Abstract
Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis, is an obligate intra-cellular bacterium that survives in neutrophils by delaying apoptosis. The human promyelocytic leukemia cell line HL-60 has been the ultimate choice for culturing Anaplasma in vitro. In this study, we assessed the various events of drug-induced apoptosis in A. phagocytophilum-infected HL-60 cells. Anaplasma infection reduced the cell viability and increased the apoptosis in HL-60 cells and staurosporine or etoposide-induced apoptosis was further exacerbated with Anaplasma infection. Altogether our results suggest that A. phagocytophilum infection is proapoptotic in HL-60 cells unlike in neutrophils where it is antiapoptotic.
Keywords: Anaplasma, Apoptosis, HL-60, Neutrophils, Anaplasmosis
Introduction
Human granulocytic anaplasmosis (HGA) has emerged as one of the most prevalent life-threatening tick-borne zoonoses in North America and Europe (Ismail et al. 2010). The disease is caused by an infection with Anaplasma phagocytophilum, an obligate intracellular gram negative bacterium. HGA is transmitted to humans by the bite of deer tick and western black-legged tick. Clinical manifestations of HGA can range from mild to life-threatening depending on the patient’s age and general health. Onset of anaplasmosis generally begins within a week of a tick bite, and often includes fever, severe headaches, malaise, muscle pains, and chills with other possible symptoms including confusion, hemorrhages, and renal failure. The unique capability of A. phagocytophilum to infect neutrophils has established this bacterium as an interesting choice to study the mechanism of bacterial pathogenesis (Carlyon and Fikrig 2003). A. phagocytophilum, survives within human neutrophils using several strategies, including delaying apoptosis, inhibiting NADPH oxidase activity, and subverting phagolysosome biogenesis to reside in an inclusion that does not fuse with lysosomes (Choi et al. 2005; Rikihisa and Lin 2010; Rikihisa et al. 2010).
The ability of pathogens to inhibit apoptosis in eukaryotic cells is essential to establish a replicative niche inside the host. Neutrophils are the most abundant cells of the innate immune system and they are responsible for effective killing of invading micro-organisms by releasing a cytotoxic and proteolytic cocktail. Neutrophils undergo spontaneous apoptosis which is essential for the timely resolution of inflammation (Borjesson et al. 2005). A. phagocytophilum infection of neutrophils has been shown to inhibit spontaneous and induced apoptosis of neutrophils by several in vitro, ex vivo and in vivo studies (Borjesson et al. 2005; Choi et al. 2005; Ge and Rikihisa 2006; Ge et al. 2005; Scaife et al. 2003). This delay of apoptosis has been attributed to allow sufficient time for the survival and propagation of Anaplasma within neutrophils. The cellular mechanisms behind the inhibition of neutrophil apoptosis by A. phagocytophilum infection involve modulation of multiple apoptotic pathways including the inhibition of loss of mitochondrial membrane potential, Bax translocation to mitochondria and the activation of caspase-3 (Ge and Rikihisa 2006; Ge et al. 2005). The p-38 mitogen-activated protein kinase signaling pathway has been reported to involve in this apoptosis delay process (Choi et al. 2005). Human promyelocytic leukemia cell line HL-60 has been employed as the choice of cells for in vitro culture of Anaplasma (Bayard-Mc Neeley et al. 2004). The HL-60 cells can be induced to neutrophil-like cells in response to a variety of chemical stimuli (Mollinedo et al. 2008). Although many studies have been done on the effect on apoptosis in A. phagocytophilum infected neutrophils, there are very few studies carried out to study the similar role in HL-60 cells. In this study, we examined the effects on various events of apoptotic signaling pathways after A. phagocytophilum infection in HL-60 cells.
Materials and methods
Materials
Routine chemicals were of analytical grade and were purchased from Sigma (St Louis, MO, USA) or Fisher Scientific (Fairlawn, NJ, USA). Ac-DEVD-AMC was from Enzo Life Sciences (Farmingdale, NY, USA).
Cell culture
HL-60 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum. For differentiation, cells were induced with 1.25% DMSO for 4–5 days (Mollinedo et al. 2008). Neutrophils from healthy volunteers were purified from peripheral blood as described previously (Schwartz et al. 2009). Donor consent was obtained from each individual following the protocol approved by the Institutional Review Board for Human Subjects at the University of Iowa. Isolated neutrophils were suspended in sterile endotoxin-free HBSS without divalent cations, counted and diluted to a final density of no greater than 20 × 106/ml and kept on ice until use.
Cultivation and infection of A. phagocytophilum
HL-60 cells were infected with A. phagocytophilum and the level of infection was monitored routinely by Hema-3 staining kit in microscope. The Anaplasma strain used in all of these experiments was the HGE bacterial isolate NCH-1 (Telford et al. 1996). When >90% cells were infected, host cell free A. phagocytophilum was prepared to infect neutrophils and fresh HL-60 cells (Borjesson 2008). Briefly, the highly infected cells were allowed to lyse by passing through 21″ gauge needle syringe for 5 times and the lysate was centrifuged at 1800 rpm for 5 min to pellet the host cells. Thus obtained host cell free bacterium was used immediately for further experiments. For mock-infections, everything was done similar from the uninfected HL-60 cells.
Apoptosis assays
Cytotoxicity assay
Mitochondrial dehydrogenase activity assay was performed to assess the viability of HL-60 cells grown in the presence or absence of Anaplasma as described previously (Mosmann 1983). The method is based on the conversion of a tetrazolium salt 3,4,5-dimethyl thiazol-2,5-diphenyl tetrazolium bromide (MTT) to insoluble formazan by mitochondrial dehydrogenases of living cells. Briefly, 20 μL of MTT (5 mg/ml) was added to the growing cells in culture with the fresh medium and incubated for 4 h. Then formed formazan crystals were dissolved in 100 μL of lysis buffer (20% Sodium dodecyl sulfate in 50% N,N-dimethyl formamide) and another 12 h incubation was allowed to dissolve the formazan. The absorbance was measured at 570 nm using a microplate spectrophotometer (Molecular Devices, CA, USA).
Annexin-V staining
The flow cytometric detection of Annexin –V and propidium iodide staining was done with the Annexin-V-Fluos staining kit as described by the manufacturer (Roche diagnostics, Germany). Briefly, the harvested cells were washed once in phosphate-buffered saline and resuspended the cell pellet in 100 μL of staining solution. After incubation for 10–15 min at room temperature, the analysis was done in accuri c6 flow cytometer (Accuri Cytometers Inc., Ann Arbor, MI, USA).
Caspase activity assays
Cells cultured in T-25 flasks were harvested and washed once with ice-cold phosphate buffered saline. Then, 100 μl of cell lysis buffer (20 mM HEPES–NaOH, pH 7.5, 100 mM NaCl, 0.25% Triton-X, 1 mM EDTA, 1 mM Dithiothreitol, 1 mM phenylmethanesulfonylfluoride, 10 μg/ml leupeptin) was added and incubated on ice for 10 min. After centrifugation at 14000 × g for 10 min, the supernatant was collected and protein concentration was determined. The reaction was carried out in 96-well plates and started by adding equal amounts of proteins (20 μg) in caspase assay buffer (20 mM HEPES–NaOH, pH 7.0, 100 mM NaCl, 1 mM EDTA, 10% glycerol and 10 mM dithiothreitol) with 50 μM of Ac-DEVD-AMC, Ac-IETD-AMC or Ac-LEHD-AMC. The caspase activity was measured at the spectrofluorometer (Molecular Devices, CA, USA) at excitation and emission wavelengths of 380 and 460 nm respectively. All the statistical analysis was done with student’s t test.
Results and discussion
A. phagocytophilum infection reduces cell viability in HL-60 cells
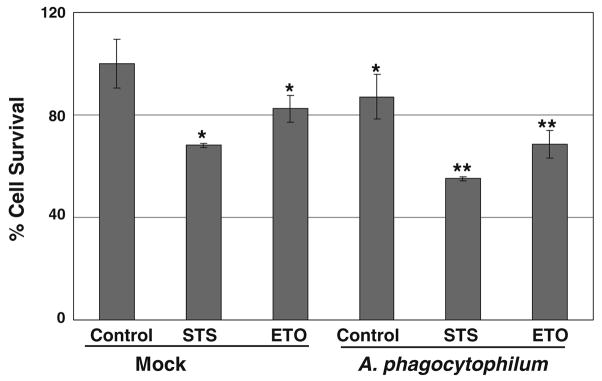
Although HL-60 cells have been the predominant choice for the in vitro cultivation and propagation of Anaplasma, the cytopathic effect associated with the bacterial infection has been an issue that ultimately results in the complete lysis of the host cells (Hsieh et al. 1997). We investigated the spontaneous and drug-induced cell death in uninfected and A. phagocytophilum infected HL-60 cells. There was a significant decrease in cell viability in Anaplasma-infected cells as compared to the mock-infected cells (Fig. 1). Similarly, staurosporine (STS) and etoposide (ETO) induced cell death in HL-60 cells as described previously (Lindsay and Wallace 1999; Matsura et al. 2002) and the cell death was further enhanced in Anaplasma-infected cells (Fig. 1). The differentiated HL-60 cells showed spontaneous death but there was already high reduction in cell viability even in mock-infected cells and Anaplasma infection resulted in further more cell death. So we opted to focus our study on normal HL-60 cells considering the quite unhealthy status of the differentiated cells making them unsuitable for cell death studies by the time of differentiation. However, with this cell viability assay, we were able to confirm that Anaplasma infection indeed enhances both spontaneous and drugs-induced cell death in HL-60 cells.
Fig. 1.
A. phagocytophilum infection reduces cell viability in HL-60 cells. HL-60 cells were mock-infected or infected with freshly isolated host-free A. phagocytophilum for 4 h and MTT assay was done as described in Materials and methods. The cells were also treated with 1 μM staurosporine (STS) and 100 μM etoposide (ETO) for 4 h where indicated. The cell viability is expressed as % of control. *significantly different from mock at P <0.05, **significantly different from mock treated STS and ETO respectively at P <0.05
A. phagocytophilum infection induces apoptosis in HL-60 cells
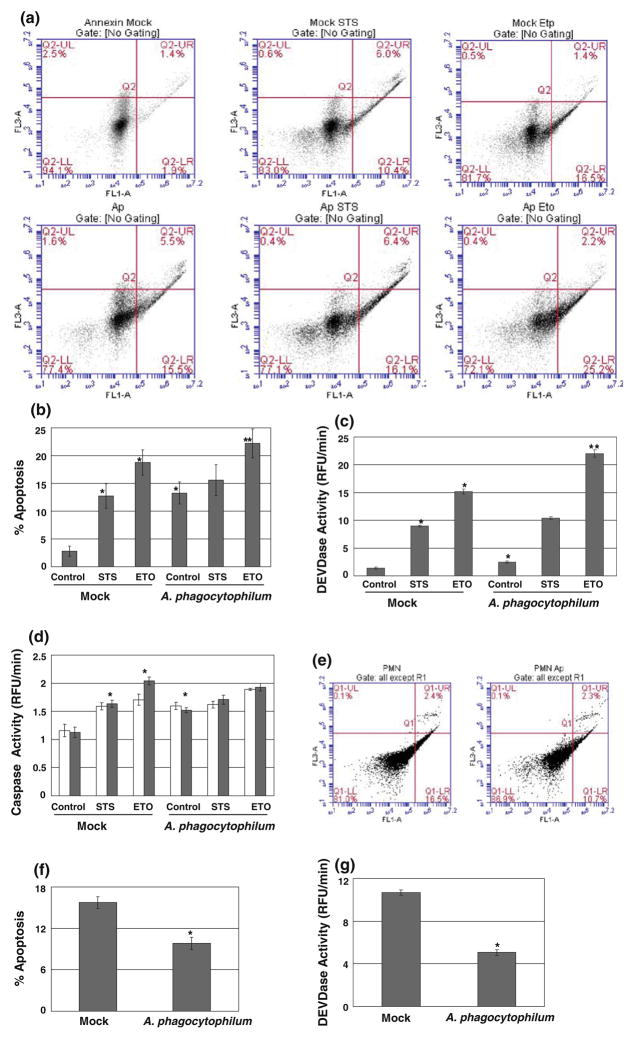
The major question we wished to address in this study was whether Anaplasma infection in HL-60 cells has same anti-apoptotic effect as in neutrophils. Initially, we analyzed the plasma membrane integrity as a marker of early apoptosis by Annexin-V staining. With the Anaplasma infection, there was a huge increase (15.5% vs. 1.9%) in apoptotic cell population as compared to mock-infected cells (Fig. 2a, b). STS also significantly increased the apoptotic population in mock-infected cells but the apoptosis almost maintained in the same level in Anaplasma infected cells after the STS treatment. It could be due to the involvement of the similar signaling pathways with Anaplasma infection as in STS treatment and thus the drug failed to show the additive effect on apoptosis. The role of STS as multi-kinases inhibitor and activation of p38 MAP kinase during Anaplasma infection in neutrophils suggests such possibility of overlapping pathways between two. However, with another drug etoposide, the increment in the apoptotic population was significantly higher in Anaplasma-treated cells as compared to those with mock-infected cells. It clearly suggests that the proapoptotic capability of Anaplasma varies with the apoptogens.
Fig. 2.
A. phagocytophilum induces apoptosis in HL-60 cells but inhibits in neutrophils. HL-60 cells were mock-infected or infected with A. phagocytophilum for 4 h and also treated with 1 μM STS and 100 μM ETO for 4 h, where indicated. Then, the cells were harvested and allowed to Annexin-V staining as described in Materials and methods. Results are represented in quadrants, the lower right quadrant being the apoptotic population (a). The percentage of apoptotic cells are shown in (b). Next, the cells were treated exactly as in (a) and caspase activity assays were performed with 50 μM of Ac-DEVD-AMC (c), Ac-IETD-AMC (d, white bars) or Ac-LEHD-AMC (d, black bars) for caspase-3, caspase-8 or caspase-9-like activity, respectively. The increase in the activity was calculated from the 1 h readings obtained from the spectrofluorometer. *significantly different from mock at P < 0.05, **significantly different from mock treated ETO at P < 0.05. Freshly isolated neutrophils were analyzed for spontaneous apoptosis with Annexin-V staining (e, f) and Caspase-3-like activity (g). The results are expressed as above for both assays. *significantly different from mock at P < 0.01. Error bars represent ± Standard deviation from Mean values in all cases
To further confirm the role of Anaplasma infection in HL-60 cells, we analyzed the caspase-3-like activity as a marker of late apoptosis. Caspase-3 is a key enzyme involved in apoptotic signaling pathway that reflects the endpoint of both mitochondrial and receptor-mediated pathways. Anaplasma infection increased the caspase-3 activity from that of the mock-infected cells (Fig. 2c). STS and etoposide treatments also increased the caspase-3 activity which was further elevated in Anaplasma infected cells. Again, the increase in the caspase activity was not significantly different in Anaplasma-infected cells with or without STS consistent with Annexin-V results. Apoptosis initiating pathways are broadly categorized into two major categories: the extrinsic pathway mediated by the death receptors and the intrinsic or mitochondrial pathway. In the extrinsic pathway, the recruitment of death receptor to its ligand results in the formation of a death-inducing signaling complex that leads to the activation of procaspase-8. In the intrinsic pathway, apoptotic stimuli result in the per-meabilization of the outer mitochondrial membrane releasing the apoptotic factors like cytochrome c which in turn forms the Apaf-1 apoptosome and activates caspase-9. We assayed both the caspase-8 and -9 activities to have an idea which of these pathways are involved in Anaplasma infection. Our results showed that Anaplasma infection involves both of these pathways as evidenced by the increase in the activity of both caspase-8 and caspase-9 in Anaplasma-infected HL-60 cells (Fig. 2d). Considering the fact that bacteria are able to trigger apoptosis by a whole variety of mechanisms, it is not surprising that both the extrinsic and intrinsic pathways were activated by Anaplasma infection, but further studies are required to identify the exact mechanism of initiation of either of these pathways during Anaplasma infection. The effect on both extrinsic and intrinsic apoptotic pathways during the delay of neutrophils apoptosis by Anaplasma also suggests that the bacterium interferes with both of these pathways (Ge and Rikihisa 2006). Finally, to validate and compare our results, we looked at the effect of Anaplasma infection in neutrophils. In complete agreement with earlier studies, we found that Anaplasma infection leads to the inhibition of apoptosis in neutrophils as evidenced by the decrease in apoptotic population and caspase-3 like activity in Anaplasma-infected neutrophils from that of mock-infected neutrophils (Fig. 2d, e, f). From these results we were able to conclude that Anaplasma infection behaves differently in HL-60 cells from neutrophils, the effect being proapoptotic in the former but antiapoptotic in the latter cell types.
Mature neutrophils die rapidly via spontaneous apoptosis in vivo and in vitro (Lee and Goodman 2006; Scaife et al. 2003). The speedy induction of apoptosis in neutrophils is important for the timely resolution of inflammation. A. phagocytophilum is unique in the sense that it survives in neutrophils by inhibiting spontaneous apoptosis although the mechanism behind it is not clear. The interference in the apoptosis signaling pathways after the bacterial infection has been credited for this antiapoptotic phenomenon (Ge and Rikihisa 2006). HL-60 cells have been popularly employed for the in vitro cultivation of Anaplasma, so the effect of the bacterial infection in the viability of these cells has always been a matter of curious concern in this filed. There have been some studies done in the past showing the less or none upregulation of antiapoptotic genes in HL-60 cells as compared to neutrophils suggesting the different effect rather than apoptosis inhibitory of Anaplasma infection in these cells (Lee and Goodman 2006; Lee et al. 2008). However, there are some studies which have shown the increased transcription of antiapoptotic genes including MCL1 and BFL1 during Anaplasma infection in NB4 promyelocytic leukemic cells and HL-60 cells (de la Fuente et al. 2005; Pedra et al. 2005). But a more recent comprehensive study compared the Anaplasma-induced gene expression in human neutrophils and HL-60 cells and found that although the same pathways are affected in both cell types, the infection altered the gene expression much slower and to lesser degree in HL-60 cells as compared to neutrophils (Lee et al. 2008). Our study confirmed that Anaplasma infection induces apoptotic cell death in HL-60 cells and the apoptosis induced by various drugs is further exacerbated with the bacterial infection. Future studies will elaborate the possible mechanism (s) behind this proapoptotic capability of A. phagocytophilum and will also examine whether recently discovered virulent proteins of the bacterium including AnkA, Ats-1 and AptA have some role in the process (JW et al. 2007; Niu et al. 2010; Sukumaran et al. 2010).
Acknowledgments
This study was supported in part by a grant from National Institute of Health (RO1AI076244) to JWI. We would like to thank Mark Ver Meer and Elizabeth L. Kennedy for their helpful discussions.
Contributor Information
Pratap Karki, Email: pratap-karki@uiowa.edu, Inflammation Program and Division of Rheumatology, Department of Internal Medicine, University of Iowa and Veterans Administration Medical Center, 2501 Crosspark Road, MTF E104, Coralville, Iowa City, IA 52241, USA.
Jacob W. IJdo, Inflammation Program and Division of Rheumatology, Department of Internal Medicine, University of Iowa and Veterans Administration Medical Center, 2501 Crosspark Road, MTF E104, Coralville, Iowa City, IA 52241, USA
References
- Bayard-Mc Neeley M, Bansal A, Chowdhury I, Girao G, Small CB, Seiter K, Nelson J, Liveris D, Schwartz I, Mc Neeley DF, Wormser GP, Aguero-Rosenfeld ME. In vivo and in vitro studies on Anaplasma phagocytophilum infection of the myeloid cells of a patient with chronic myelogenous leukaemia and human granulocytic ehrlichiosis. J Clin Pathol. 2004;57(5):499–503. doi: 10.1136/jcp.2003.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjesson DL. Culture, isolation, and labeling of Anaplasma phagocytophilum for subsequent infection of human neutrophils. Methods Mol Biol. 2008;431:159–171. doi: 10.1007/978-1-60327-032-8_13. [DOI] [PubMed] [Google Scholar]
- Borjesson DL, Kobayashi SD, Whitney AR, Voyich JM, Argue CM, Deleo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. 2005;174(10):6364–6372. doi: 10.4049/jimmunol.174.10.6364. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Fikrig E. Invasion and survival strategies of Anaplasma phagocytophilum. Cell Microbiol. 2003;5(11):743–754. doi: 10.1046/j.1462-5822.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- Choi KS, Park JT, Dumler JS. Anaplasma phagocytophilum delay of neutrophil apoptosis through the p38 mitogen-activated protein kinase signal pathway. Infect Immun. 2005;73(12):8209–8218. doi: 10.1128/IAI.73.12.8209-8218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J, Ayoubi P, Blouin EF, Almazan C, Naranjo V, Kocan KM. Gene expression profiling of human promyelocytic cells in response to infection with Anaplasma phagocytophilum. Cell Microbiol. 2005;7(4):549–559. doi: 10.1111/j.1462-5822.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- Ge Y, Rikihisa Y. Anaplasma phagocytophilum delays spontaneous human neutrophil apoptosis by modulation of multiple apoptotic pathways. Cell Microbiol. 2006;8(9):1406–1416. doi: 10.1111/j.1462-5822.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- Ge Y, Yoshiie K, Kuribayashi F, Lin M, Rikihisa Y. Anaplasma phagocytophilum inhibits human neutrophil apoptosis via upregulation of bfl-1, maintenance of mitochondrial membrane potential and prevention of caspase 3 activation. Cell Microbiol. 2005;7(1):29–38. doi: 10.1111/j.1462-5822.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Aguero-Rosenfeld ME, Wu JM, Ng C, Papanikolaou NA, Varde SA, Schwartz I, Pizzolo JG, Melamed M, Horowitz HW, Nadelman RB, Wormser GP. Cellular changes and induction of apoptosis in human promyelocytic HL-60 cells infected with the agent of human granulocytic ehrlichiosis (HGE) Biochem Biophys Res Commun. 1997;232(2):298–303. doi: 10.1006/bbrc.1997.6276. [DOI] [PubMed] [Google Scholar]
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30(1):261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JWIJ, Carlson AC, Kennedy EL. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell Microbiol. 2007;9(5):1284–1296. doi: 10.1111/j.1462-5822.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Lee HC, Goodman JL. Anaplasma phagocytophilum causes global induction of antiapoptosis in human neutrophils. Genomics. 2006;88(4):496–503. doi: 10.1016/j.ygeno.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Lee HC, Kioi M, Han J, Puri RK, Goodman JL. Anaplasma phagocytophilum-induced gene expression in both human neutrophils and HL-60 cells. Genomics. 2008;92(3):144–151. doi: 10.1016/j.ygeno.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Lindsay GS, Wallace HM. Changes in polyamine catabolism in HL-60 human promyelogenous leukaemic cells in response to etoposide-induced apoptosis. Biochem J. 1999;337(Pt 1):83–87. [PMC free article] [PubMed] [Google Scholar]
- Matsura T, Serinkan BF, Jiang J, Kagan VE. Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett. 2002;524(1–3):25–30. doi: 10.1016/s0014-5793(02)02990-3. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Lopez-Perez R, Gajate C. Differential gene expression patterns coupled to commitment and acquisition of phenotypic hallmarks during neutrophil differentiation of human leukaemia HL-60 cells. Gene. 2008;419(1–2):16–26. doi: 10.1016/j.gene.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Niu H, Kozjak-Pavlovic V, Rudel T, Rikihisa Y. Anaplasma phagocytophilum Ats-1 is imported into host cell mitochondria and interferes with apoptosis induction. PLoS Pathog. 2010;6(2):e1000774. doi: 10.1371/journal.ppat.1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedra JH, Sukumaran B, Carlyon JA, Berliner N, Fikrig E. Modulation of NB4 promyelocytic leukemic cell machinery by Anaplasma phagocytophilum. Genomics. 2005;86(3):365–377. doi: 10.1016/j.ygeno.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol. 2010;13(1):59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Lin M, Niu H. Type IV secretion in the obligatory intracellular bacterium Anaplasma phagocytophilum. Cell Microbiol. 2010;12(9):1213–1221. doi: 10.1111/j.1462-5822.2010.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife H, Woldehiwet Z, Hart CA, Edwards SW. Anaplasma phagocytophilum reduces neutrophil apoptosis in vivo. Infect Immun. 2003;71(4):1995–2001. doi: 10.1128/IAI.71.4.1995-2001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Leidal KG, Femling JK, Weiss JP, Nauseef WM. Neutrophil bleaching of GFP-expressing staphylococci: probing the intraphagosomal fate of individual bacteria. J Immunol. 2009;183(4):2632–2641. doi: 10.4049/jimmunol.0804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran B, Mastronunzio JE, Narasimhan S, Fankhauser S, Uchil PD, Levy R, Graham M, Colpitts TM, Lesser CF, Fikrig E. Anaplasma phagocytophilum AptA modulates Erk1/2 signalling. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93(12):6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]