLEDGEMENTS
Supported in part by RO1EY06819 and RO1EY019872 (to SCGT) and by P30EY014801 from the National Eye Institute, National Institutes of Health, Bethesda, MD, USA, and in part by a research grant from TissueTech, Inc., Miami, FL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was supported by Grant NHRI-99A1-PDCO-0108117 from the National Health Research Institutes, Taiwan.
REFERENCES
- 1.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 3.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]; (b) Romano AC, Espana EM, Budak MT, Wolosin JM, Tseng SC. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci. 2003;12:5125–5129. doi: 10.1167/iovs.03-0628. [DOI] [PubMed] [Google Scholar]
- 5.Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47:4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- 7.Ebato B, Friend J, Thoft RA. Comparison of central and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1987;28:1450–1456. [PubMed] [Google Scholar]
- 8.Kruse FE, Tseng SC. A tumor promoter-resistant subpopulation of progenitor cells is larger in limbal epithelium than in corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34:2501–2511. [PubMed] [Google Scholar]
- 9.Lindberg K, Brown ME, Chaves HV, Kenyon KR, Rheinwald JG. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993;34:2672–2679. [PubMed] [Google Scholar]
- 10.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen WY, Mui MM, Kao WW, Liu CY, Tseng SC. Conjunctival epithelial cells do not transdifferentiate in organotypic cultures: expression of K12 keratin is restricted to corneal epithelium. Curr Eye Res. 1994;13:765–778. doi: 10.3109/02713689409047012. [DOI] [PubMed] [Google Scholar]
- 12.Liu CY, Zhu G, Converse R, et al. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;269:24627–24636. [PubMed] [Google Scholar]
- 13.Matic M, Petrov IN, Rosenfeld T, Wolosin JM. Alterations in connexin expression and cell communication in healing corneal epithelium. Invest Ophthalmol Vis Sci. 1997;38:600–609. [PubMed] [Google Scholar]
- 14.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 15.Lauweryns B, van den Oord JJ, Missotten L. The transitional zone between limbus and peripheral cornea. An immunohistochemical study. Invest Ophthalmol Vis Sci. 1993;34:1991–1999. [PubMed] [Google Scholar]
- 16.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–9528. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 19.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715–1724. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stepp MA, Zhu L, Sheppard D, Cranfill RL. Localized distribution of alpha 9 integrin in the cornea and changes in expression during corneal epithelial cell differentiation. J Histochem Cytochem. 1995;43:353–362. doi: 10.1177/43.4.7534781. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi R, Yamato M, Sugiyama H, et al. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;25:289–296. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- 24.Schlotzer-Schrehardt U, Dietrich T, Saito K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845–860. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci USA. 1993;90:7391–7395. doi: 10.1073/pnas.90.15.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brittan M, Wright NA. The gastrointestinal stem cell. Cell Prolif. 2004;37:35–53. doi: 10.1111/j.1365-2184.2004.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes S, Vig P, Poulsom R, Thomas H, Alison M. Hepatic stem cells. J Pathol. 2002;197:510–518. doi: 10.1002/path.1163. [DOI] [PubMed] [Google Scholar]
- 30.Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg MF, Bron AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–171. [PMC free article] [PubMed] [Google Scholar]
- 32.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 34.Shanmuganathan VA, Foster T, Kulkarni BB, et al. Morphological characteristics of the limbal epithelial crypt. Br J Ophthalmol. 2007;91:514–519. doi: 10.1136/bjo.2006.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung AM, Schlotzer-Schrehardt U, Kulkarni B, Tint NL, Hopkinson A, Dua HS. Limbal epithelial crypt: a model for corneal epithelial maintenance and novel limbal regional variations. Arch Ophthalmol. 2008;126:665–669. doi: 10.1001/archopht.126.5.665. [DOI] [PubMed] [Google Scholar]
- 36.Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, Daniels JT. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 37.Espana EM, Kawakita T, Romano A, et al. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130–5135. doi: 10.1167/iovs.03-0584. [DOI] [PubMed] [Google Scholar]
- 38.Blazejewska EA, Schlotzer-Schrehardt U, Zenkel M, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–652. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng SCG, Sun TT. Stem cells: ocular surface maintenance. In: Brightbill FS, editor. Corneal Surgery: Theory, Technique, and Tissue. Mosby: St. Louis; 1999. pp. 9–18. [Google Scholar]
- 41.Wolosin JM, Xiong X, Schutte M, Stegman Z, Tieng A. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog Retin Eye Res. 2000;19:223–255. doi: 10.1016/s1350-9462(99)00005-1. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita S, Adachi W, Sotozono C, et al. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20:639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 43.Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990;31:1301–1314. [PubMed] [Google Scholar]
- 44.Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2219–2233. [PubMed] [Google Scholar]
- 45.Kruse FE, Chen JJ, Tsai RJ, Tseng SC. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990;31:1903–1913. [PubMed] [Google Scholar]
- 46.Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:96–105. [PubMed] [Google Scholar]
- 47.Tseng SC. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep. 1996;23:47–58. doi: 10.1007/BF00357072. [DOI] [PubMed] [Google Scholar]
- 48.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 49.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. discussion 722-03. [DOI] [PubMed] [Google Scholar]
- 50.Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Tseng SC, Chen JY, Huang AJW, Kruse FE, Maskin SL, Tsai RJF. Classification of conjunctival surgeries for corneal disease based on stem cell concept. Ophthalmol Clin North Am. 1990;3:595–610. [Google Scholar]
- 52.Holland EJ, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15:549–556. [PubMed] [Google Scholar]
- 53.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–484. [PubMed] [Google Scholar]
- 54.Dua HS, Azuara-Blanco A. Amniotic membrane transplantation. Br J Ophthalmol. 1999;83:748–752. doi: 10.1136/bjo.83.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruse FE, Rohrschneider K, Volcker HE. Transplantation of amniotic membrane for reconstruction of the eye surface. Ophthalmologe. 1998;95:114–119. doi: 10.1007/s003470050247. [DOI] [PubMed] [Google Scholar]
- 56.Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12:269–281. doi: 10.1097/00055735-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Tseng SC. Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep. 2001;21:481–489. doi: 10.1023/a:1017995810755. [DOI] [PubMed] [Google Scholar]
- 58.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–575. doi: 10.1136/bjo.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomes JA, dos Santos MS, Cunha MC, Mascaro VL, Barros Jde N, de Sousa LB. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–473. doi: 10.1016/s0161-6420(02)01888-2. [DOI] [PubMed] [Google Scholar]
- 61.Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008;145:787–794. doi: 10.1016/j.ajo.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003;110:1585–1592. doi: 10.1016/S0161-6420(03)00503-7. [DOI] [PubMed] [Google Scholar]
- 63.Kheirkhah A, Raju VK, Tseng SC. Minimal conjunctival limbal autograft for total limbal stem cell deficiency. Cornea. 2008;27:730–733. doi: 10.1097/QAI.0b013e31815cea8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 65.Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 67.De Luca M, Pellegrini G, Green H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med. 2006;1:45–57. doi: 10.2217/17460751.1.1.45. [DOI] [PubMed] [Google Scholar]
- 68.Espana EM, Romano AC, Kawakita T, Di Pascuale M, Smiddy R, Tseng SC. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281. doi: 10.1167/iovs.03-0089. [DOI] [PubMed] [Google Scholar]
- 69.Kawakita T, Shimmura S, Higa K, et al. Greater growth potential of p63-positive epithelial cell clusters maintained in human limbal epithelial sheets. Invest Ophthalmol Vis Sci. 2009;50:4611–4617. doi: 10.1167/iovs.08-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W, Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48:605–613. doi: 10.1167/iovs.06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 72.Grueterich M, Espana EM, Tseng SC. Modulation of keratin and connexin expression in limbal epithelium expanded on denuded amniotic membrane with and without a 3T3 fibroblast feeder layer. Invest Ophthalmol Vis Sci. 2003;44:4230–4236. doi: 10.1167/iovs.02-0943. [DOI] [PubMed] [Google Scholar]
- 73.Miyashita H, Shimmura S, Higa K, et al. A novel NIH/3T3 duplex feeder system to engineer corneal epithelial sheets with enhanced cytokeratin 15-positive progenitor populations. Tissue Eng Part A. 2008;14:1275–1282. doi: 10.1089/ten.tea.2007.0212. [DOI] [PubMed] [Google Scholar]
- 74.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 76.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 77.Umemoto T, Yamato M, Nishida K, et al. Rat limbal epithelial side population cells exhibit a distinct expression of stem cell markers that are lacking in side population cells from the central cornea. FEBS Lett. 2005;579:6569–6574. doi: 10.1016/j.febslet.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 78.Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24:86–94. doi: 10.1634/stemcells.2005-0064. [DOI] [PubMed] [Google Scholar]
- 79.Park KS, Lim CH, Min BM, et al. The side population cells in the rabbit limbus sensitively increased in response to the central cornea wounding. Invest Ophthalmol Vis Sci. 2006;47:892–900. doi: 10.1167/iovs.05-1006. [DOI] [PubMed] [Google Scholar]
- 80.Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- 81.Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Gap junctional communication in microinjected human limbal and peripheral corneal epithelial cells cultured on intact amniotic membrane. Exp Eye Res. 2003;76:303–314. doi: 10.1016/s0014-4835(02)00314-7. [DOI] [PubMed] [Google Scholar]
- 82.Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Expression of Delta Np63 in response to phorbol ester in human limbal epithelial cells expanded on intact human amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:2959–2965. doi: 10.1167/iovs.02-0776. [DOI] [PubMed] [Google Scholar]
- 83.Baharvand H, Heidari M, Ebrahimi M, Valadbeigi T, Salekdeh GH. Proteomic analysis of epithelium-denuded human amniotic membrane as a limbal stem cell niche. Mol Vis. 2007;13:1711–1721. [PubMed] [Google Scholar]
- 84.Chen YT, Li W, Hayashida Y, et al. Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells. 2007;25:1995–2005. doi: 10.1634/stemcells.2006-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Omoto M, Miyashita H, Shimmura S, et al. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest Ophthalmol Vis Sci. 2009;50:2109–2115. doi: 10.1167/iovs.08-2262. [DOI] [PubMed] [Google Scholar]
- 86.Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol. 2005;167:381–393. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W, Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48:605–613. doi: 10.1167/iovs.06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li W, Hayashida Y, Chen YT, et al. Air exposure induced squamous metaplasia of human limbal epithelium. Invest Ophthalmol Vis Sci. 2008;49:154–162. doi: 10.1167/iovs.07-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Tsai RJF, Li L-M, Chen J-K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Eng J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 91.Nakamura T, Inatomi T, Sotozono C, Koizumi N, Kinoshita S. Successful primary culture and autologous transplantation of corneal limbal epithelial cells from minimal biopsy for unilateral severe ocular surface disease. Acta Ophthalmol Scand. 2004;82:468–471. doi: 10.1111/j.1395-3907.2004.00285.x. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura T, Inatomi T, Sotozono C, Ang LP, Koizumi N, Yokoi N, Kinoshita S. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology. 2006;113:1765–1772. doi: 10.1016/j.ophtha.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 93.Sangwan VS, Vemuganti GK, Iftekhar G, Bansal AK, Rao GN. Use of autologous cultured limbal and conjunctival epithelium in a patient with severe bilateral ocular surface disease induced by acid injury: a case report of unique application. Cornea. 2003;22:478–481. doi: 10.1097/00003226-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 94.Shimazaki J, Aiba M, Goto E, Kato N, Shimmura S, Tsubota K. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290. doi: 10.1016/s0161-6420(02)01089-8. [DOI] [PubMed] [Google Scholar]
- 95.Kawashima M, Kawakita T, Satake Y, Higa K, Shimazaki J. Phenotypic study after cultivated limbal epithelial transplantation for limbal stem cell deficiency. Arch. Ophthalmol. 2007;125:1337–1344. doi: 10.1001/archopht.125.10.1337. [DOI] [PubMed] [Google Scholar]