Abstract
Background
Previous reports indicate that both distribution and amount of body fat confers susceptibility to metabolic syndrome. However, the relative contributions of these two different parameters of body fat to the various components of the metabolic syndrome have not been well defined.
Methods
Dual-energy X-ray absorptiometry (DXA) was used to measure and compare the relative amounts of total body fat, truncal fat, and lower body fat in a representative sample of 2587 black, white, and Hispanic men and women from the Dallas Heart Study (DHS). The relationships among these variables and fasting plasma levels of lipids, glucose, insulin, C-reactive protein (CRP), and leptin as well as blood pressure were analyzed.
Results
Beyond total body fat, fat distribution had the greatest impact on plasma triglycerides in all subjects and on high-density lipoprotein cholesterol (HDL-C) levels in women only. An intermediate effect of fat distribution was observed for homeostasis model assessment of insulin resistance (HOMA-IR) and for blood pressure. Plasma CRP levels were much more sensitive to body fat content than to body fat distribution and leptin levels were determined almost exclusively by body fat content. Although there were minor differences among the different ethnic groups, the major relationship patterns between these variables were similar.
Conclusion
For most metabolic risk factors, both body fat content and distribution independently contributed to levels, although significant differences were seen between the relative contributions of each variable to individual risk factors.
Introduction
The metabolic syndrome is an aggregation of several metabolic risk factors: dyslipidemia, dysglycemia, hypertension, and prothrombotic and proinflammatory states.1 Most individuals having multiple metabolic risk factors are overweight or obese,2 and often have upper body obesity.3 However, the relative contributions of total body fat and body fat distribution to the metabolic syndrome and the different metabolic syndrome risk factors remain an area of considerable dispute. This issue was examined recently in the Dallas Heart Study (DHS),3 which is a large multiethnic study.4 The current study is an extension of our previous study,3 in which we compared specifically the relative contributions of upper and lower body fat to metabolic risk factors as contrasted to total body fat. The essential questions being asked were whether body fat distribution contributed significantly to metabolic parameters beyond total body fat content, and, if so, how much more.
Methods
Study population
The study included 2587 men and women (ages 30– 65 years) who participated in the DHS.4 The sample contained 1449 women (50.7% black, 31.3% white, 18.1% Hispanic) and 1138 men (46.5% black, 37.3% white, 16.3% Hispanic). DHS study participants of other ethnicities (n = 62) were excluded from the studies as were 331 individuals with type 2 diabetes mellitus. All participants consented to an Institutional Review Board-approved study.3
Risk factor measurements
Height, weight, waist circumference, blood pressure, fasting plasma lipids, glucose, insulin, and C-reactive protein (CRP) were measured as described.3 Insulin resistance (IR) was estimated by homeostasis model assessment (HOMA-IR) (HOMA Calculator version 2.2).5 CRP was measured in the Roche/Hitachi 912 System, Tina-quant assay (Roche Diagnostics, Indianapolis, IN)6 and leptin by commercial radioimmunoassay (Linco Research Inc., St. Charles, Missouri).7
Body fat
Total fat mass (kg), fat-free mass (kg), and bone mineral mass (kg) were quantified by dual energy X-ray absorptiometry (DXA)8 in the trunk, upper and lower extremities, and head as recently described3 (Delphi W scanner, Hologic Inc., Bedford, MA and Discovery software [version 12.2]). A total of 49 subjects did not complete DXA scan for various reasons.
Statistical analysis
Continuous variables are summarized as medians and interquartile range or means ± standard deviation (SD). The association between body fat content, body fat distribution, and metabolic risk factors was assessed with Spearman correlation coefficients. Spearman partial correlation coefficients were used to adjust body fat content correlations for body distribution or to adjust body distribution correlations for percent body fat. The method of Meng et al.9 was used to compare dependent coefficient correlations within each gender. Two-way analysis of variance (ANOVA) models were constructed for each metabolic risk factor to evaluate the independent variables representing three fat content (percent body fat) and three fat distribution (truncal-to-lower body fat ratio) variables and to test for statistical interactions between percent body fat and the truncal-to-lower body fat ratio. In the two-way ANOVA, a significant interaction between percent body fat and truncal/lower-fat ratio indicates that the effects of these two variables are not independent of each other. To evaluate the effect of ethnicity, three-way ANOVA was used, adding a between group factor with three levels: black, non-Hispanic white, and Hispanic. Because most variables were not normally distributed, rank transformations were performed prior to analysis. Analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
Body fat contents
Study subjects were divided into three categories of percent body fat (body fat contents): for men, categories were <20%, 20–24.9%, and ≥25%; for women, <35%, 35–39.9%, and >40% (Table 1).These ranges approximate standard body mass index (BMI) categories for normal (<25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2) categories; such correspondence was reported previously3 and was observed through inspection of DHS data. Body fat content for a given BMI category is approximately 15% greater in women than in men. Mean (and/or median) levels of each of the metabolic risk factors in the three categories of percent body fat are provided in Table 1.
Table 1.
Metabolic Risk Variables According to Categories of Percentage Body Fat in Men and Women
| Variable | Men | Women | ||||
|---|---|---|---|---|---|---|
| Body fat contenta | <20% | 20–24.9% | ≥25% | <35% | 35–39.9% | ≥40% |
| Number | 287 | 344 | 507 | 410 | 381 | 658 |
| Race B/W/H (n) | 186/76/25 | 165/110/69 | 178/238/91 | 193/149/68 | 200/117/64 | 341/187/130 |
| Age (years) | 42.6 ± 9.7 | 43.5 ± 9.3 | 44.7 ± 9.6 | 40.8 ± 9.5 | 44.0 ± 9.8 | 45.8 ± 9.8 |
| BMI (kg/m2) | 23.8 ± 3.0 | 27.5 ± 3.5 | 31.6 ± 4.2 | 24.0 ± 3.8 | 28.7 ± 3.9 | 36.0 ± 6.5 |
| Waist (cm) | 85.4 ± 7.5 | 96.3 ± 7.1 | 108.1 ± 10.5 | 80.9 ± 9.9 | 92.3 ± 10.3 | 106.1 ± 13.9 |
| Body fat content (%) | 15.0 ± 3.6 | 22.7 ± 1.4 | 29.4 ± 3.5 | 29.5 ± 4.8 | 37.5 ± 1.4 | 44.4 ± 3.2 |
| Trunk/lower body fat ratio | 1.41 ± 0.42 | 1.69 ± 0.46 | 1.77 ± 0.41 | 1.07 ± 0.42 | 1.25 ± 0.33 | 1.35 ± 0.33 |
| TG (mg/dL) | 76 (58–113) | 104 (72–152) | 121 (82–180) | 72 (54–102) | 95 (69–128) | 95 (70–135) |
| HDL-C (mg/dL) | 50 (41–61) | 45 (38.5–51) | 41 (35–49) | 57 (48–67) | 51 (43–60) | 50 (43–59) |
| HOMA-IR | 0.8 (0.5–1.4) | 1.3 (0.9–1.8) | 2.0 (1.3–2.9) | 1.0 (0.6–1.5) | 1.6 (1.0–2.3) | 2.2 (1.4–3.0) |
| Systolic BP (mm/Hg) | 121 (111–132) | 123 (115–135) | 125 (117–136) | 111 (104–123) | 118 (109–129) | 122 (111–134) |
| CRP (mg/L) | 1.1 (0.5–2.9) | 1.6 (0.8–3.3) | 2.4 (1.4–4.4) | 1.4 (0.6–3.4) | 2.8 (1.5–5.2) | 6.3 (3.0–12.1) |
| Leptin (ng/mL) | 1.8 (1.0–2.7) | 4.4 (2.9–6.2) | 8.8 (6.4–12.6) | 9.4 (5.7–13.9) | 20.1 (15.3–26.5) | 36.8 (27.2–47.3) |
Data are presented as mean ± standard deviation or median and interquartile range (25th–75th percentile).
For continuous variables BMI through leptin, p < 0.001 comparing the three percent body fat categories with the Kruskal–Wallis test. For age, p < 0.01. The Kruskal–Wallis test was run for men and women separately.
Note: B, black; W, white; H, Hispanic, n, number; TG, triglycerides; HDL-C, high-density lipoproteins cholesterol; HOMA-IR, homeostatis assessment model for insulin resistance; BP, blood pressure; BMI, body mass index; CRP, C-reactive protein.
Body fat distributions
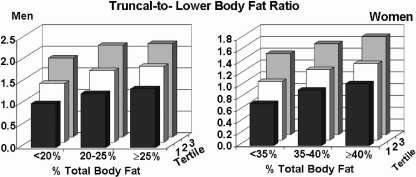
Body fat distribution was defined as the ratio of truncal fat-to-lower body fat. Distribution was categorized by tertile for each category of body fat content (Fig. 1). Mean truncal/lower fat ratios rose for each higher category of total body fat content. Thus, as described previously,3 higher weights associated with a disproportional increase in truncal fat relative to lower body fat, resulting in a higher truncal/lower fat ratio. For every tertile in each category of percent body fat, men had higher truncal/lower fat ratios than women (ANOVA p < 0.0001).
FIG. 1.
Truncal-to-lower body fat ratios in men and women according to total percent body fat and tertiles of ratios. With increasing percent body fat, both men and women showed an increase in ratio of truncal-to-lower body fat. Nonetheless, within a given range of percent total body fat, there remained considerable variation in ratio among the two groups.
Metabolic risk factors
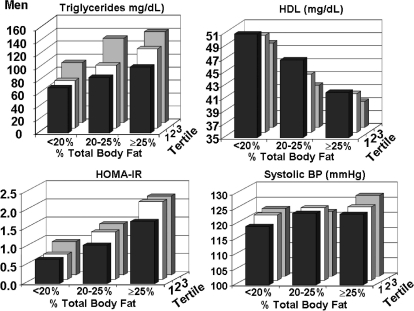
Values for four metabolic risk factors—median fasting plasma levels of triglycerides and mean high-density lipoprotein cholesterol (HDL-C), HOMA-IR, and systolic blood pressure—are plotted against categories of body fat content and distributions for men in Fig. 2. Statistical correlations in men are shown in Table 2. Median triglyceride concentrations rose moderately with progressively higher body fat categories, but body fat distribution had an even stronger effect on triglyceride levels (p = 0.005 comparing correlations). Both increasing body fat content and body fat distribution accompanied a fall in HDL-C levels. In comparison, neither fat content nor distribution dominated over the other in statistical correlation with HDL-C. HOMA-IR values in men rose with increasing body fat content and correlated more strongly with body fat content than did body fat distribution. However, significant independent effects of HOMA-IR were seen for both fat content and distribution with two-way ANOVA (p < 0.0001). Further, systolic blood pressure had significant but weak correlations with both body fat content and body fat distribution. However, only body fat content remained statistically significant in the ANOVA model.
FIG. 2.
For men, plasma levels of triglycerides, high density lipoprotein cholesterol (HDL), homeostasis model assessment of insulin resistance (HOMA-IR), and systolic blood pressure are plotted against percent body fat and tertiles of ratios of truncal-to-lower body fat.
Table 2.
Summary of Statistical Analysis in Men
| |
Spearman correlation |
|
|
|||
|---|---|---|---|---|---|---|
| |
|
|
|
|
Two-way analysis of variance |
|
| Men Variable | n | r1 versus percent total body fat | r2 versus truncal fat to lower body fat ratio | p value (r1 vs. r2) | ANOVA factor | ANOVA p value |
| Percent total body fat | 1138 | 0.32 | ||||
| TG | 1137 | 0.29 | 0.38 | 0.005 | %Total body fat | <0.0001 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.32 | |||||
| HDL | 1137 | −0.30 | −0.26 | 0.26 | %Total body fat | <0.0001 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.74 | |||||
| HOMA-IR | 1134 | 0.48 | 0.30 | <0.0001 | %Total body fat | <0.0001 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.67 | |||||
| SBP | 1138 | 0.13 | 0.09 | 0.36 | %Total body fat | 0.0005 |
| T/L ratio | 0.13 | |||||
| Interactiona | 0.41 | |||||
Interaction between percent body fat and truncal-to-lower body fat (T/L) ratio.
Note: TG, triglyceride; HDL, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; SBP, systolic blood pressure; T/L ratio, truncal-to-lower body fat ratio.
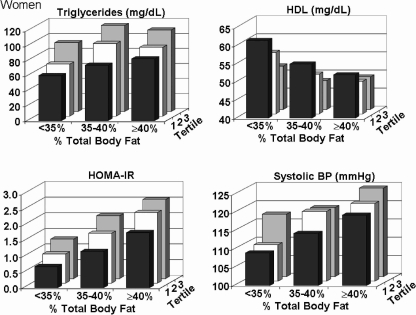
In women, body fat distribution correlated more strongly with triglycerides than did body fat content (Fig. 3, Tables 2 and 3). However, there was for triglycerides a statistically significant interaction noted between percent body fat and truncal/lower body fat ratio (p = 0.03) as depicted in Fig. 3, where the triglyceride concentration increases more consistently with increasing truncal-to-lower body fat ratio than with percent body fat; i.e., the triglyceride levels plateau in the two higher fat categories. HDL-C levels declined with an increasing body fat content and increasing body fat distribution. Fat distribution was also more strongly related to HDL-C than was body fat content (p < 0.0001 comparing correlations) in women. For HOMA-IR in women, body fat distribution also had a strong effect, although body fat content was influential as well. Body fat distribution had a moderate effect on systolic blood pressure in women, more so than in men (Fig. 2, Tables 2 and 3). Although in men the rise in systolic blood pressure across truncal-to-lower-fat ratios was not significant (see Fig. 2), the effect of body fat distribution in women was significant (p < 0.0001 by two-way ANOVA).
FIG. 3.
For women, plasma levels of triglycerides, high density lipoprotein cholesterol (HDL), homeostasis model assessment of insulin resistance (HOMA-IR), and systolic blood pressure are plotted against percent body fat and tertiles of ratios of truncal-to-lower body fat.
Table 3.
Summary of Statistical Analysis in Women
| |
Spearman correlation |
|
|
|||
|---|---|---|---|---|---|---|
| |
|
|
|
|
Two-way analysis of variance |
|
| Women Variable | n | r1 versus Percent total body fat | r2 versus truncal fat to lower body fat ratio | P value (r1 vs. r2) | ANOVA factor | ANOVA p value |
| Percent total body fat | 1448 | 0.36 | ||||
| TG | 1448 | 0.19 | 0.40 | <0.0001 | %Total body fat | 0.0003 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.03 | |||||
| HDL | 1448 | −0.16 | −0.29 | <0.0001 | %Total body fat | <0.0001 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.36 | |||||
| HOMA-IR | 1435 | 0.46 | 0.43 | 0.327827 | %Total body fat | <0.0001 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.11 | |||||
| SBP | 1448 | 0.27 | 0.25 | 0.45 | %Total body fat | <0.0001 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.11 | |||||
Interaction between percent body fat and truncal-to-lower body fat (T/L) ratio.
Note: TG, triglyceride; HDL, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; SBP, systolic blood pressure; T/L ratio, truncal-to-lower body fat ratio.
C-reactive protein and leptin
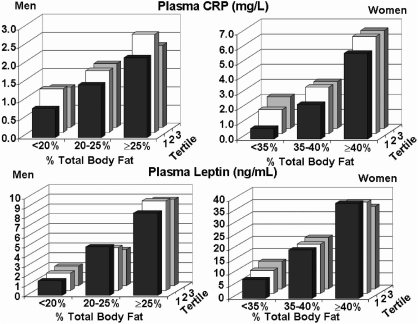
Figure 4 plots CRP and leptin levels against body fat content and distribution in men and women. Statistics are given in Table 4. For CRP, correlations with fat content predominated over distribution in both sexes (p < 0.0001) (Table 2).In men, there was no longer a significant relationship between CRP and fat distribution after adjusting for percent body fat. In women, a significant interaction between the body fat content and fat distribution categories was found (p = 0.02). On the basis of multiple comparisons, CRP fat distribution tertiles were similar within >40% body fat category whereas CRP significantly increased with fat distribution in the <35% and the 35–40% fat categories.
FIG. 4.
For men and women, plasma levels of CRP and leptin are plotted against percent body fat and tertiles of ratios of truncal-to-lower body fat.
Table 4.
C-Reactive Protein and Leptin
| |
Spearman correlation |
|
|
|||
|---|---|---|---|---|---|---|
| |
|
|
|
|
Two-way analysis of variance |
|
| Variable | n | r1 versus Percent total body fat | r2 versus truncal fat to lower body fat ratio | P value (r1 vs. r2) | ANOVA factor | ANOVA p value |
| CRP (men) | 1128 | 0.29 | 0.11 | <0.0001 | %Total body fat | <0.0001 |
| T/L ratio | 0.08 | |||||
| Interactiona | 0.85 | |||||
| CRP (women) | 1438 | 0.53 | 0.30 | <0.0001 | %Total body fat | <0.0001 |
| T/L ratio | <0.0001 | |||||
| Interactiona | 0.02 | |||||
| Leptin (men) | 1137 | 0.79 | 0.23 | <0.0001 | %Total body fat | <0.0001 |
| T/L ratio | 0.13 | |||||
| Interactiona | 0.20 | |||||
| Leptin (women) | 1438 | 0.81 | 0.28 | <0.0001 | %Total body fat | <0.0001 |
| T/L ratio | 0.49 | |||||
| Interactiona | 0.003 | |||||
Interaction between % body fat and truncal-to-lower body fat (T/L) ratio.
Note: CRP, C-reactive protein.
In the case of leptin, levels were much more closely associated with the body-fat content than was distribution in both men and women (Fig. 4, Table 4).In the two-way ANOVA models, percent body fat was significant in men and women (p < 0.0001). In men, both the truncal-to-lower body fat ratio and the interaction between body fat content and distribution were nonsignificant. In women, a significant interaction between body fat content and body fat distribution (p = 0.003) was seen, attributable to the inconsistent effect of fat distribution within fat categories. In particular, an increase in leptin was observed with increasing truncal/lower fat ratio in the leaner body fat <35% group (p = 0.003), but no difference was detected between truncal-to-lower body fat ratio tertiles in the 35–40% and >40% fat categories (p = 0.93 and p = 0.13, respectively).
Ethnicity
Differences between the ethnic groups were statistically significant (p < 0.0001) for percent body fat, truncal-to-lower body fat ratio, triglycerides, HDL-C, HOMA2, CRP, leptin, and systolic blood pressure (data not shown). Moreover, when controlling for ethnicity in the analysis of variance models, a few interactions between ethnicity and body fat or body distribution were observed. For HDL-C, a significant interaction between ethnic group and truncal-to-lower fat ratio tertiles was found in men (p = 0.006). This interaction stemmed from a weaker, nonsignificant relationship between truncal-to-lower fat ratio groups and HDL-C in black men (p = 0.90), whereas HDL-C was different between truncal-to-lower fat ratio groups for non-Hispanic white men (p = 0.0002) and Hispanic men (p = 0.01). In females, an interaction between ethnicity and truncal-to-lower fat ratio was observed for CRP, stemming from no differences observed in Hispanic women when comparing truncal-to-lower fat ratio tertiles (p = 0.59). But for black and non-Hispanic white women, CRP increased with increasing truncal/lower fat ratio tertiles (p < 0.01 for both ethnic groups). Apart from these interactions, the three ethnic groups generally showed consistent associations between body fat or body distribution and metabolic risk variables. This justifies pooling the data for simplicity of presentation (Figs. 1–4).
Discussion
This study showed that the relative contributions of fat content and distribution differ for different metabolic risk factors. Some studies10,11 have claimed that total body fat, not fat distribution, is the major correlate of the metabolic syndrome; many others12,13 identify fat distribution as the culprit. Our study showed that both are important and influential on risk factors. This study must be interpreted in the light of our recent report on the black and white cohorts of the DHS.3 Here body fat distribution was also found to have some influence beyond total body fat content on metabolic risk factors. Waist circumference still correlated significantly with risk factors after adjusting for total body fat. But unexpectedly, individual body fat compartments, such as visceral fat, accounted for only a portion of the variation in risk-factor levels beyond total body fat and waist circumference. The current paper focuses exclusively on two factors—body fat content and distribution—and more clearly reveals relative contributions of the two on particular metabolic risk correlates. Our recent study3 suggested that DXA data provide most of the information relating body fat to risk factors; measurement of subcompartments by magnetic resonance imaging (MRI) appeared to add little more information. Therefore, it was not examined in the current study.
Although upper body fat has been widely touted as the major driving force behind the metabolic syndrome,1,12,13 recent reports3,14–16 suggest that lower body fat protects against metabolic risk. Lower body adipose tissue may provide a reservoir for fat storage that is reduced in persons who manifest predominant upper body fat; more lower body adipose tissue should provide a defense against overloading of upper body adipocytes with triglycerides, which will make them resistant to the antilipolytic effects of insulin.17,18 A deficiency of lower body fat may result from diminished adipogenesis19 or maturation of small adipocytes into larger lipid-storing cells.20 Either should result in overloading of upper body adipocytes, higher levels of nonesterified fatty acids (NEFA), which in turn should suppress systemic insulin sensitivity and predispose to dyslipidemia. Thus, a lower truncal-to-lower body fat ratio should be an indicator of insufficient lower body fat needed to protect against triglyceride overload of upper body adipocytes.
Body fat content/distribution and metabolic parameters
Increasing body fat content caused a rise in mean plasma triglycerides; but body fat distribution also powerfully affected triglycerides—both in men and women (Figs. 2 and 3). Body fat distribution affected triglycerides even in the lowest body fat category (“normal weight”). Fat distribution was particularly influential in the intermediate “overweight” category, and remained so among obese men and women. HDL-C levels changed opposite to triglyceride levels. HDL-C fell with higher percent body fat, but also fell with higher truncal-to-lower-fat ratios. In this study, body fat content and distribution were both related to insulin resistance, as indicated by HOMA-IR. However, the effects of body fat distribution were not as strong as for lipid parameters. In our analysis, increasing body fat content was accompanied by higher blood pressure, particularly evident in women. Several reports suggest that blood pressure is also sensitive to body fat distribution as well as total body fat.21–23 We also found that increases in truncal-to-lower body fat ratios were somewhat more strongly associated with higher blood pressure in women than in men.
With increasing body fat content, CRP levels rose in both men and women (Fig. 4); the effect of body weight on CRP levels has been reported before for this population.24 Overall, women had higher CRP levels than men, presumably because they had higher percent body fat. CRP levels are a reflection of amounts of inflammatory cytokines released by adipose tissue. Body fat distribution had little effect on CRP concentrations, except that in those with the lowest truncal-to-lower body ratios usually had somewhat lower CRP levels than did other ratio tertiles in the same body fat category. Adipose tissue is the source of circulating leptin, and, as shown in Fig. 4, leptin levels are dependent almost exclusively on body fat content. Body fat distribution appears to play almost no role in leptin release.
Ethnicity
Our previous studies3 have shown that metabolic risk factors differ among different ethnic groups. For example, black men have lower triglycerides and higher HDL-C than do white men. These risk factor differences may be related in part to differences in body fat content and distribution For example, black men appeared to have lower ratios of truncal-to-lower body fat than whites and Hispanics. Even so, the current study did not have the power to define precisely how differences in metabolic risk factors in different ethnic groups are related to differences in body fat distribution.
Summary
This analysis of the DHS data provides a visual depiction of the relative contributions of body fat content and body fat distribution on metabolic parameters related to the metabolic syndrome (Figs. 1–4). It shows clearly that both factors significantly influence cardiovascular risk factors. However, the contributions of each measure to metabolic risk factors vary depending on the risk factor. Beyond total body fat, fat distribution had the greatest impact on plasma triglycerides and the least effect on CRP and leptin levels. An intermediate effect of fat distribution was observed for HOMA-IR and for blood pressure. Although a great emphasis has been placed on upper body obesity as a cause of the metabolic syndrome, our findings on body fat distribution raise the possibility that a reduction in lower body fat may be a key factor in causation of the syndrome.
Acknowledgments
This work was supported by the Donald W. Reynolds Cardiovascular Clinical Research Center at Dallas and General Clinical Research Center Grant MO1-RR0633, the Moss Heart Foundation, and a Veterans Affairs Merit grant. The authors acknowledge the valuable comments of Dr. Helen Hobbs on this manuscript.
Author Disclosure Statement
The authors have no commercial disclosures to make with regard to this manuscript. No competing financial interests exist.
References
- 1.Grundy SM. Cleeman JI. Daniels SR. Donato KA. Eckel RH. Franklin BA. Gordon DJ. Krauss RM. Savage PJ. Smith SC., Jr Spertus JA. Costa F. Diagnosis and Management of the Metabolic Syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Park YW. Zhu S. Palaniappan L. Heshka S. Carnethon MR. Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vega GL. Adams-Huet B. Peshock R. Willett D. Shah B. Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 4.Victor RG. Haley RW. Willett DL. Peshock RM. Vaeth PC. Leonard D. Basit M. Cooper RS. Iannacchione VG. Visscher WA. Staab JM. Hobbs HH Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 5.Wallace TM. Levy JC. Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 6.Roberts WL. Moulton L. Law TC. Farrow G. Cooper-Anderson M. Savory J. Rifai N. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47:418–425. [PubMed] [Google Scholar]
- 7.Abdullah SM. Khera A. Leonard D. Das SR. Canham RM. Kamath SA. Vega GL. Grundy SM. McGuire DK. de Lemos JA. Sex differences in the association between leptin and CRP: Results from the Dallas Heart Study. Atherosclerosis. 2007;195:404–410. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Kelly TL. Berger N. Richardson TL. DXA body composition: theory and practice. Appl Radiat Isot. 1998;49:511–513. doi: 10.1016/s0969-8043(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 9.Meng X –L. Rosenthal R. Rubin DB. Comparing correlated correlation coefficients. Psycholog Bull. 1992;111:172–175. [Google Scholar]
- 10.Farin HM. Abbasi F. Reaven GM. Comparison of body mass index versus waist circumference with the metabolic changes that increase the risk of cardiovascular disease in insulin-resistant individuals. Am J Cardiol. 2006;98:1053–1056. doi: 10.1016/j.amjcard.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Bosy-Westphal A. Geisler C. Onur S. Korth O. Selberg O. Schrezenmeir J. Muller MJ. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes. 2006;30:475–483. doi: 10.1038/sj.ijo.0803144. [DOI] [PubMed] [Google Scholar]
- 12.Abate N. Garg A. Peshock RM. Stray-Gundersen J. Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despres JP. Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 14.Snijder MB. Dekker JM. Visser M. Bouter LM. Stehouwer CD. Yudkin JS. Heine RJ. Nijpels G. Seidell JC. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH. Krishnaswami S. Harris TB. Katsiaras A. Kritchevsky SB. Simonsick EM. Nevitt M. Holvoet P. Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S. Hawken S. Ounpuu S. Bautista L. Franzosi MG. Commerford P. Lang CC. Rumboldt Z. Onen CL. Lisheng L. Tanomsup S. Wangai P., Jr Razak F. Sharma AM. Anand SS INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 17.Jernås M. Palming J. Sjöholm K. Jennische E. Svensson PA. Gabrielsson BG. Levin M. Sjögren A. Rudemo M. Lystig TC. Carlsson B. Carlsson LM. Lönn M. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 18.Chandalia M. Lin P. Seenivasan T. Livingston EH. Snell PG. Grundy SM. Abate N. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS ONE. 2007;2:e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal AK. Garg A. Genetic basis of lipodystrophies and management of metabolic complications. Annu Rev Med. 2006;57:297–311. doi: 10.1146/annurev.med.57.022605.114424. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin T. Sherman A. Tsao P. Gonzalez O. Yee G. Lamendola C. Reaven GM. Cushman SW. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50:1707–1715. doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 21.Chuang SY. Chou P. Hsu PF. Cheng HM. Tsai ST. Lin IF. Chen CH. Presence and progression of abdominal obesity are predictors of future high blood pressure and hypertension. Am J Hypertens. 2006;19:788–795. doi: 10.1016/j.amjhyper.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Gus M. Fuchs SC. Moreira LB. Moraes RS. Wiehe M. Silva AF. Albers F. Fuchs FD. Association between different measurements of obesity and the incidence of hypertension. Am J Hypertens. 2004;17:50–53. doi: 10.1016/j.amjhyper.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Canoy D. Luben R. Welch A. Bingham S. Wareham N. Day N. Khaw KT. Fat distribution, body mass index and blood pressure in 22,090 men and women in the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk) study. J Hypertens. 2004;22:2067–2074. doi: 10.1097/00004872-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Niskanen LK. Haffner S. Karhunen LJ. Turpeinen AK. Miettinen H. Uusitupa MI. Serum leptin in obesity is related to gender and body fat topography but does not predict successful weight loss. Eur J Endocrinol. 1997;137:61–67. doi: 10.1530/eje.0.1370061. [DOI] [PubMed] [Google Scholar]