Abstract
In modern medicine, vigorous efforts are being made in the prediction and prevention of diseases. Mental disorders are suitable candidates for the application of this program. The currently known neurobiological and psychosocial risk indicators for schizophrenia do not have a predictive power sufficient for selective prevention in asymptomatic patients at risk. However, once predictive basic and later pre-psychotic high risk symptoms of psychosis develop into the five-year initial prodrome, the impending outbreak of the disease can be predicted with high accuracy. Research findings suggest a differential strategy of indicated prevention with cognitive behavioral therapy in early initial prodromal states and low dosage atypical antipsychotics in late initial prodromal states. The most important future tasks are the improvement of the predictive power by risk enrichment and stratification, as well as the confirmation of the existing and the development of new prevention strategies, with a stronger focus on the etiology of the disorder. In addition, the prediction and prevention approach would benefit from the inclusion of risk symptoms in the DSM-5 criteria.
Keywords: Schizophrenia, risk factors, early course, basic symptoms, high risk symptoms, risk staging, differentiated prevention
Since the traditional clinical paradigm has been replaced by the modern molecular one, medicine set off into new directions. “Prediction”, “prevention” and “personalization” are the programmatic key words of this new approach. Like other medical disciplines, psychiatry has broadened its focus from diagnosis and treatment to the detection and estimation of the risk of disease development, the prediction of its onset and strategies to avoid its manifestation 1,2,3,4.
Although treatment of schizophrenia has greatly advanced over the last decades, a significant number of patients continue to take an unfavorable chronic course 5,6. This makes schizophrenia the leading cause for permanent occupational disability among people under 40 years of age in Germany 7, and the 8th most common cause for disability adjusted life years (DALYs) lost among the 15 to 34-year olds worldwide 8, despite its low prevalence. Moreover, schizophrenia involves tremendous direct and indirect societal costs 9 and a huge burden on patients and their families 8,10.
It is becoming increasingly clear that schizophrenia is a complex disorder with polygenic heredity and that its pathogenesis is greatly influenced by interactions between different genes and between genes and environment. Associations to variants of the genes for dysbindin and neuregulin-1, the genetic locus G72 and the DAOA (D-amino acid oxidase activator) gene have now been repeatedly confirmed. As with all other complex diseases, research is focusing now on characterizing the polygenetic predisposition and clarifying its influence on the development of the phenotype 11. Research methods range from molecular genetics via proteome research to cell biology, neurophysiology, brain structural and functional imaging and neuropsychology. With all these methods, several indicators for an increased risk of schizophrenia have been identified. However, the currently recognized neurobiological risk factors are not sufficiently predictive to allow the development and application of “selective” prevention measures targeting asymptomatic persons at risk. For neuro-psychological risk factors, this has just become evident in the large-scale attempt of the North American Prodrome Longitudinal Study (NAPLS) group to improve their multivariate model by integrating the examined neurocognitive variables 12.
There are also established environmental risk factors for schizophrenia, such as pregnancy or birth complications, growing up in a large city, IQ low but normal and drug consumption. However, with odds ratios around 2, each of these factors appears to increase the lifetime risk of the disease only slightly 13. Thus, the currently known risk factors, either alone or taken together, cannot be used for prediction and prevention without knowledge of the complete predispositional basis and the gene-gene and gene-environment interactions, which are probably numerous.
In view of this situation, it may be argued that the current efforts towards prediction and prevention are still premature and that further progress of etiological research is needed. However, a different perspective has emerged from the work of the centers for early recognition and prevention, established first in Melbourne, Australia and in Cologne, Germany in the mid 1990s, and later on in many other places around the world. This resulted from retrospective research of the early course of psychosis, in which the pathophysiologically active disturbances in brain development extend beyond early abnormalities in behavior into psychopathologically definable early risk and ultra high risk (UHR) symptoms, depending on the individual combination of stressors and resilience factors. First episode psychosis (FEP) research has shown that the outbreak of the disease is preceded in about 70% to nearly 100% of cases by an initial prodrome, which lasts for an average of five to six years. Even in highly developed health care systems, an average of one year thereafter elapses from the first manifestation of psychotic positive symptoms to the initiation of adequate treatment 14,15.
The period over which the FEP remains untreated (duration of untreated psychosis, DUP) correlates with: delayed and incomplete remission of the symptoms; necessity of more protracted treatment and greater risk of relapse; lower compliance, greater burden on the family, and a higher level of “expressed emotion”; increased risk of depression and suicide; greater impact on the individual’s employment or education; increased drug abuse and delinquent behavior; markedly increased costs of treatment 16.
These correlations have recently been confirmed by a meta-analysis 17, with coefficients ranging from 0.285 to 0.434 (95% CI). This does not only provide strong arguments in favor of treating the FEP as early as possible, but has also led to a systematic effort to decrease the incidence of psychosis through indicated prevention.
PREDICTION OF SCHIZOPHRENIA USING BASIC SYMPTOM CRITERIA
Two important studies concerning the early stage prior to the conversion to FEP have demonstrated that the earliest and most common symptoms, which generally dominate during the prodrome, are unspecific and cannot be distinguished from impairment in mood, drive, contact, and concentration of depressive episodes. These are the Age-Beginning-Course (ABC) study of schizophrenia, a retrospective study with optimized methods 14, and the Cologne Early Recognition (CER) Study, a long-term prospective study with an average follow-up period just below 10 years 18. These studies also found striking cognitive impairments in the form of self-experienced disturbances in thought, speech, and perception processes. This subgroup of so-called basic symptoms, which were found in more than a quarter of patients, had high specificity and a high positive predictive power, accompanied by only low rates of false positive predictions 19,20,21.
Basic symptoms were first operationalized in the Bonn Scale for the Assessment of Basic Symptoms (BSABS). Shorter versions of the scale for adults and for children and adolescents – the Schizophrenia Proneness Instrument, Adult version (SPI-A) and the Schizophrenia Proneness Instrument, Child and Youth version (SPI-CY) – were later developed from dimensional analyses 22,23,24. While the BSABS only allows an assessment of the current state, the SPI-A and the SPI-CY also allow severity ratings according to the maximum frequency of occurrence within the past 3 months.
In the CER study, 385 patients who were presumably in the prodromal phase of schizophrenia were followed up for an average of 9.6 (±7.6) years past baseline. Twenty percent of the initial criterion-positive cases (1 of 66 basic symptoms) who agreed to be followed up developed schizophrenia after 12 months, a further 17% after 24 months, a further 13% after 36 months, and finally a total of 70% after an average of 4.5 years. Thus, only 30% did not convert to schizophrenia. The overall presence/absence of at least one basic symptom correctly predicted presence/absence of a subsequent transition to schizophrenia in 78.1% of cases. From further analyses, two partially overlapping basic symptom criteria for defining at risk mental states (ARMS) for psychosis, primarily schizophrenia, were developed (Table 1).
| Criterion | Predictive accuracy |
| Cognitive-perceptive basic symptoms (COPER) | sensitivity = .87 |
| At least any 1 of the following 10 basic symptoms with a | specificity = .54 |
| SPI-A/SPI-CY score of ≥3 within the last 3 months and first | positive predictive value = .65 |
| occurrence ≥12 months ago: thought interference; thought | negative predictive value = .82 |
| perseveration; thought pressure; thought blockages; disturbance of | positive likelihood ratio = 1.89 |
| receptive speech; decreased ability to discriminate between ideas and | negative likelihood ratio = .24 |
| perception, fantasy and true memories; unstable ideas of reference; | odds ratio = 7.86 |
| derealization; visual perception disturbances (excluding blurred vision | false positives = 23.1% |
| and hypersensitivity to light); acoustic perception disturbances | false negatives = 6.3% |
| (excluding hypersensitivity to sounds/noises) | |
| Cognitive disturbances (COGDIS) | sensitivity = .67 |
| At least any 2 of the following 9 basic symptoms with a SPI-A/SPI-CY | specificity = .83 |
| score of ≥ 3 within the last 3 months: inability to divide attention; thought | positive predictive value = .79 |
| interference; thought pressure; thought blockages; disturbance of | negative predictive value = .72 |
| receptive speech; disturbance of expressive speech; unstable ideas of | positive likelihood ratio = 3.94 |
| reference; disturbances of abstract thinking; captivation of attention by | negative likelihood ratio = .40 |
| details of the visual field | odds ratio = 9.91 |
| false positives = 8.8% | |
| false negatives = 16.3% |
The first criterion, which consists of ten cognitive-perceptive basic symptoms and is abbreviated as COPER, was based on findings concerning the predictive accuracy of individual basic symptoms 18,25. The second was based on a methodological re-analysis of the same data set, in which a cluster of nine cognitive basic symptoms had repeatedly been selected as the most predictive. This cluster was called “cognitive disturbances” (COGDIS). In terms of general predictive accuracy, the two criteria slightly differed in the CER study, as COGDIS tended to be more conservative than COPER, i.e. to perform better in ruling in subsequent schizophrenia at the cost of performing worse in ruling it out. The transition rate throughout the average follow-up period of roughly 10 years was 65% for COPER and 79% for COGDIS, with the majority of transitions occurring within the first 3 years past baseline.
In a second prospective study 26, conducted with the SPI-A and with a systematic follow-up of 24 months, 38% of the initially included 146 at-risk subjects developed a frank psychosis, mainly schizophrenia, within 12.3 (±10.4) months on average (1-48; median=9) according to COPER. Thus, the positive results of the CER study were confirmed. Again, COGDIS appeared to be more specific but less sensitive than COPER.
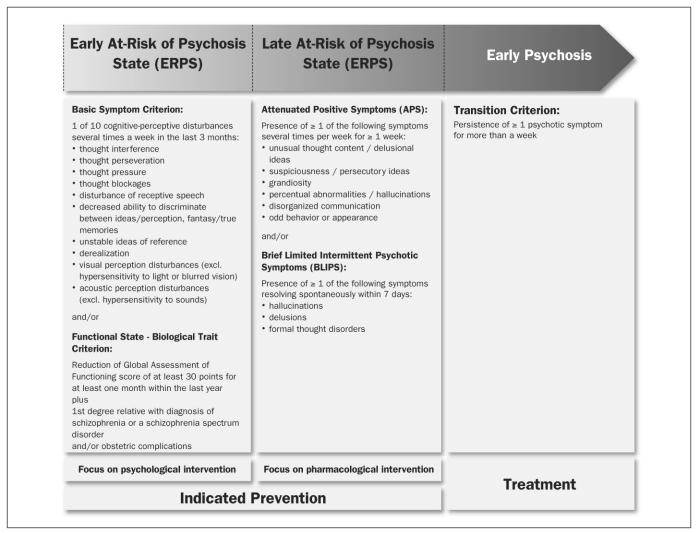
As a consequence of these findings, predictive basic symptoms have been established as a set of criteria for risk assessment in international research on the early recognition of psychosis. In particular, the German Research Network on Schizophrenia used these symptoms, together with a combined criterion of functional deterioration and biological risk, in defining an “early at-risk of psychosis state” (ERPS), thereby suggesting a clinical risk staging model (Figure 1).
Figure 1 Early and late initial prodromal state: a clinical staging approach.
Table 1.
Table 1 Definitions of a mental state at risk for psychosis based on basic symptoms and their predictive accuracy in the Cologne Early Recognition (CER) study
PREDICTION OF SCHIZOPHRENIA USING ULTRA-HIGH RISK CRITERIA
The positive symptoms typical of schizophrenia – such as delusions, hallucinations or formal thought disorders – often first appear in an attenuated or transient form during the initial prodromal phase. These symptoms provide a valid prediction of conversion into FEP, particularly in the short term. Warning signs of this sort have been used as ultra-high risk (UHR) criteria 27,28. Notwithstanding their differences across studies, these criteria are generally composed of three alternative elements: attenuated positive symptoms (APS), brief limited intermittent psychotic symptoms (BLIPS), or a combination of one or more risk factors (always including genetic risk) and functional decline within a certain recent period.
For the ascertainment of the UHR criteria, the Melbourne group gradually developed a specific instrument, the Comprehensive Assessment of At Risk Mental States (CAARMS) 29. Based on the Australian definition of the UHR criteria, the Structured Interview for Prodromal Syndromes (SIPS), the Scale for Prodromal Syndromes (SOPS) and, subsequently, the Criteria of Prodromal Syndromes (COPS) were developed 30,31. Different UHR-related approaches to an early detection of FEP, particularly schizophrenia, were developed by the Hillside Recognition and Prevention (RAP) program in New York 32 and the Basel Früherkennung von Psychosen (FEPSY) study 33.
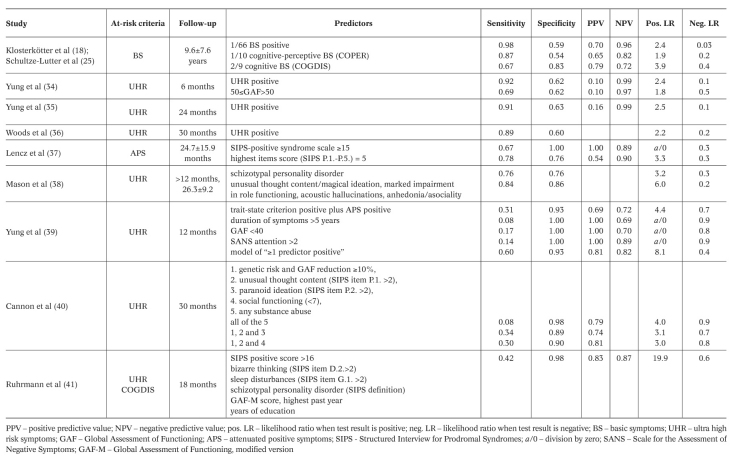
There have been at least 15 prediction studies using UHR criteria, some of which with large samples 34,35,36,37,38,39,40,41. The 12-month rates of transition into FEP published so far range between approximately 13% and 50%. A substantial variance is even observed with comparable observation periods in the same center 34,35. Yet, as the annual incidence for all forms of psychosis in the general population is only about 0.034% 42, even the lowest conversion rates still indicate a dramatic increase in the relative risk of illness, at least in the help-seeking samples of specialized centers. Table 2 depicts the predictive accuracy measures published so far, with the last five listed studies representing secondary predictor analyses of samples meeting at risk criteria. As a result, in the German Research Network on Schizophrenia, the UHR approach was combined with the basic symptom approach and applied in a slightly modified form for the definition of “late at-risk of psychosis state” (LRPS) (Figure 1). This clinical staging model, which suggests a syndromal sequence for the development of FEP progressing from unspecific prodromal symptoms to predictive basic symptoms, and then to APS, to BLIPS and to full-blown psychotic symptoms, was recently strongly supported 15.
Table 2 Prognostic accuracy of different predictors of psychosis.
PREVENTION OF SCHIZOPHRENIA WITH A DIFFERENTIATED PREVENTION STRATEGY
Universal or selective prevention measures target healthy population groups or clinically still healthy risk carriers, respectively 43. Indicated prevention, instead, targets individuals with basic symptoms and UHR symptoms. Even at the early stages when these individuals seek advice and help at the early recognition and prevention centers, they must be regarded as ill and in need of treatment. Furthermore, the impending deterioration of psychosocial performance in schizophrenia often already occurs in the initial prodromal phase, even prior to the conversion into FEP 14,15. These clinical and psychosocial impairments justify defining the interventions in EPRS and LPRS as indicated prevention, pursuing the following three objectives: a) improvement in the current burden of prodromal symptoms; b) avoidance or perhaps delay in the development of psychosocial handicap; c) prevention of or at least delay or attenuation of psychosis.
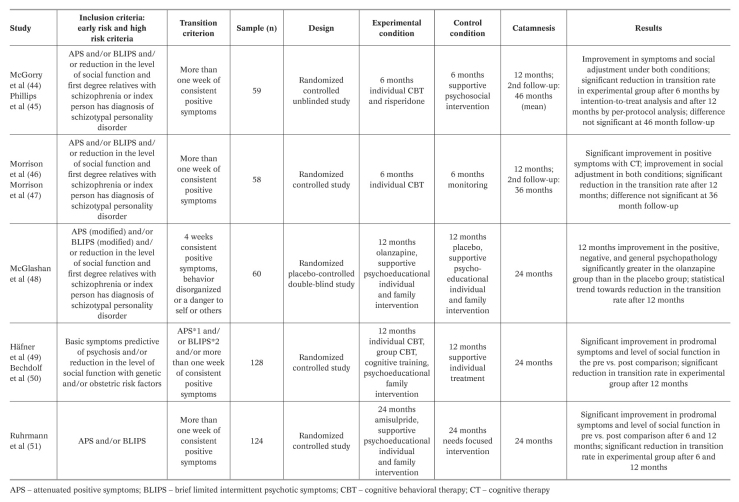
Five international intervention studies have attempted to find out whether or to what extent these three objectives can be reached 44,45,46,47,48,49,50,51 (Table 3). The preventive measures used were either cognitive behavioral therapy (CBT), adapted to the requirements of the persons at risk, or atypical antipsychotics (risperidone, olanzapine, and amisulpride). These were randomized controlled studies, but there were problems with the blinding condition in the two CBT interventions. This and other methodological shortcomings currently limit conclusions and have encouraged the research groups working in this area to set up new, optimized intervention studies. For example, the protocol of the ongoing parallel group PREVENT study includes careful comparative analyses and superiority and inferiority tests of the psychological and pharmacological treatments 52.
Table 3 Prospective, randomized, controlled prevention studies in persons with increased risk of psychosis.
A staging of risk, thereby implying a temporal dimension, was considered for the first time in the two intervention studies of the German Research Network on Schizophrenia. One of these studies covered ERPS and only offered CBT as a preventive measure 49,50. The other study was designed for LRPS and used only preventive treatment with amisul-pride 51. When the symptom development in the initial prodromal state follows the sequence shown in Figure 1, it would be beneficial for scientific and especially ethical reasons to focus on psychological interventions in ERPS, which are well tolerated and highly accepted. As soon as the first attenuated or transient psychotic symptoms occur, it seems justifiable to apply well tolerated antipsychotics with few side effects. This differential prevention strategy is now pursued in all German early recognition centers and is also increasingly gaining support in other countries.
Another pharmacological option is aripiprazole, tested in a pilot study in UHR states 53. Its possible preventive effects are currently being analyzed in the PREVENT study. Antidepressants were used in a naturalistic, non-randomized observational study of an adolescent sample employing only the APS criterion for inclusion, but, for methodological reasons, this study does not allow any conclusion about differential preventive effects of these medications 54.
FUTURE TASKS
A critical evaluation of the achievements over the past 15 years through continuous efforts to enhance prediction and prevention of psychoses, particularly of schizophrenia, reveals quite impressive results. However, the results achieved thus far need to be evaluated in the light of the ambitious, initially mentioned objectives of modern predictive and preventive medicine. Once predictive basic symptoms and UHR symptoms have occurred, the underlying pathophysiological process might have already progressed. For such a complex disease with a long-term course and a pre-dispositional basis, this kind of risk identification and risk-oriented prevention may possibly come too late. A more substantial reduction in incidence could be reached with selective and universal prevention measures. Therefore, symptom-based prediction and prevention need to be further developed into the direction of selective prevention for symptom-free risk carriers. In the future, it is necessary to strive for: a) an improvement of risk enrichment with the inclusion of biological risk factors; b) a stronger individualization of the risk estimation by stratification; c) the inclusion of sub-psychotic mental states, as cross-sectionally defined by current at risk criteria, in the diagnostic systems; d) the application of prevention strategies more closely associated with the etiology of the disease.
Risk enrichment
If the initial prodromal phase persists for as much as 5 years, then most of the follow-up periods shown in Table 2 are not sufficient to acquire the true transition rates. A significant number of later converters may be classified as non-converters and, thereby, the predictive power of the risk syndromes may be systematically underestimated 12. Therefore, the first and most important future task is to carry out new, methodologically optimized large-scale studies with long follow-up periods spanning the full duration of the initial prodromal phase, as in the CER study 18.
The risk enrichment can also be advanced through the inclusion of biomarkers, following the example of recent research on the prediction of Alzheimer’s dementia through the mild cognitive impairment (MCI) syndrome 55. This condition indicates a risk for Alzheimer’s dementia with a conversion rate comparable to the risk syndrome for FEPS. If, however, the MCI patients simultaneously show certain imaging and biochemical markers, the predictive power increases significantly. Such risk enrichment may be possible for FEPS using brain morphological changes, but also impairments of processing speed and verbal memory, which are associated with the psychosis risk syndrome, and are more frequent and severe in those cases with a later transition to schizophrenia and other psychoses 12,56,57,58,59,60. Only new large-scale studies with sufficiently long observation periods could clarify whether the risk enrichment can be achieved by means of such biomarkers. The success of this strategy is dependent on the progress of research on biological and environmental risk factors and their interactions, as is currently attempted in the European Network of national schizophrenia networks studying Gene-Environment Interactions (EU-GEI) study 61.
Risk stratification
In other medical disciplines, such as oncology or pneumology, a well-established risk modeling procedure, which does not result in a loss of sensitivity, is using prognostic indices (PI) for multivariate clinical staging by risk stratification. In the European Prediction of Psychosis (EPOS) study, this approach was introduced into psychosis prediction research for the first time 41. A clinical model was developed based on a Cox regression equation including six variables (SIPS positive score, SIPS bizarre thinking score, SIPS sleep disturbances score, SIPS schizotypal personality disorder, highest Global Assessment of Functioning score in the past year, and years of education). Based on the individual regression scores, a multivariate PI for further stratifying the risk of transition to psychosis into four risk classes was suggested, each delineating a significantly increased relative risk compared to the general population, increasing with each class.
This 4-class model was argued to significantly improve the prediction of psychosis by enabling a differentiation of the individual risk in terms of magnitude and time. Such a more individualized risk estimation or clinical staging of risk, if validated in future studies, could significantly advance the development of risk adapted inclusion criteria for future randomized preventive trials. In the first application of this approach in the EPOS, only clinical and demographic variables were considered. It remains to be explored whether a multilevel model including neurocognitive, neurobiological, socio-biographical or environmental variables would increase the predictive accuracy even further. In addition, future studies will have to examine whether such models can also be applied to the prediction of psychosis within different time frames.
Introduction of at risk mental states (ARMS) in diagnostic systems
The currently ongoing revision of the DSM has stimulated a debate about the inclusion of a risk syndrome for psychosis in order to facilitate prevention 62. Several researchers initially argued against this project and drew attention to the dangers that the application of ARMS as diagnostic criteria could imply. They emphasized that the high rate of false-positive predictions in specialist clinics (60-70%) would be expected to increase up to 90% in general outpatient clinics. This criticism is certainly justified and should receive attention prior to deciding whether to include the ARMS in the upcoming revisions of the diagnostic systems. The debate, however, almost exclusively focuses on the predictive validity of at risk criteria, thereby disregarding the main finding: persons meeting at risk criteria already suffer from multiple mental and functional disturbances, for which they seek help. Moreover, they exhibit various psychological and cognitive deficits along with morphological and functional cerebral changes. Thereby, the majority of help-seeking at risk persons fulfil DSM-IV general criteria for mental disorder (i.e., a clinically significant behavioral or psychological syndrome associated with disability and/or severe distress) and have to be considered as ill, i.e. as people with the need and right to be treated. Keeping these considerations in mind, there are good reasons for the inclusion of a clinical profile in the diagnostic system as delineated by current at risk criteria, not as a prodromal risk syndrome for first psychosis, but as an independent disorder. Besides allowing access to standard medical care, the introduction of such an independent diagnosis would have the additional advantage of avoiding the stigmatization potentially caused by explicitly linking the current mental state to a threatening and negatively labeled outcome. Although an increased risk of psychosis would continue to be a characteristic of such a diagnosis, the psychological and medical focus would be shifted from an uncertain future outcome to psychopathology and needs. At this current state of knowledge, the DSM-5 criteria would be the right framework for the inclusion of this syndrome. A great impetus for the planning and implementation of a new generation of international and national studies would be triggered with this inclusion in DSM-5 and later on also in ICD-11.
More etiologically oriented prevention strategies
A new prevention approach is driven by the idea of neuroprotection 63,64 and studies indicating a progressive loss of gray matter volume before the onset of psychosis 56,58,60. Among the various substances with potential neuroprotective properties, the first results are available for high-dose omega-3 fatty acids, glycine and low-dose lithium. The 12-week transition rate was significantly lower in an omega-3 fatty acids-treated group of UHR adolescents than in a placebo group 65, and this effect was maintained at a 6-month follow-up. Glycine, an N-methyl-d-aspartate receptor coagonist, was evaluated in 10 patients in an open pilot trial, and a significant improvement in different psychopathological domains was reported 66. In an open proof-of-concept study, hippocampal T2 relaxation time was significantly reduced in an UHR group treated with low-dose lithium, as compared with a similar group receiving supportive standard treatment, suggesting a protection of hippocampal microstructure 58,67. This was the first study providing imaging data on neuro-protective effects in individuals at risk. The apparent preventive effect of omega-3 fatty acids is currently in the process of getting reviewed in the North-American, European, Australian Prodrome (NEURAPRO) large-sample study 68.
CONCLUSIONS
With the exception of Alzheimer’s dementia, schizophrenia is the first mental disorder to which the prediction and prevention program of modern medicine has hitherto systematically been applied. The results are promising and justify the expectation that in the years to come it will be possible to provide preventive strategies tailored specifically to the individual risk of illness of each person seeking advice. In order to attain a major reduction in incidence, symptom-oriented risk assessment has to be enriched by neurobiological and psychosocial risk factors, and indicated prevention has to be further developed towards selective prevention. This requires a new generation of large sample studies for prediction as well as prevention, with significantly longer observation periods. In these studies, promising combinations of risk indicators, selected to maximize predictive values, must be evaluated, psychological and pharmacological interventions need to be assessed on a long-term basis, more etiologically oriented prevention strategies have to be tested. In order to be able to plan and conduct such studies, it would certainly be helpful to include subpsychotic mental states, as defined by the currently used risk symptoms, in the upcoming revision of the diagnostic systems.
References
- 1.Bundesministeriums für Gesundheit. Maßnahmen des Bundesministeriums für Gesundheit zur Umsetzung nationaler Gesundheitsziele. Berlin: Bundesministerium für Gesundheit; 2007. [Google Scholar]
- 2.Commonwealth Department of Health and Aged Care. Promotion, prevention and early intervention for mental health – a monograph. Canberra: Commonwealth Department of Health and Aged Care; 2000. [Google Scholar]
- 3.European Commission. Green Paper – Improving mental health of the population: towards a strategy on mental health for the European Union. Brussels: European Commission; 2005. [Google Scholar]
- 4.World Health Organization. Prevention of mental disorders: effective interventions and policy options. Geneva: World Health Organization; 2004. [Google Scholar]
- 5.Häfner H, an der Heiden W. In: Clinical handbook of schizophrenia. Mueser KT, Jeste DV, editors. New York: Guilford; 2008. pp. 100–113. [Google Scholar]
- 6.Harrison G, Hopper K, Craig T. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J Psychiatry. 2001;178:506–517. doi: 10.1192/bjp.178.6.506. [DOI] [PubMed] [Google Scholar]
- 7.Clouth J. Costs of early retirement – the case of schizophrenia. Psychiatr Prax. 2004;31(Suppl. 2):S238–S245. doi: 10.1055/s-2004-828476. [DOI] [PubMed] [Google Scholar]
- 8.Rössler W, Salize HJ, van Os J. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. 2005;15:399–409. doi: 10.1016/j.euroneuro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Samnaliev M, Clark RE. The economics of schizophrenia. In: Mueser KT, Jeste DV, editors. Clinical handbook of schizophrenia. New York: Guilford; 2008. pp. 507–515. [Google Scholar]
- 10.Gutiérrez-Maldonado J, Caqueo-Urízar A, Kavanagh D. Burden of care and general health in families of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2005;40:899–904. doi: 10.1007/s00127-005-0963-5. [DOI] [PubMed] [Google Scholar]
- 11.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression and neuropathology on the matter of their convergence. Mol Psychiatry. 2005;10(Suppl. 3):40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 12.Seidman LJ, Giuliano AJ, Meyer EC. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 14.Häfner H, Maurer K, Löffler W. The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol. 1998;33:380–386. doi: 10.1007/s001270050069. [DOI] [PubMed] [Google Scholar]
- 15.Schultze-Lutter F, Ruhrmann S, Berning J. Basic symptoms and ultrahigh risk criteria: symptom development in the initial prodromal state. Schizophr Bull. 2010;36:182–191. doi: 10.1093/schbul/sbn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Early detection and intervention in the initial prodromal phase of schizophrenia. Pharmacopsychiatry. 2003;36(Suppl. 3):S162–S167. doi: 10.1055/s-2003-45125. [DOI] [PubMed] [Google Scholar]
- 17.Marshall M, Lewis S, Lockwood A. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62:975–983. doi: 10.1001/archpsyc.62.9.975. [DOI] [PubMed] [Google Scholar]
- 18.Klosterkötter J, Hellmich M, Steinmeyer EM. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 19.Huber G, Gross G. The concept of basic symptoms in schizophrenic and schizoaffective psychoses. Recent Prog Med. 1989;80:646–652. [PubMed] [Google Scholar]
- 20.Gross G. The ‘basic’ symptoms of schizophrenia. Br J Psychiatry. 1989;7:21–25. [PubMed] [Google Scholar]
- 21.Gross G, Huber G, Klosterkötter J. Bonner Skala für die Beurteilung von Basissymptomen (BSABS; Bonn Scale for the Assessment of Basic Symptoms) Berlin: Springer; 1987. [Google Scholar]
- 22.Schultze-Lutter F, Addington J, Ruhrmann S. Schizophrenia Proneness Instrument, Adult version (SPI-A) Rome: Fioriti; 2007. [Google Scholar]
- 23.Schultze-Lutter F, Koch E. Schizophrenia Proneness Instrument, Child & Youth version (SPI-CY) Rome: Fioriti; 2010. [DOI] [PubMed] [Google Scholar]
- 24.Schultze-Lutter F, Steinmeyer EM, Ruhrmann S. The dimensional structure of self-reported ‘prodromal’ disturbances in schizophrenia. Clin Neuropsychiatry. 2008;5:140–150. [Google Scholar]
- 25.Schultze-Lutter F, Ruhrmann S, Klosterkötter J. Evolving psychosis. In: Johannessen JO, Martindale B, Cullberg J, editors. Different stages, different treatments. London: Routledge; 2006. pp. 104–123. [Google Scholar]
- 26.Schultze-Lutter F, Klosterkötter J, Picker H. Predicting first-episode psychosis by basic symptom criteria. Clin Neuropsychiatry. 2007;4:11–22. [Google Scholar]
- 27.Yung AR, Phillips LJ, McGorry PD. Prediction of psychosis. Br J Psychiatry. 1998;172(Suppl. 33):14–20. [PubMed] [Google Scholar]
- 28.Phillips LJ, Yung AR, McGorry PD. Identification of young people at risk of psychosis: validation of personal assessment and crisis evaluation clinic intake criteria. Aust N Z J Psychiatry. 2000;34:S164–S169. doi: 10.1080/000486700239. [DOI] [PubMed] [Google Scholar]
- 29.Yung AR, Yuen HP, McGorry PD. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller TJ, McGlashan TH, Rosen JL. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 31.McGlashan T, Walsh B, Woods S, editors. The psychosis-risk syndrome. Handbook for diagnosis and follow-up. New York: Oxford University Press; 2010. [Google Scholar]
- 32.Cornblatt B. The New York High-Risk Project to the Hillside Recognition and Prevention (RAP) Program. Am J Med Genet. 2002;114:956–966. doi: 10.1002/ajmg.b.10520. [DOI] [PubMed] [Google Scholar]
- 33.Riecher-Rössler A, Geschwandtner U, Aston J. The Basel early-detection-of-psychosis (FEPSY)-study – design and preliminary results. Acta Psychiatr Scand. 2007;115:114–125. doi: 10.1111/j.1600-0447.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 34.Yung AR, Stanford C, Cosgrave E. Testing the ultra high risk (prodromal) criteria for the prediction of psychosis in a clinical sample of young people. Schizophr Res. 2006;84:57–66. doi: 10.1016/j.schres.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Yung AR, Nelson B, Stanford C. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Woods SW, Addington J, Cadenhead KS. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lencz T, Smith CW, Auther A. The assessment of “prodromal schizophrenia”: unresolved issues and future directions. Schizophr Bull. 2003;29:717–728. doi: 10.1093/oxfordjournals.schbul.a007041. [DOI] [PubMed] [Google Scholar]
- 38.Mason O, Startup M, Halpin S. Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states’. Schizophr Res. 2004;71:227–237. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Yung AR, Phillips LJ, Yuen HP. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 40.Cannon TD, Cadenhead K, Cornblatt B. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruhrmann S, Schultze-Lutter F, Salokangas RK. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European Prediction of Psychosis Study (EPOS). Arch Gen Psychiatry. 2010;67:241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- 42.Kirkbride JB, Fearon P, Morgan C. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AESOP study. Arch Gen Psychiatry. 2006;63:250–258. doi: 10.1001/archpsyc.63.3.250. [DOI] [PubMed] [Google Scholar]
- 43.Mrazek PJ, Haggerty RJ, editors. Reducing risks for mental disorders: frontiers for preventive intervention research. Washington: National Academy Press; 1994. [PubMed] [Google Scholar]
- 44.McGorry PD, Yung AR, Phillips LJ. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 45.Phillips LJ, McGorry PD, Yuen HP. Medium-term follow-up of a randomized controlled trial of interventions for young people at ultra high risk of psychosis. Schizophr Res. 2007;96:25–33. doi: 10.1016/j.schres.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 46.Morrison AP, French P, Walford L. Cognitive therapy for the prevention of psychosis in people at ultra-high risk. Br J Psychiatry. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 47.Morrison AP, French P, Parker S. Three-year follow-up of a randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultrahigh risk. Schizophr Bull. 2007;33:682–687. doi: 10.1093/schbul/sbl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGlashan TH, Zipursky RB, Perkins D. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophr Res. 2003;61:7–18. doi: 10.1016/s0920-9964(02)00439-5. [DOI] [PubMed] [Google Scholar]
- 49.Häfner H, Maurer K, Ruhrmann S. Early detection and secondary prevention of psychosis: facts and visions. Eur Arch Psychiatry Clin Neurosci. 2004;254:117–128. doi: 10.1007/s00406-004-0508-z. [DOI] [PubMed] [Google Scholar]
- 50.Bechdolf A, Wagner M, Harrigan S. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry. doi: 10.1192/bjp.bp.109.066357. in press. [DOI] [PubMed] [Google Scholar]
- 51.Ruhrmann S, Schultze-Lutter F, Maier W. Pharmacological intervention in the initial prodromal phase of psychosis. Eur Psychiatry. 2005;20:1–6. doi: 10.1016/j.eurpsy.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Bechdolf A, Müller H, Stützer H. Rationale and baseline characteristics of PREVENT: a second generation intervention trial in subjects at-risk (prodromal) of developing first episode psychosis evaluating cognitive behaviour therapy, aripiprazole and placebo for the prevention of psychosis. Schizophr Bull. doi: 10.1093/schbul/sbr083. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods SW, Tully EM, Walsh BC. Aripiprazole in the treatment of the psychosis prodrome. An open-label pilot study. Br J Psychiatry. 2007;191(Suppl. 51):96–101. doi: 10.1192/bjp.191.51.s96. [DOI] [PubMed] [Google Scholar]
- 54.Cornblatt BA, Lencz T, Smith CW. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry. 2007;68:546–557. doi: 10.4088/jcp.v68n0410. [DOI] [PubMed] [Google Scholar]
- 55.Hampel H, Frank R, Broich K. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 56.Borgwardt SJ, McGuire PK, Aston J. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry. 2007;191(Suppl. 51):69–75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- 57.Brockhaus-Dumke A, Schultze-Lutter F, Mueller R. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Pantelis C, Velakoulis D, Wood SJ. Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. Int Rev Psychiatry. 2007;19:371–381. doi: 10.1080/09540260701512079. [DOI] [PubMed] [Google Scholar]
- 59.Pukrop R, Schultze-Lutter F, Ruhrmann S. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychiatry. 2006;28:1388–1407. doi: 10.1080/13803390500434425. [DOI] [PubMed] [Google Scholar]
- 60.Witthaus H, Kaufmann C, Bohner G. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 2008;173:163–169. doi: 10.1016/j.pscychresns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 61.The European Network of Schizophrenia Networks for the Study of Gene-Environment Interactions (EU-GEI) Schizophrenia aetiology: do gene-environment interactions hold the key? Schizophr Res. 2008;102:21–26. doi: 10.1016/j.schres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Probably at-risk, but certainly ill - Advocating the introduction of a psychosis spectrum disorder in DSM-V. Schizophr Res. 2010;120:23–37. doi: 10.1016/j.schres.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 63.Lieberman JA. Neuroprotection: a new strategy in the treatment of schizophrenia. Neurobiological basis of neurodegeneration and neuroprotection. CNS Spectr. 2007;12(Suppl. 18):4–6. [PubMed] [Google Scholar]
- 64.Berger G, Dell’Olio M, Amminger P. Neuroprotection in emerging psychotic disorders. Early Interv Psychiatry. 2007;1:114–127. [Google Scholar]
- 65.Amminger GP, Schäfer MR, Papageorgiou K. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 66.Woods SW, Walsh B, Pearlson GD. Glycine treatment of prodromal symptoms. Schizophr Res. 2006;86:S7–S17. [Google Scholar]
- 67.Berger G, Wood SJ, Dell’Olio M. Neuroprotective effects of low dose lithium in individuals at ultra-high risk for psychosis. A longitudinal MRI/MRS study. Schizophr Res. 2008;102:39–40. doi: 10.2174/138161212799316163. [DOI] [PubMed] [Google Scholar]
- 68.Nelson B, McGorry P, Yung A. The NEURAPRO (North America, Europe, Australia Prodrome) Study: a multicenter RCT of treatment strategies for symptomatic patients at ultra-high risk for progression to schizophrenia and related disorders. Design and study plan. Schizophr Res. 2008;102(Suppl. 2):295–295. [Google Scholar]