Abstract
Adiponectin is an adipocyte hormone that links visceral adiposity with insulin resistance and atherosclerosis. It is unique among adipocyte-derived hormones in that its circulating concentrations are inversely proportional to adiposity, and low adiponectin concentrations predict the development of type 2 diabetes and cardiovascular disease. Consequently, in the decade since its discovery, adiponectin has generated immense interest as a potential therapeutic target for the metabolic syndrome and diabetes.
This review summarizes current research regarding the regulation of circulating adiponectin concentrations by physiological, pharmacological, and nutritional factors, with an emphasis on human studies. In humans, plasma adiponectin concentrations are influenced by age and gender, and are inversely proportional to visceral adiposity. In vitro studies suggest that adiponectin production may be determined primarily by adipocyte size and insulin sensitivity, with larger, insulin-resistant adipocytes producing less adiponectin. While adiponectin concentrations are unchanged after meal ingestion, they are increased by significant weight loss, such as after bariatric surgery. In addition, adiponectin production is inhibited by a number of hormones, including testosterone, prolactin, glucocorticoids and growth hormone, and by inflammation and oxidative stress in adipose tissue. Smoking decreases, while moderate alcohol consumption increases, circulating adiponectin concentrations. Dietary fatty acid composition in rodents influences adiponectin production via ligand-activated nuclear receptors (PPARs); however, current evidence in humans is equivocal. In addition to PPAR agonists (such as thiazolidinediones and fibrates), a number of pharmacological agents (angiotensin receptor type 1 blockers, ACE inhibitors, and cannabinoid receptor antagonists) used in treatment of the metabolic syndrome also increase adiponectin concentrations in humans.
Introduction
Adipose tissue is now well recognized as an important source of hormones that influence body adiposity, glucose homeostasis, inflammation, and cardiovascular disease.1,2 Due to its involvement in each of these physiological processes, the adipocyte-derived hormone adiponectin (also referred to as Acrp30, AdipoQ, apM1, and GBP28 in initial reports)3–6 has been intensively studied. This review will focus on the physiological, pharmacological, and nutritional factors that influence circulating adiponectin concentrations, with an emphasis on studies conducted in humans.
Adiponectin is an Adipocyte-Specific Secreted Protein Dysregulated in Obesity, Type 2 Diabetes, and Cardiovascular Disease
Adiponectin mRNA is highly expressed in and is relatively specific for mature adipocytes.3,4 The human adiponectin gene encodes a 244 amino acid, 30 kDa secreted protein, which contains a putative signal sequence, a collagen-like domain, and a globular domain. Adiponectin shares structural similarity with collagens VIII and X, tumor necrosis factor alpha (TNF-α), and complement factor C1q.
In plasma, adiponectin circulates at very high concentrations for a hormone, usually in the range 3 to 30 μg/mL. Adiponectin concentrations are decreased in a variety of human metabolic and cardiovascular disease states, including obesity,7 type 2 diabetes mellitus,8 lipodystrophy,9 nonalcoholic hepatic steatosis,10 essential hypertension,11 and coronary artery disease.12 Low adiponectin levels precede the development of insulin resistance13 and myocardial infarction14 in humans. Interestingly, adiponectin concentrations increase with age15 and are elevated in type 1 diabetes.16 Adiponectin is detectable in cerebrospinal fluid (CSF), with CSF concentrations in humans typically 0.1% of corresponding plasma concentrations.17,18
Adiponectin Action in Peripheral Tissues
Adiponectin has insulin-sensitizing actions in the liver, and lowers blood glucose levels in diabetic animals by improving insulin-mediated suppression of gluconeogenesis.19 In liver and skeletal muscle, adiponectin also improves glucose utilization and stimulates fatty acid oxidation via a pathway that involves AMP kinase (AMPK) and acetyl-CoA carboxylase (ACC).20 Adiponectin also prevents TNF-α–stimulated expression of adhesion molecules in cultured human endothelial cells21 by inhibiting IKKβ phosphorylation and NF-κB activation,22 and inhibits the transformation of macrophages into foam cells.23 Together, these effects have been shown to prevent plaque formation in apoE-deficient mice, a mouse model of atherosclerosis.24,25 Adiponectin may also prevent excessive cardiac remodeling following injury. In response to pressure overload, adiponectin-deficient mice exhibit an exaggerated hypertrophic response compared to wild-type mice.26 This response is prevented by intravenous administration of an adenovirus expressing adiponectin prior to injury.27
Adiponectin's diverse actions in these tissues are mediated by its receptors, AdipoR1 and AdipoR2.28 In humans, AdipoR1 is ubiquitously expressed, with highest levels of expression in heart and skeletal muscle; while AdipoR2 expression is more restricted to skeletal muscle and liver.28 Overexpression of each receptor in the livers of leptin-deficient mice revealed their divergent functions: overexpression of AdipoR1 increased AMPK phosphorylation and reduced the expression of genes involved in hepatic gluconeogenesis; while overexpression of AdipoR2 increased peroxisome proliferator-activated receptor alpha (PPAR-α) mRNA and reduced the expression of inflammatory cytokines and markers of oxidative stress.29 In this model, overexpression of either receptor reduced hepatic triglyceride content. In addition to AdipoR1 and AdipoR2, adiponectin also binds to T-cadherin, a receptor localized on vascular endothelium and muscle cells.30 This interaction may underlie some antiatherogenic, vascular-protective actions of adiponectin.
Adiponectin may also have actions in the central nervous system (CNS) to influence the control of body weight, although its specific role is controversial.31 Adiponectin is detectable in CSF, and its receptors are abundantly expressed in hypothalamic areas that control food intake.18 In mice, although central administration of adiponectin was initially shown to reduce body weight by increasing energy expenditure,32 intravenous adiponectin treatment has been recently found to increase feeding in mice by activating AMP kinase in the hypothalamus.33 Further studies are needed to better define the potential role of adiponectin in the CNS regulation of energy homeostasis.
Adiponectin Circulates as Multimers that Activate Differential Signaling Pathways
Adiponectin's biological effects depend upon the formation of multimeric complexes and may require proteolytic cleavage. The formation of higher-order structures in plasma is similar to other proteins with collagen-like domains, such as complement factor C1q.34 Adiponectin's basic unit consists of a trimer, formed by interactions within the globular domain and stabilized by a collagenous coiled-coil structure.6 These trimers associate by disulfide bonds to form hexamers, dodecamers (12 subunits), and octadecamers (18 subunits).35,36 Trimeric, hexameric, and larger forms of adiponectin are referred to as low, medium, and high molecular weight (LMW, MMW, HMW), respectively.37 Globular adiponectin, a fragment of human adiponectin that includes the C-terminal globular domain, has demonstrated biological activity in some studies, but is present at a much lower concentration than the other forms of adiponectin.38
Growing evidence indicates that HMW adiponectin is the most active form with respect to insulin sensitivity. Type 2 diabetes is associated with a lower proportion of adiponectin in the HMW form, and this ratio (termed SA) is improved by treatment with antidiabetic thiazolidinediones (TZDs).39 Injection of HMW, but not MMW, adiponectin reduced blood glucose in adiponectin-deficient mice,39 and mutations in the adiponectin gene that interfere with the assembly of HMW adiponectin are associated with insulin resistance and type 2 diabetes in humans.37 Other forms of adiponectin may have oligomer-specific functions, as LMW and globular adiponectin, but not MMW or HMW, activate AMPK in rat skeletal muscle.40,41 In monocytes, only the LMW form inhibits NFκβ activity and proinflammatory cytokine release,42 while HMW and adiponectin may instead exert the opposite effect.43
Adiponectin Synthesis and Secretion
Circulating adiponectin concentrations are the end result of a complex, highly regulated secretory pathway in adipocytes.44 Adiponectin mRNA expression is enhanced by a variety of adipogenic transcription factors, including PPAR-γ,45 C/EBPα,46 C/EBPβ,47 FOXO1, SIRT1,48 and by Sp1, which is induced during adipogenesis.49 Studies of humans with obesity, type 2 diabetes, or gestational diabetes have shown dysregulation of adiponectin mRNA in human adipose tissue,50–52 however, changes in adiponectin mRNA expression do not always correspond to changes in plasma adiponectin concentrations.53,54 This latter observation supports the involvement of post-transcriptional and post-translational mechanisms in the regulation of adiponectin production.
Prior to secretion, adiponectin undergoes extensive posttranslational modifications including hydroxylation of proline and lysine residues and glycosylation of hydroxylysines.55 These modifications are necessary for HMW multimer formation56,57 and are therefore likely to determine HMW-induced activation of hepatic AMPK and its effects to stimulate fatty acid oxidation and reduce liver triglyceride deposition.57 Type 2 diabetes is associated with reduced glycosylation of adiponectin as well as lower concentrations of HMW adiponectin in the circulation.57
The assembly of hexameric and HMW adiponectin from trimers requires the formation of disulfide bonds, at Cys-36 of the human protein and Cys-39 in the murine equivalent.35,37,40 These disulfide bonds are crucial for the release of intracellular adiponectin58 via a process known as thiol-mediated protein retention.59 This process involves two endoplasmic reticulum chaperones, ERp44 and Ero1-Lα. ERp44 retains adiponectin within the cell, and Ero1-Lα competes with adiponectin for binding to ERp44. Accordingly, increasing the amount of ERp44 in a heterologous system (cultured human embryonic kidney cells) dose-dependently reduced adiponectin secretion, while reducing ERp44 levels in adipocytes increased adiponectin secretion. The in vivo relationships between these chaperones and circulating adiponectin concentrations are likely to be complex, however, as levels of ERp44 protein in adipose tissue were greater in female mice relative to male mice, and higher in wild-type mice relative to ob/ob mice, both conditions under which ERp44 protein levels would be expected to be reduced.35,37 The specific mechanisms by which adiponectin is assembled and secreted are currently under investigation, and may yield new pharmacological targets to increase adiponectin production.
Adiponectin may also exert negative feedback inhibition of its own production, as adiponectin mRNA expression in cultured adipocytes is suppressed by treatment with physiological concentrations of adiponectin.60 This is likely to be due to degradation of about half of the synthesized adiponectin prior to secretion.45 In a similar manner, transgenic mice designed to overexpress adiponectin specifically in adipose tissue actually displayed lower circulating adiponectin levels relative to wild-type mice.60 To circumvent this problem, it was necessary for Combs and colleagues45 to use transgenic mice overexpressing a mutated form of adiponectin in order to elevate circulating adiponectin concentrations.
Circulating adiponectin concentrations may also be affected by renal clearance, as adiponectin levels are elevated in states characterized by impaired renal function, such as macroalbuminuria61 and end-stage kidney disease.62,63
Role of Adipose Distribution and Adipocyte Size
Visceral adiposity is an important determinant of plasma adiponectin concentrations in humans. Direct assessments of visceral fat in humans have repeatedly shown an independent and inverse relationship between visceral adiposity and plasma adiponectin concentrations.15,64–66 While this relationship is not well supported by analyses of adiponectin mRNA expression,51,67,68 in one study of 36 morbidly obese nondiabetic subjects it was reported that adiponectin mRNA expression in visceral fat, but not in subcutaneous fat, was positively correlated with the serum adiponectin level.69 This finding is supported by the results of in vitro experiments in which adiponectin secretion from omental, but not subcutaneous, adipocytes under basal conditions was found to be reduced in obesity.7 In another study, both insulin-stimulated and rosiglitazone-stimulated adiponectin secretion were found to be significantly higher in omental relative to subcutaneous adipocytes, although basal adiponectin secretion did not differ between the two depots.70 Studies of cultured human adipose tissue also suggest that subcutaneous adipocytes do not make a major contribution (∼10%) to interindividual variations in circulating adiponectin and insulin sensitivity.71 The importance of the visceral depot in determining circulating adiponectin concentrations is also supported by a recent study in mice.72
Adiponectin production may also be determined by adipocyte size.73 Larger adipocytes are more insulin-resistant,74 and one mechanism by which TZDs improve insulin sensitivity is by increasing the number of small, insulin-sensitive adipocytes, at the expense of large, insulin-resistant ones.75 TZDs also increase adiponectin secretion.76 Our laboratory has reported data from in vitro experiments indicating a strong inverse relationship between adipocyte volume and adiponectin secretion from isolated rat adipocytes.77 Although our results are not consistent with recent data obtained from human subcutaneous adipocytes,78 due to their anatomical source, the contribution of subcutaneous adipocytes to circulating adiponectin concentrations appears likely to be minor compared with adipocytes in visceral depots.
Diurnal Variation of Circulating Adiponectin Concentrations and Effects of Meals, Glucose, and Insulin
Adiponectin concentrations in plasma are fairly stable throughout the day, exhibiting only a minor fluctuation (∼20%) from the 24-hour mean, with levels declining modestly during the night to a nadir in the early morning.79 This diurnal variation appears to be greater in females than in males,79,80 and may increase in amplitude following significant weight loss.80,81 Diurnal changes in adiponectin concentrations may be related to meals, as a study of 110 subjects found that adiponectin concentrations decreased by 6% two hours after a 75 g glucose load and by 8% five hours after a high-fat mixed meal.82 Other studies have reported little or no diurnal variation in adiponectin levels,8,83,84 and have indicated that adiponectin concentrations are unchanged after 72 hours of fasting,85 however some of these studies may have been underpowered to detect such small effects. In one study, a four-fold postprandial increase in adiponectin in obese, but not in normal weight, subjects was reported.86 In contrast, we have observed that plasma adiponectin concentrations do not change in either normal-weight or overweight/obese subjects over the course of a day during which 3 meals accompanied by glucose-sweetened beverages were consumed, despite large postprandial increases of glucose and insulin (unpublished observations). Consistent with these results, infusion of 200 mg/m2/min glucose for 48 hours in normal weight, insulin-sensitive humans produced moderate increases of plasma glucose, insulin, and leptin concentrations, but did not change circulating adiponectin concentrations at all.87 Overall, the diurnal variation in circulating adiponectin concentrations is much less than that observed for leptin.88
Although a consistent inverse relationship between plasma insulin and adiponectin concentrations has been shown in cross-sectional studies,8,89 the effects of insulin on adiponectin secretion and circulating adiponectin levels reported in both in vivo and in vitro studies are inconsistent. Short-term elevations in insulin, such as those measured during a hyperinsulinemic-euglycemic clamp, modestly lower circulating adiponectin concentrations, particularly the HMW form,90 in human subjects.91–93 In type 1 diabetic patients, however, nearly two years of insulin replacement did not significantly increase adiponectin concentrations.16 Inconsistent effects of insulin on adiponectin synthesis have been observed in isolated cells: pulse-chase studies of 3T3-L1 adipocytes have shown that supraphysiological concentrations of insulin (160 nM) roughly doubled adiponectin secretion over two hours.94 This is supported by data from our laboratory indicating that adiponectin secretion from isolated rat adipocytes was increased by a 96-hour exposure to physiological insulin concentrations, and that both insulin-stimulated glucose utilization and adiponectin secretion were reduced in large adipocytes from obese animals compared with smaller, more insulin-sensitive adipocytes from nonobese rats.77 The effects of insulin on adiponectin secretion may be cell-and time-dependent, however, as adiponectin secretion from cultured human subcutaneous and omental adipocytes was reportedly unaffected by 24 hours of 100 nM insulin treatment.70
Effects of Caloric Restriction and Weight Loss on Circulating Adiponectin Concentrations
There is little consistent evidence to indicate that adiponectin concentrations in humans are regulated by short-term caloric restriction, prior to significant weight loss. In obese women, consumption of a a very low calorie diet (550 kcal/day) for three weeks reduced weight by approximately 5% but did not change adiponectin concentrations.95 In men, similarly, adiponectin levels were unchanged following consumption of an 800 kcal/day diet for four days.96 We have observed a modest (∼10%) but significant increase in serum adiponectin in normal-weight women following consumption of a calorie-restricted (600 kcal/day) diet for one week; interestingly, the opposite effect (an ∼20% decrease of adiponectin) was observed in men restricted to 800 kcal/day.97 One study of healthy normal-weight women restricted to 1000 to 1200 kcal/day for four weeks showed a significant reduction in adiponectin concentrations (16%), despite an average weight loss of 3.4 kg.98 Analogously, exercise does not produce changes in circulating adiponectin concentrations independently of weight loss.99–101
In contrast, longer-term caloric restriction producing significant weight loss (>8–9% of initial weight) has been repeatedly shown to increase adiponectin concentrations (reviewed recently in Imbeault101). Weight changes of this magnitude usually result from either long-term caloric restriction or various forms of weight loss surgery. In these studies, the increase in adiponectin concentration appears to be more related to the amount of weight lost than the method used.102 We and others have reported that increases in adiponectin following weight loss are strongly and negatively correlated with changes in body weight, body mass index (BMI) and fat mass.103–107 The failure of some adequately-powered studies to observe changes in adiponectin concentrations after significant weight loss may be attributable to a redistribution of adiponectin oligomers, towards the higher molecular weight forms, that is not apparent when the total adiponectin concentration is examined.108–110 We have observed that although total adiponectin concentrations were unchanged one month after Roux-en-Y gastric bypass surgery, absolute concentrations of HMW adiponectin and the proportion of HMW adiponectin were increased.103 Notably, adiponectin levels are also elevated in patients with anorexia nervosa.111–112
Regulation of Adiponectin by Peroxisome Proliferator-Activated Receptors
Peroxisome proliferator-activated receptors alpha, delta and gamma (PPAR-α, -δ and -γ) are ligand-activated nuclear receptors involved in the regulation of lipid and carbohydrate metabolism and adipogenesis.113 PPARs are thought to function as sensors for dietary fatty acids and their metabolic derivatives,114 and are also activated by synthetic ligands such as fibrates (PPAR-α), GW501516 (PPAR-δ) and TZDs (PPAR-γ). Both fibrates and TZDs increase adiponectin concentrations in humans115–117 through an induction of adiponectin mRNA in adipose tissue.76,118 These effects are mediated through a functional peroxisome proliferator response element in the proximal adiponectin promoter.119 Adipose-specific PPAR-γ knockout mice have reduced plasma adiponectin concentrations.120
Of the dietary fatty acids, n-3 (or omega-3) polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have received a great deal of attention as prophylactic agents for cardiovascular disease and insulin resistance.121 DHA is a ligand for both PPAR-α and -γ,122 and oxidized EPA is a potent regulator of PPAR-α.123 n-3 fatty acids readily undergo oxidation at ambient temperatures, even in the absence of exogenous oxidizing reagents. In rats, the addition of high levels of EPA/DHA as part of a high-fat diet (15% of the 35% fat by weight) for five weeks increased adiponectin concentrations by approximately 20 to 30%.124 In ob/ob mice, similarly, consumption of EPA at 5% of diet by weight for four weeks reduced adipocyte size and increased adiponectin concentrations.125 Coculture experiments, involving both 3T3-L1 adipocytes and macrophages, have recently shown that the stimulatory effects of EPA on adiponectin concentrations are likely to involve inhibition of TNF-α secretion from neighboring macrophages rather than direct effects on adiponectin mRNA in adipocytes.125 Interestingly, micromolar concentrations of EPA have also been shown to induce PPAR-γ1 mRNA in isolated human subcutaneous adipocytes,126 suggesting an additional mechanism by with EPA could increase adiponectin concentrations. In obese humans with the metabolic syndrome, Itoh et al.125 recently found that consumption of 1.8g/day of highly-purified EPA for three months increased adiponectin concentrations by approximately 60%. The relationship between n-3 fatty acid intake and adiponectin concentrations is further supported by the observation that plasma DHA levels, which are indicative of dietary DHA intake, are proportional to adiponectin concentrations in humans.127
Fish oil is rich in both EPA and DHA, and in rodents, dietary supplementation with fish oil improves insulin resistance with up to a two-fold increase of adiponectin concentrations.128,129 This effect likely involves PPAR-γ rather than PPAR-α, as fish oil–mediated increases in plasma adiponectin concentrations were prevented by pharmacological inhibition of PPAR-γ, and were still observed in PPAR-α-null mice.129 In humans, while diets high in fish oil or n-3 PUFAs themselves are associated with a reduced risk of cardiovascular disease,130,131 studies have yet to conclusively demonstrate that they increase adiponectin concentrations132 independently of weight loss.81 The constrasting effects of EPA125 and fish oil81 on adiponectin concentrations in humans may be due to the use in the former study of highly-purified EPA preparations or subjects with preexisting metabolic syndrome components. However, the amounts of EPA administered were similar in both studies. Further investigation will be required to conclusively determine whether dietary supplementation with fish oil/EPA/DHA increases adiponectin concentrations in humans.
Another dietary fatty acid, conjugated linoleic acid (CLA), has been intensively studied for its effects to reduce fat mass,133 especially in mice.134 Dietary CLA is derived from dairy products and ruminant meats, such as beef and lamb, and when added to human diets, it has been shown to modestly reduce adiposity.135 Studies in isolated rat adipocytes have shown that CLA inhibits adiponectin production, possibly via reductions in adipocyte glucose utilization and PPAR-γ mRNA expression.136
Regulation of Adiponectin by Other Pharmacological Agents
Treatment of hypertension by inhibition of the renin-angiotensin system, using either angiotensin converting enzyme (ACE) inhibitors or angiotensin type 1 receptor (AT1R) blockers, concomitantly improves insulin sensitivity and reduces new-onset type 2 diabetes in humans.137 The insulin-sensitizing effects of ACE inhibitors and AT1R blockers are likely to involve adiponectin, as the low adiponectin concentrations observed in hypertensive patients11 are increased by treatment with these compounds.138,139 There are several potential mechanisms involved: angiotensin II inhibits differentiation of adipocytes, via the AT1 receptor,140 and also inhibits key elements of the insulin signaling pathway in cultured smooth muscle cells.141 Accordingly, treatment of obese rats with AT1R blockers not only increases adipocyte differentiation but also reduces TNF-α expression in adipose tissue.142 AT1R blockers have also been shown to be partial agonists of PPAR-γ,143,144 and their ability to increase adiponectin concentrations may be, at least partially PPAR-γ–dependent, as eprosartan, which does not activate PPAR-γ, had no effect on adiponectin secretion from 3T3-L1 adipocytes.145 Interestingly, olmesartan, another AT1R blocker, prevented decreases in circulating adiponectin levels in genetically and diet-induced obese mice by reducing oxidative stress in adipose tissue.146
Adiponectin secretion is also influenced by the endocannabinoid system. Receptors for endocannabinoids are present on human adipocytes,147,148 and treatment with the CB1 antagonist, rimonabant, increases adiponectin mRNA and protein in cultured mouse adipocytes.147 In human patients, rimonabant treatment not only reduces body weight and waist circumference, but also increases adiponectin concentrations and HDL-cholesterol.149 Notably, the increases in adiponectin concentrations are larger than would be expected from the magnitude of the reductions of body weight and adiposity. Although it has been approved and is used in treatment of the metabolic syndrome in 38 countries worldwide, rimonabant has not been approved for use in the U.S., due to concerns about psychiatric side effects (depression and suicidal thoughts) reported in some patients.
Adiponectin secretion in cultured adipocytes and in mice is inhibited by treatment with valproic acid, an anticonvulsant agent used therapeutically for the treatment of epilepsy.150 Common side effects of valproate treatment in humans include obesity151 and insulin resistance.152 Valproic acid inhibits adiponectin gene expression and decreases plasma adiponectin levels in mice by reducing the amount of C/EBPα,150 an adipogenic transcription factor which stimulates adiponectin transcription.46,153
Endocrine Influences on Adiponectin Concentrations
Adiponectin concentrations in rodents and humans are sexually dimorphic, with higher concentrations observed in females compared with males. This appears to be due to a selective increase in the HMW oligomer of the hormone.35 These differences develop during puberty and are a result of inhibition of adiponectin production by circulating androgens.154 In mice, HMW adiponectin concentrations are increased by castration and are decreased by testosterone replacement,155 and testosterone replacement therapy significantly reduces adiponectin concentrations in hypogonadal men.156 Studies in 3T3-L1 adipocytes indicate that testosterone-mediated decreases in adiponectin secretion are due to enhanced intracellular retention of HMW adiponectin.155
Adiponectin concentrations are stable throughout the menstrual cycle.157 During pregnancy, however, both adiponectin mRNA expression and circulating adiponectin concentrations decline during the third trimester158 and postpartum159 when insulin sensitivity is reduced. This may be a mechanism to ensure greater nutrient availability for the developing fetus.160 Reduced adiponectin concentrations during pregnancy do not appear to be attributable to central fat accumulation and weight gain,159 rather, they are likely to result from inhibition by prolactin, which decreases adiponectin content and secretion in cultured human adipocytes and adipose tissue.159,161 Human adipocytes express prolactin receptors,159,162 and elevated prolactin levels in humans have been associated with insulin resistance.163 Support for this inverse relationship has also been obtained in mice: female, but not male, transgenic mice overexpressing prolactin have reduced adiponectin levels.161 Interestingly, adiponectin concentrations are not increased in prolactin receptor–knockout mice,161 suggesting that this particular pathway may exist to favor the suppression of adiponectin, and thereby ensure fetal growth and development.
Adiponectin mRNA expression and secretion in human adipocytes are also inhibited by glucocorticoids.164 In healthy subjects, similarly, acute intravenous administration of 25 mg hydrocortisone transiently decreased adiponectin by approximately 25% after one hour.165 This effect may be dose-dependent, however, as no changes of adiponectin were observed in men treated for 5 days with 3 mg dexamethasone.166 Excessive endogenous glucocorticoid production (Cushing disease) is associated with central obesity and insulin resistance, conditions under which adiponectin concentrations would already be expected to be reduced. However, at the present time, there are no convincing data to suggest that adiponectin levels are reduced in Cushing patients, independently of obesity.165 In fact, Libè et al.167 reported that there were no differences in adiponectin concentrations between normal-weight Cushing patients and BMI-matched control subjects with similar levels of insulin resistance, and adiponectin concentrations did not change after treatment of the disease with transsphenoidal resection of the pituitary adenomas.
The discrepant effects of glucocorticoids on adiponectin observed between in vivo and in vitro studies might possibly be resolved by considering the effects of intracellular steroid metabolism, which appears to be an important determinant of glucocorticoid action. 11β hydroxysteroid dehydrogenase type 1 (11β HSD-1) regulates intracellular glucocorticoid levels by converting inactive cortisone to active cortisol, and its activity is elevated in subcutaneous adipose tissue from obese subjects.168 11β HSD-1 may indirectly regulate adiponectin gene transcription, as adipose-specific overexpression of this enzyme in transgenic mice decreased adiponectin mRNA in mesenteric adipose tissue.169 Conversely, knockout of 11β HSD-1 in all tissues was associated with increased adiponectin mRNA expression in epididymal fat, although not in visceral mesenteric fat.170 Plasma adiponectin concentrations were not measured in either of these mouse studies, however, so the contribution of local glucocorticoid action in regulating circulating adiponectin concentrations remains to be determined.
Adiponectin may also be inhibited by growth hormone (GH). Adiponectin secretion in cultured explants of human adipose tissue is reduced by incubation with GH, and GH-overexpressing transgenic mice of both sexes have lower circulating adiponectin concentrations than wild-type littermates.161 This effect appears to be independent of energy balance and adiposity, as GH-overexpressing transgenic mice have reduced body fat and are resistant to diet-induced weight gain on a high-fat diet,171 conditions when circulating adiponectin concentrations would be expected to be elevated. The inverse relationship between GH and adiponectin in mice is further supported by the observation that GH receptor deficiency is associated with increased adiponectin concentrations in both sexes.161 The underlying mechanism has been recently shown to involve GH-mediated increases in the expression of the p85 subunit of phosphatidylinositol 3-kinase (PI3K), a negative regulator of insulin signaling, in adipose tissue.172 In humans, however, there is presently a lack of consensus on whether elevated GH levels (such as in acromegaly) are associated with reduced adiponectin concentrations.173–176 There is one positive report, however, of patients with acromegaly having low adiponectin levels that were reversed following GH-lowering therapy.177 However, treatment of HIV-associated lipodystropy patients with recombinant human GH increased circulating adiponectin by approximately 20% and the increase of adiponectin was correlated with increases of HDL cholesterol.178
Adiponectin synthesis and secretion also appear to be inhibited by activation of the sympathetic nervous system. Adiponectin gene expression in human visceral adipose tissue is inhibited by β-adrenergic agonists.179 Similarly, in both mouse adipose tissue explants and in vivo, β-adrenergic agonists reduce adiponectin mRNA, secretion and plasma concentrations, with β3-agonists having the greatest effect.179 Consistent with these findings, six months of treatment with rilmenidine, which reduces the firing rate of sympathetic neurons, increased adiponectin concentrations by approximately 35% in hypertensive human subjects, independently of changes in weight or visceral adiposity.180 In contrast, studies examining adiponectin concentrations in response to cold exposure-mediated activation of the sympathetic nervous system have been much less consistent: one study in humans suggests that cold exposure at 10°C decreases adiponectin concentrations after 90 minutes,101 while studies in rodents have reported increases,181 decreases,182 and no change183 in adiponectin mRNA or circulating concentrations in response to cold exposure (18–24 hours at 4–6°C). These discrepancies may reflect the different timepoints studied or species differences in responses to cold exposure.
Finally, a role for bone-derived hormones in the regulation of insulin sensitivity has been suggested by the recent observation that mice deficient in osteocalcin, a hormone secreted by osteoblasts, exhibit glucose intolerance and insulin resistance, likely due to reduced adiponectin concentrations.184 The influence of such osteogenic factors on glucose homeostasis is likely to be an active area of future research.
Together, these observations indicate that during periods of growth, stress, reproduction (and male sexual development), a number of endocrine systems may act to decrease circulating adiponectin concentrations, potentially increasing nutrient availability via a transient reduction in insulin sensitivity. Prolonged suppression of adiponectin production, however, as occurs in response to visceral adipocyte hypertrophy associated with weight gain, may prove maladaptive and lead to the development of insulin resistance and type 2 diabetes in susceptible individuals.
Effects of Inflammation and Oxidative Stress
In humans and in laboratory animals, obesity is frequently characterized by macrophage infiltration into adipose tissue, resulting in chronic, low-grade inflammation.185–187 This inflammatory state, characterized by elevated adipose tissue TNF-α expression188–189 and circulating concentrations of C-reactive protein (CRP),190 interleukin-6 (IL-6),191 and monocyte chemoattractant protein-1 (MCP-1),192 has been implicated in development of many of the complications of severe obesity, in particular, atherosclerosis, insulin resistance, and type 2 diabetes.193 Cross-sectional studies have consistently demonstrated an inverse relationship between adiponectin concentrations and those of inflammatory markers such as CRP, TNF-α, and IL-6.194,195
The inverse relationship between adiponectin and inflammation is well supported by in vitro data, as TNF-α,164,196 IL-6,197 and CRP,198 inhibit adiponectin synthesis in human and murine adipocytes. These inflammatory factors have all been shown to interfere with insulin signaling in adipocytes, reinforcing the idea that adiponectin secretion is likely to be determined by adipocyte insulin sensitivity. TNF-α induces serine phosphorylation of insulin receptor substrate-1 (IRS-1), which inhibits insulin receptor kinase activity and downstream signaling via PI3K activation.199 Inhibition of adiponectin mRNA by CRP is also at least partially dependent upon the PI3K pathway.198 Both IL-6 and TNF-α reduce the expression of IRS-1, GLUT-4, and PPAR-γ in 3T3-L1 adipocytes.200 Furthermore, IL-6 induces the expression of suppressor of cytokine signaling (SOCS) proteins, which inhibit insulin signaling by binding to the insulin receptor and IRS-1.201,202 Lastly, interleukin-15, an anabolic cytokine produced by skeletal muscle, increases adiponectin secretion from 3T3-L1 adipocytes, suggesting the involvement of muscle-to-adipocyte endocrine signaling.203
There are abundant data from studies in mice to indicate that oxidative stress also regulates adiponectin secretion. Oxidative stress is defined as a persistent imbalance between the production of highly reactive molecular species (chiefly oxygen and nitrogen) and the capacity of antioxidant defense systems to inactivate or remove them.204 Oxidative stress is elevated in human obesity and insulin resistance.205 Results obtained from experiments in mice suggest that lipid accumulation in adipocytes, and a concomitant rise in ROS production, may be a key trigger for the development of insulin resistance via reduced adiponectin secretion.206 Exposure of cultured primary rat adipocytes to hyperglycemic conditions (15 mM glucose, 100 nM insulin) increases intracellular nutrient availability and ROS production, leading to a reduction in insulin sensitivity.207 Similarly, exposure of 3T3-L1 adipocytes to hydrogen peroxide, a powerful oxidizing agent, reduces adiponectin mRNA expression within 10 minutes.208 The mechanism(s) linking ROS production to adiponectin secretion are currently under investigation, and studies in 3T3-L1 adipocytes have suggested roles for uncoupling protein-2 (UCP2), a protein which increases mitochondrial respiration, as well as the transcription factor CHOP-10, which interferes with the C/EBP-binding region in the promoter of the adiponectin gene.209
Cigarette smoking also reduces adiponectin concentrations.210–212 Acute exposure to cigarette smoke significantly reduced adiponectin levels by 9% after 3 hours, and the maximum decrease (15%) was observed after 12 hours.211 There is some in vitro evidence to suggest that smoking suppresses adiponectin secretion, either via generation of ROS or by direct effects of nicotine on adipocytes,211 but this may also be due to smoking-related inflammation,213 tissue hypoxia,214 or possibly via activation of the sympathetic nervous system activity via nicotinic receptors in sympathetic ganglia.179 The inhibitory effects of smoking on adiponectin contrast unfavorably with those of alcohol consumption, as several studies have shown that adiponectin concentrations are increased by moderate alcohol consumption.215,217
Conclusions
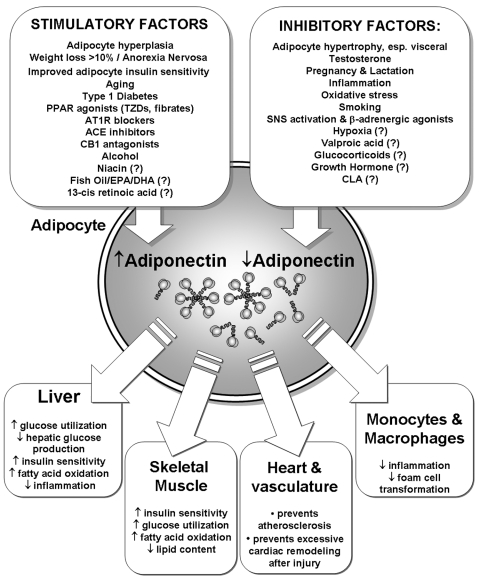
In the decade since its discovery, the adipocyte-derived hormone adiponectin has been revealed to be a key component in the relationships between excess adiposity, insulin resistance, inflammation and cardiovascular disease. Decreased adiponectin production by adipocytes, leading to reduced circulating adiponectin levels, is likely to be an important mechanism by which visceral adipose deposition and a number of other hormones promote insulin resistance in extra-adipose tissues, such as liver and skeletal muscle (Figure 1). Adiponectin concentrations are reduced in obesity, pregnancy, inflammation, and states of metabolic and oxidative stress, while adiponectin levels are increased following weight loss and in anorexia nervosa. Collectively, these observations can be drawn together by the recent proposal that increases of adiponectin may act as a systemic “starvation signal”218 indicating the availability of excess storage capacity in adipocytes.219 As discussed by Behre,218 in starvation the presence of high adiponectin concentrations in concert with reduced insulin-stimulated glucose uptake.112 would act to increase lipid oxidation in liver and muscle, limiting the use of amino acids as a source of energy and sparing carbohydrate (glucose) for use by the CNS. As a consequence of its actions to promote lipid metabolism as an energy source, adiponectin is able to prevent the ectopic deposition of triglyceride in liver and skeletal muscle, which can occur in obesity and is associated with the development of insulin resistance in these tissues.220,221 The “starvation signal” hypothesis is further supported by data indicating that adiponectin can stimulate food intake in mice by enhancing hypothalamic AMP kinase activity,33 however the question of whether adiponectin has physiological actions within the CNS is still controversial. Nonetheless, strategies directed at increasing adiponectin production and its circulating concentrations, whether by lifestyle interventions (diet and weight loss), pharmacological therapy, or possibly with nutritional supplements, will likely be effective approaches for the prevention and treatment of insulin resistance/metabolic syndrome, type 2 diabetes, and cardiovascular disease, diseases that are rapidly increasing in prevalence worldwide.
FIG. 1.
Summary of factors that regulate adiponectin concentrations in humans. Factors with limited or conflicting data in humans (including extended-release niacin222 and 13-cis-retinoic acid223) are indicated with a question mark.
Acknowledgments
Peter Havel's laboratory receives research support from National Institutes of Health Grants HL-075675, AT-002599, AT-002993, and AT-003645, and from the American Diabetes Association. The authors do not have any conflicts of interest to declare. Michael Swarbrick is supported by a New Investigator Research Award from NAASO, the Obesity Society.
References
- 1.Trujillo ME. Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 2.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53:S143–151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE. Williams S. Fogliano M. Baldini G. Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 4.Hu E. Liang P. Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K. Okubo K. Shimomura I. Funahashi T. Matsuzawa Y. Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 6.Nakano Y. Tobe T. Choi-Miura NH. Mazda T. Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem (Tokyo) 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 7.Arita Y. Kihara S. Ouchi N. Takahashi M. Maeda K. Miyagawa J. Hotta K. Shimomura I. Nakamura T. Miyaoka K. Kuriyama H. Nishida M. Yamashita S. Okubo K. Matsubara K. Muraguchi M. Ohmoto Y. Funahashi T. Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 8.Hotta K. Funahashi T. Arita Y. Takahashi M. Matsuda M. Okamoto Y. Iwahashi H. Kuriyama H. Ouchi N. Maeda K. Nishida M. Kihara S. Sakai N. Nakajima T. Hasegawa K. Muraguchi M. Ohmoto Y. Nakamura T. Yamashita S. Hanafusa T. Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 9.Haque WA. Shimomura I. Matsuzawa Y. Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 10.Targher G. Bertolini L. Scala L. Poli F. Zenari L. Falezza G. Decreased plasma adiponectin concentrations are closely associated with nonalcoholic hepatic steatosis in obese individuals. Clin Endocrinol (Oxf) 2004;61:700–703. doi: 10.1111/j.1365-2265.2004.02151.x. [DOI] [PubMed] [Google Scholar]
- 11.Adamczak M. Wiecek A. Funahashi T. Chudek J. Kokot F. Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16:72–75. doi: 10.1016/s0895-7061(02)03197-7. [DOI] [PubMed] [Google Scholar]
- 12.Kumada M. Kihara S. Sumitsuji S. Kawamoto T. Matsumoto S. Ouchi N. Arita Y. Okamoto Y. Shimomura I. Hiraoka H. Nakamura T. Funahashi T. Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 13.Spranger J. Kroke A. Mohlig M. Bergmann MM. Ristow M. Boeing H. Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 14.Pischon T. Girman CJ. Hotamisligil GS. Rifai N. Hu FB. Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 15.Cnop M. Havel PJ. Utzschneider KM. Carr DB. Sinha MK. Boyko EJ. Retzlaff BM. Knopp RH. Brunzell JD. Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 16.Imagawa A. Funahashi T. Nakamura T. Moriwaki M. Tanaka S. Nishizawa H. Sayama K. Uno S. Iwahashi H. Yamagata K. Miyagawa J. Matsuzawa Y. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care. 2002;25:1665–1666. doi: 10.2337/diacare.25.9.1665. [DOI] [PubMed] [Google Scholar]
- 17.Kusminski CM. McTernan PG. Schraw T. Kos K. O'Hare J P. Ahima R. Kumar S. Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634–642. doi: 10.1007/s00125-006-0577-9. [DOI] [PubMed] [Google Scholar]
- 18.Kos K. Harte AL. da Silva NF. Tonchev A. Chaldakov G. James S. Snead DR. Hoggart B. O'Hare JP. McTernan PG. Kumar S. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 19.Combs TP. Berg AH. Obici S. Scherer PE. Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi T. Kamon J. Minokoshi Y. Ito Y. Waki H. Uchida S. Yamashita S. Noda M. Kita S. Ueki K. Eto K. Akanuma Y. Froguel P. Foufelle F. Ferre P. Carling D. Kimura S. Nagai R. Kahn BB. Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 21.Ouchi N. Kihara S. Arita Y. Maeda K. Kuriyama H. Okamoto Y. Hotta K. Nishida M. Takahashi M. Nakamura T. Yamashita S. Funahashi T. Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N. Kihara S. Arita Y. Okamoto Y. Maeda K. Kuriyama H. Hotta K. Nishida M. Takahashi M. Muraguchi M. Ohmoto Y. Nakamura T. Yamashita S. Funahashi T. Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N. Kihara S. Arita Y. Nishida M. Matsuyama A. Okamoto Y. Ishigami M. Kuriyama H. Kishida K. Nishizawa H. Hotta K. Muraguchi M. Ohmoto Y. Yamashita S. Funahashi T. Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto Y. Kihara S. Ouchi N. Nishida M. Arita Y. Kumada M. Ohashi K. Sakai N. Shimomura I. Kobayashi H. Terasaka N. Inaba T. Funahashi T. Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi T. Kamon J. Waki H. Imai Y. Shimozawa N. Hioki K. Uchida S. Ito Y. Takakuwa K. Matsui J. Takata M. Eto K. Terauchi Y. Komeda K. Tsunoda M. Murakami K. Ohnishi Y. Naitoh T. Yamamura K. Ueyama Y. Froguel P. Kimura S. Nagai R. Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 26.Shibata R. Ouchi N. Ito M. Kihara S. Shiojima I. Pimentel DR. Kumada M. Sato K. Schiekofer S. Ohashi K. Funahashi T. Colucci WS. Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata R. Sato K. Pimentel DR. Takemura Y. Kihara S. Ohashi K. Funahashi T. Ouchi N. Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamauchi T. Kamon J. Ito Y. Tsuchida A. Yokomizo T. Kita S. Sugiyama T. Miyagishi M. Hara K. Tsunoda M. Murakami K. Ohteki T. Uchida S. Takekawa S. Waki H. Tsuno NH. Shibata Y. Terauchi Y. Froguel P. Tobe K. Koyasu S. Taira K. Kitamura T. Shimizu T. Nagai R. Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T. Nio Y. Maki T. Kobayashi M. Takazawa T. Iwabu M. Okada-Iwabu M. Kawamoto S. Kubota N. Kubota T. Ito Y. Kamon J. Tsuchida A. Kumagai K. Kozono H. Hada Y. Ogata H. Tokuyama K. Tsunoda M. Ide T. Murakami K. Awazawa M. Takamoto I. Froguel P. Hara K. Tobe K. Nagai R. Ueki K. Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 30.Hug C. Wang J. Ahmad NS. Bogan JS. Tsao TS. Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spranger J. Verma S. Gohring I. Bobbert T. Seifert J. Sindler AL. Pfeiffer A. Hileman SM. Tschop M. Banks WA. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. [PubMed] [Google Scholar]
- 32.Qi Y. Takahashi N. Hileman SM. Patel HR. Berg AH. Pajvani UB. Scherer PE. Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 33.Kubota N. Yano W. Kubota T. Yamauchi T. Itoh S. Kumagai H. Kozono H. Takamoto I. Okamoto S. Shiuchi T. Suzuki R. Satoh H. Tsuchida A. Moroi M. Sugi K. Noda T. Ebinuma H. Ueta Y. Kondo T. Araki E. Ezaki O. Nagai R. Tobe K. Terauchi Y. Ueki K. Minokoshi Y. Kadowaki T. Adiponectin Stimulates AMP-Activated Protein Kinase in the Hypothalamus and Increases Food Intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Kishore U. Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 35.Pajvani UB. Du X. Combs TP. Berg AH. Rajala MW. Schulthess T. Engel J. Brownlee M. Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki S. Wilson-Kubalek EM. Wert D. Tsao TS. Lee DH. The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 2007;581:809–814. doi: 10.1016/j.febslet.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waki H. Yamauchi T. Kamon J. Ito Y. Uchida S. Kita S. Hara K. Hada Y. Vasseur F. Froguel P. Kimura S. Nagai R. Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 38.Fruebis J. Tsao TS. Javorschi S. Ebbets-Reed D. Erickson MR. Yen FT. Bihain BE. Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pajvani UB. Hawkins M. Combs TP. Rajala MW. Doebber T. Berger JP. Wagner JA. Wu M. Knopps A. Xiang AH. Utzschneider KM. Kahn SE. Olefsky JM. Buchanan TA. Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 40.Tsao TS. Tomas E. Murrey HE. Hug C. Lee DH. Ruderman NB. Heuser JE. Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure, signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 41.Tomas E. Tsao TS. Saha AK. Murrey HE. Zhang Cc C. Itani SI. Lodish HF. Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neumeier M. Weigert J. Schaffler A. Wehrwein G. Muller-Ladner U. Scholmerich J. Wrede C. Buechler C. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 43.Haugen F. Drevon CA. Activation of nuclear factor-κB by high molecular weight and globular adiponectin. Endocrinology. 2007;148:5478–5486. doi: 10.1210/en.2007-0370. [DOI] [PubMed] [Google Scholar]
- 44.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 45.Combs TP. Pajvani UB. Berg AH. Lin Y. Jelicks LA. Laplante M. Nawrocki AR. Rajala MW. Parlow AF. Cheeseboro L. Ding YY. Russell RG. Lindemann D. Hartley A. Baker GR. Obici S. Deshaies Y. Ludgate M. Rossetti L. Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 46.Qiao L. Maclean PS. Schaack J. Orlicky DJ. Darimont C. Pagliassotti M. Friedman JE. Shao J. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes. 2005;54:1744–1754. doi: 10.2337/diabetes.54.6.1744. [DOI] [PubMed] [Google Scholar]
- 47.Kita A. Yamasaki H. Kuwahara H. Moriuchi A. Fukushima K. Kobayashi M. Fukushima T. Takahashi R. Abiru N. Uotani S. Kawasaki E. Eguchi K. Identification of the promoter region required for human adiponectin gene transcription: Association with CCAAT/enhancer binding protein-beta and tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2005;331:484–490. doi: 10.1016/j.bbrc.2005.03.205. [DOI] [PubMed] [Google Scholar]
- 48.Qiao L. Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 49.Barth N. Langmann T. Scholmerich J. Schmitz G. Schaffler A. Identification of regulatory elements in the human adipose most abundant gene transcript-1 (apM-1) promoter: role of SP1/SP3 and TNF-alpha as regulatory pathways. Diabetologia. 2002;45:1425–1433. doi: 10.1007/s00125-002-0895-5. [DOI] [PubMed] [Google Scholar]
- 50.Fisher FM. McTernan PG. Valsamakis G. Chetty R. Harte AL. Anwar AJ. Starcynski J. Crocker J. Barnett AH. McTernan CL. Kumar S. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res. 2002;34:650–654. doi: 10.1055/s-2002-38246. [DOI] [PubMed] [Google Scholar]
- 51.Statnick MA. Beavers LS. Conner LJ. Corominola H. Johnson D. Hammond CD. Rafaeloff-Phail R. Seng T. Suter TM. Sluka JP. Ravussin E. Gadski RA. Caro JF. Decreased expression of apM1 in omental and subcutaneous adipose tissue of humans with type 2 diabetes. Int J Exp Diabetes Res. 2000;1:81–88. doi: 10.1155/EDR.2000.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ranheim T. Haugen F. Staff AC. Braekke K. Harsem NK. Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 53.Combs TP. Berg AH. Rajala MW. Klebanov S. Iyengar P. Jimenez-Chillaron JC. Patti ME. Klein SL. Weinstein RS. Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 54.Behre CJ. Gummesson A. Jernas M. Lystig TC. Fagerberg B. Carlsson B. Carlsson LM. Dissociation between adipose tissue expression and serum levels of adiponectin during and after diet-induced weight loss in obese subjects with and without the metabolic syndrome. Metabolism. 2007;56:1022–1028. doi: 10.1016/j.metabol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y. Xu A. Knight C. Xu LY. Cooper GJ. Hydroxylation, glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–19529. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 56.Richards AA. Stephens T. Charlton HK. Jones A. Macdonald GA. Prins JB. Whitehead JP. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20:1673–1687. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y. Lam KS. Chan L. Chan KW. Lam JB. Lam MC. Hoo RC. Mak WW. Cooper GJ. Xu A. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 58.Wang ZV. Schraw TD. Kim JY. Khan T. Rajala MW. Follenzi A. Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anelli T. Alessio M. Bachi A. Bergamelli L. Bertoli G. Camerini S. Mezghrani A. Ruffato E. Simmen T. Sitia R. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. Embo J. 2003;22:5015–5022. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bauche IB. Ait El Mkadem S. Rezsohazy R. Funahashi T. Maeda N. Miranda LM. Brichard SM. Adiponectin down-regulates its own production and the expression of its AdipoR2 receptor in transgenic mice. Biochem Biophys Res Commun. 2006;345:1414–1424. doi: 10.1016/j.bbrc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 61.Looker HC. Krakoff J. Funahashi T. Matsuzawa Y. Tanaka S. Nelson RG. Knowler WC. Lindsay RS. Hanson RL. Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:4010–4017. doi: 10.1210/jc.2003-031916. [DOI] [PubMed] [Google Scholar]
- 62.Shen YY. Charlesworth JA. Kelly JJ. Loi KW. Peake PW. Up-regulation of adiponectin, its isoforms and receptors in end-stage kidney disease. Nephrol Dial Transplant. 2007;22:171–178. doi: 10.1093/ndt/gfl552. [DOI] [PubMed] [Google Scholar]
- 63.Zoccali C. Mallamaci F. Tripepi G. Benedetto FA. Cutrupi S. Parlongo S. Malatino LS. Bonanno G. Seminara G. Rapisarda F. Fatuzzo P. Buemi M. Nicocia G. Tanaka S. Ouchi N. Kihara S. Funahashi T. Matsuzawa Y. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 64.Park KG. Park KS. Kim MJ. Kim HS. Suh YS. Ahn JD. Park KK. Chang YC. Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135–142. doi: 10.1016/j.diabres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Yatagai T. Nagasaka S. Taniguchi A. Fukushima M. Nakamura T. Kuroe A. Nakai Y. Ishibashi S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 66.Bacha F. Saad R. Gungor N. Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 67.Lihn AS. Bruun JM. He G. Pedersen SB. Jensen PF. Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219:9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Yang WS. Chen MH. Lee WJ. Lee KC. Chao CL. Huang KC. Chen CL. Tai TY. Chuang LM. Adiponectin mRNA levels in the abdominal adipose depots of nondiabetic women. Int J Obes Relat Metab Disord. 2003;27:896–900. doi: 10.1038/sj.ijo.0802367. [DOI] [PubMed] [Google Scholar]
- 69.Fredriksson J. Carlsson E. Orho-Melander M. Groop L. Ridderstrale M. A polymorphism in the adiponectin gene influences adiponectin expression levels in visceral fat in obese subjects. Int J Obes (Lond) 2006;30:226–232. doi: 10.1038/sj.ijo.0803138. [DOI] [PubMed] [Google Scholar]
- 70.Motoshima H. Wu X. Sinha MK. Hardy VE. Rosato EL. Barbot DJ. Rosato FE. Goldstein BJ. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 71.Hoffstedt J. Arvidsson E. Sjolin E. Wahlen K. Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004;89:1391–1396. doi: 10.1210/jc.2003-031458. [DOI] [PubMed] [Google Scholar]
- 72.Bullen JW., Jr. Bluher S. Kelesidis T. Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2007;292:E1079–1086. doi: 10.1152/ajpendo.00245.2006. [DOI] [PubMed] [Google Scholar]
- 73.Bahceci M. Gokalp D. Bahceci S. Tuzcu A. Atmaca S. Arikan S. The correlation between adiposity, adiponectin, tumor necrosis factor alpha, interleukin-6, high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–214. doi: 10.1007/BF03347427. [DOI] [PubMed] [Google Scholar]
- 74.Salans LB. Knittle JL. Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968;47:153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okuno A. Tamemoto H. Tobe K. Ueki K. Mori Y. Iwamoto K. Umesono K. Akanuma Y. Fujiwara T. Horikoshi H. Yazaki Y. Kadowaki T. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeda N. Takahashi M. Funahashi T. Kihara S. Nishizawa H. Kishida K. Nagaretani H. Matsuda M. Komuro R. Ouchi N. Kuriyama H. Hotta K. Nakamura T. Shimomura I. Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 77.Stanhope KL. Graham JL. Sinha M. Peter HJ. Low circulating adiponectin levels and reduced adipocyte adiponectin production in obese, insulin-resistant Sprague-Dawley rats. Diabetes. 2002;51:A404–A404. [Google Scholar]
- 78.Skurk T. Alberti-Huber C. Herder C. Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 79.Gavrila A. Peng CK. Chan JL. Mietus JE. Goldberger AL. Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 80.Calvani M. Scarfone A. Granato L. Mora EV. Nanni G. Castagneto M. Greco AV. Manco M. Mingrone G. Restoration of adiponectin pulsatility in severely obese subjects after weight loss. Diabetes. 2004;53:939–947. doi: 10.2337/diabetes.53.4.939. [DOI] [PubMed] [Google Scholar]
- 81.Kratz M. Swarbrick MM. Callahan HS. Matthys CC. Havel PJ. Weigle DS. Effect of dietary n-3-polyunsaturated fatty acids on plasma total and high molecular weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr. 2008;87:347–353. doi: 10.1093/ajcn/87.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubin D. Helwig U. Nothnagel M. Lemke N. Schreiber S. Folsch UR. Doring F. Schrezenmeir J. Postprandial plasma adiponectin decreases after glucose and high fat meal and is independently associated with postprandial triacylglycerols but not with—11388 promoter polymorphism. Br J Nutr. 2007;99:76–82. doi: 10.1017/S0007114507791857. [DOI] [PubMed] [Google Scholar]
- 83.Shand B. Elder P. Scott R. Frampton C. Willis J. Biovariability of plasma adiponectin. Clin Chem Lab Med. 2006;44:1264–1268. doi: 10.1515/CCLM.2006.227. [DOI] [PubMed] [Google Scholar]
- 84.Peake PW. Kriketos AD. Denyer GS. Campbell LV. Charlesworth JA. The postprandial response of adiponectin to a high-fat meal in normal and insulin-resistant subjects. Int J Obes Relat Metab Disord. 2003;27:657–662. doi: 10.1038/sj.ijo.0802289. [DOI] [PubMed] [Google Scholar]
- 85.Merl V. Peters A. Oltmanns KM. Kern W. Born J. Fehm HL. Schultes B. Serum adiponectin concentrations during a 72-hour fast in over- and normal-weight humans. Int J Obes (Lond) 2005;29:998–1001. doi: 10.1038/sj.ijo.0802971. [DOI] [PubMed] [Google Scholar]
- 86.English PJ. Coughlin SR. Hayden K. Malik IA. Wilding JP. Plasma adiponectin increases postprandially in obese, but not in lean, subjects. Obes Res. 2003;11:839–844. doi: 10.1038/oby.2003.115. [DOI] [PubMed] [Google Scholar]
- 87.Teff KL. Petrova M. Havel PJ. Townsend RR. 48-h glucose infusion in humans: effect on hormonal responses, hunger and food intake. Physiol Behav. 2007;90:733–743. doi: 10.1016/j.physbeh.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sinha MK. Ohannesian JP. Heiman ML. Kriauciunas A. Stephens TW. Magosin S. Marco C. Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weyer C. Funahashi T. Tanaka S. Hotta K. Matsuzawa Y. Pratley RE. Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 90.Basu R. Pajvani UB. Rizza RA. Scherer PE. Selective down-regulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 91.Yu JG. Javorschi S. Hevener AL. Kruszynska YT. Norman RA. Sinha M. Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 92.Mohlig M. Wegewitz U. Osterhoff M. Isken F. Ristow M. Pfeiffer AF. Spranger J. Insulin decreases human adiponectin plasma levels. Horm Metab Res. 2002;34:655–658. doi: 10.1055/s-2002-38248. [DOI] [PubMed] [Google Scholar]
- 93.Brame LA. Considine RV. Yamauchi M. Baron AD. Mather KJ. Insulin and endothelin in the acute regulation of adiponectin in vivo in humans. Obes Res. 2005;13:582–588. doi: 10.1038/oby.2005.62. [DOI] [PubMed] [Google Scholar]
- 94.Bogan JS. Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderlova K. Kremen J. Dolezalova R. Housova J. Haluzikova D. Kunesova M. Haluzik M. The influence of very-low-calorie-diet on serum leptin, soluble leptin receptor, adiponectin and resistin levels in obese women. Physiol Res. 2006;55:277–283. doi: 10.33549/physiolres.930779. [DOI] [PubMed] [Google Scholar]
- 96.Imbeault P. Pomerleau M. Harper ME. Doucet E. Unchanged fasting and postprandial adiponectin levels following a 4-day caloric restriction in young healthy men. Clin Endocrinol (Oxf) 2004;60:429–433. doi: 10.1111/j.1365-2265.2004.01997.x. [DOI] [PubMed] [Google Scholar]
- 97.Havel PJ. Stanhope KL. Sinha M. Dubuc GR. Phinney SD. Gender differences in circulating adiponectin concentrations and in adiponectin responses to 7 days of energy restriction in normal weight men and women. Diabetes. 2002;51:A454–A454. [Google Scholar]
- 98.Wolfe BE. Jimerson DC. Orlova C. Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 2004;61:332–338. doi: 10.1111/j.1365-2265.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 99.Hulver MW. Zheng D. Tanner CJ. Houmard JA. Kraus WE. Slentz CA. Sinha MK. Pories WJ. MacDonald KG. Dohm GL. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283:E861–865. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- 100.Polak J. Klimcakova E. Moro C. Viguerie N. Berlan M. Hejnova J. Richterova B. Kraus I. Langin D. Stich V. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55:1375–1381. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 101.Imbeault P. Environmental influences on adiponectin levels in humans. Appl Physiol Nutr Metab. 2007;32:505–511. doi: 10.1139/H07-017. [DOI] [PubMed] [Google Scholar]
- 102.Kotidis EV. Koliakos GG. Baltzopoulos VG. Ioannidis KN. Yovos JG. Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—a prospective study. Obes Surg. 2006;16:1425–1432. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 103.Swarbrick MM. Austrheim-Smith IT. Stanhope KL. Van Loan MD. Ali MR. Wolfe BM. Havel PJ. Circulating concentrations of high-molecular-weight adiponectin are increased following Roux-en-Y gastric bypass surgery. Diabetologia. 2006;49:2552–2558. doi: 10.1007/s00125-006-0452-8. [DOI] [PubMed] [Google Scholar]
- 104.Lin E. Phillips LS. Ziegler TR. Schmotzer B. Wu K. Gu LH. Khaitan L. Lynch SA. Torres WE. Smith CD. Gletsu-Miller N. Increases in adiponectin predict improved liver, but not peripheral, insulin sensitivity in severely obese women during weight loss. Diabetes. 2007;56:735–742. doi: 10.2337/db06-1161. [DOI] [PubMed] [Google Scholar]
- 105.Kopp HP. Krzyzanowska K. Mohlig M. Spranger J. Pfeiffer AF. Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond) 2005;29:766–771. doi: 10.1038/sj.ijo.0802983. [DOI] [PubMed] [Google Scholar]
- 106.Santosa S. Demonty I. Lichtenstein AH. Cianflone K. Jones PJ. An investigation of hormone and lipid associations after weight loss in women. J Am Coll Nutr. 2007;26:250–258. doi: 10.1080/07315724.2007.10719608. [DOI] [PubMed] [Google Scholar]
- 107.Jung SH. Park HS. Kim KS. Choi WH. Ahn CW. Kim BT. Kim SM. Lee SY. Ahn SM. Kim YK. Kim HJ. Kim DJ. Lee KW. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2007 Jul 3; doi: 10.1016/j.jnutbio.2007.05.007. Prepublished on. as DOI. [DOI] [PubMed] [Google Scholar]
- 108.Bobbert T. Rochlitz H. Wegewitz U. Akpulat S. Mai K. Weickert MO. Mohlig M. Pfeiffer AF. Spranger J. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 109.Polak J. Kovacova Z. Jacek M. Klimcakova E. Kovacikova M. Vitkova M. Kuda O. Sebela M. Samcova E. Stich V. An increase in plasma adiponectin multimeric complexes follows hypocaloric diet-induced weight loss in obese and overweight pre-menopausal women. Clin Sci (Lond) 2007;112:557–565. doi: 10.1042/CS20060296. [DOI] [PubMed] [Google Scholar]
- 110.O'Leary VB. Jorett AE. Marchetti CM. Gonzalez F. Phillips SA. Ciaraldi TP. Kirwan JP. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am J Physiol Endocrinol Metab. 2007;293:E421–427. doi: 10.1152/ajpendo.00123.2007. [DOI] [PubMed] [Google Scholar]
- 111.Delporte ML. Brichard SM. Hermans MP. Beguin C. Lambert M. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol (Oxf) 2003;58:22–29. doi: 10.1046/j.1365-2265.2003.01702.x. [DOI] [PubMed] [Google Scholar]
- 112.Pannacciulli N. Vettor R. Milan G. Granzotto M. Catucci A. Federspil G. De Giacomo P. Giorgino R. De Pergola G. Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab. 2003;88:1748–1752. doi: 10.1210/jc.2002-021215. [DOI] [PubMed] [Google Scholar]
- 113.Ferre P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53:S43–50. doi: 10.2337/diabetes.53.2007.s43. [DOI] [PubMed] [Google Scholar]
- 114.Evans RM. Barish GD. Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 115.Hiuge A. Tenenbaum A. Maeda N. Benderly M. Kumada M. Fisman EZ. Tanne D. Matas Z. Hibuse T. Fujita K. Nishizawa H. Adler Y. Motro M. Kihara S. Shimomura I. Behar S. Funahashi T. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler Thromb Vasc Biol. 2007;27:635–641. doi: 10.1161/01.ATV.0000256469.06782.d5. [DOI] [PubMed] [Google Scholar]
- 116.Hirose H. Kawai T. Yamamoto Y. Taniyama M. Tomita M. Matsubara K. Okazaki Y. Ishii T. Oguma Y. Takei I. Saruta T. Effects of pioglitazone on metabolic parameters, body fat distribution, and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metabolism. 2002;51:314–317. doi: 10.1053/meta.2002.30506. [DOI] [PubMed] [Google Scholar]
- 117.Yilmaz MI. Sonmez A. Caglar K. Gok DE. Eyileten T. Yenicesu M. Acikel C. Bingol N. Kilic S. Oguz Y. Vural A. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist increases plasma adiponectin levels in type 2 diabetic patients with proteinuria. Endocrine. 2004;25:207–214. doi: 10.1385/ENDO:25:3:207. [DOI] [PubMed] [Google Scholar]
- 118.Combs TP. Wagner JA. Berger J. Doebber T. Wang WJ. Zhang BB. Tanen M. Berg AH. O'Rahilly S. Savage DB. Chatterjee K. Weiss S. Larson PJ. Gottesdiener KM. Gertz BJ. Charron MJ. Scherer PE. Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 119.Iwaki M. Matsuda M. Maeda N. Funahashi T. Matsuzawa Y. Makishima M. Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 120.He W. Barak Y. Hevener A. Olson P. Liao D. Le J. Nelson M. Ong E. Olefsky JM. Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruxton CH. Reed SC. Simpson MJ. Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet. 2007;20:275–285. doi: 10.1111/j.1365-277X.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 122.Krey G. Braissant O. L'Horset F. Kalkhoven E. Perroud M. Parker MG. Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 123.Sethi S. Ziouzenkova O. Ni H. Wagner DD. Plutzky J. Mayadas TN. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood. 2002;100:1340–1346. doi: 10.1182/blood-2002-01-0316. [DOI] [PubMed] [Google Scholar]
- 124.Flachs P. Mohamed-Ali V. Horakova O. Rossmeisl M. Hosseinzadeh-Attar MJ. Hensler M. Ruzickova J. Kopecky J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 125.Itoh M. Suganami T. Satoh N. Tanimoto-Koyama K. Yuan X. Tanaka M. Kawano H. Yano T. Aoe S. Takeya M. Shimatsu A. Kuzuya H. Kamei Y. Ogawa Y. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 126.Chambrier C. Bastard JP. Rieusset J. Chevillotte E. Bonne-font-Rousselot D. Therond P. Hainque B. Riou JP. Laville M. Vidal H. Eicosapentaenoic acid induces mRNA expression of peroxisome proliferator-activated receptor gamma. Obes Res. 2002;10:518–525. doi: 10.1038/oby.2002.70. [DOI] [PubMed] [Google Scholar]
- 127.Fernandez-Real JM. Vendrell J. Ricart W. Circulating adiponectin and plasma fatty acid profile. Clin Chem. 2005;51:603–609. doi: 10.1373/clinchem.2004.041350. [DOI] [PubMed] [Google Scholar]
- 128.Rossi AS. Lombardo YB. Lacorte JM. Chicco AG. Rouault C. Slama G. Rizkalla SW. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R486–R494. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]
- 129.Neschen S. Morino K. Rossbacher JC. Pongratz RL. Cline GW. Sono S. Gillum M. Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 130.Kris-Etherton PM. Harris WS. Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 131.Yokoyama M. Origasa H. Matsuzaki M. Matsuzawa Y. Saito Y. Ishikawa Y. Oikawa S. Sasaki J. Hishida H. Itakura H. Kita T. Kitabatake A. Nakaya N. Sakata T. Shimada K. Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 132.Lara JJ. Economou M. Wallace AM. Rumley A. Lowe G. Slater C. Caslake M. Sattar N. Lean ME. Benefits of salmon eating on traditional and novel vascular risk factors in young, non-obese healthy subjects. Atherosclerosis. 2007;193:213–221. doi: 10.1016/j.atherosclerosis.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 133.Gaullier JM. Halse J. Hoye K. Kristiansen K. Fagertun H. Vik H. Gudmundsen O. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr. 2005;135:778–784. doi: 10.1093/jn/135.4.778. [DOI] [PubMed] [Google Scholar]
- 134.Park Y. Albright KJ. Liu W. Storkson JM. Cook ME. Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- 135.Whigham LD. Watras AC. Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–1211. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 136.Perez-Matute P. Marti A. Martinez JA. Fernandez-Otero MP. Stanhope KL. Havel PJ. Moreno-Aliaga MJ. Conjugated linoleic acid inhibits glucose metabolism, leptin and adiponectin secretion in primary cultured rat adipocytes. Mol Cell Endocrinol. 2007;268:50–58. doi: 10.1016/j.mce.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 137.Abuissa H. Jones PG. Marso SP. O'Keefe JH., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821–826. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 138.Furuhashi M. Ura N. Higashiura K. Murakami H. Tanaka M. Moniwa N. Yoshida D. Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 139.Hermann TS. Li W. Dominguez H. Ihlemann N. Rask-Madsen C. Major-Pedersen A. Nielsen DB. Hansen KW. Hawkins M. Kober L. Torp-Pedersen C. Quinapril treatment increases insulin-stimulated endothelial function and adiponectin gene expression in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:1001–1008. doi: 10.1210/jc.2005-1231. [DOI] [PubMed] [Google Scholar]
- 140.Janke J. Engeli S. Gorzelniak K. Luft FC. Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- 141.Velloso LA. Folli F. Perego L. Saad MJ. The multi-faceted cross-talk between the insulin and angiotensin II signaling systems. Diabetes Metab Res Rev. 2006;22:98–107. doi: 10.1002/dmrr.611. [DOI] [PubMed] [Google Scholar]
- 142.Iwai M. Chen R. Imura Y. Horiuchi M. TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens. 2007;20:579–586. doi: 10.1016/j.amjhyper.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 143.Schupp M. Janke J. Clasen R. Unger T. Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 144.Benson SC. Pershadsingh HA. Ho CI. Chittiboyina A. Desai P. Pravenec M. Qi N. Wang J. Avery MA. Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 145.Clasen R. Schupp M. Foryst-Ludwig A. Sprang C. Clemenz M. Krikov M. Thone-Reineke C. Unger T. Kintscher U. PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 146.Kurata A. Nishizawa H. Kihara S. Maeda N. Sonoda M. Okada T. Ohashi K. Hibuse T. Fujita K. Yasui A. Hiuge A. Kumada M. Kuriyama H. Shimomura I. Funahashi T. Blockade of Angiotensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. Kidney Int. 2006;70:1717–1724. doi: 10.1038/sj.ki.5001810. [DOI] [PubMed] [Google Scholar]
- 147.Bensaid M. Gary-Bobo M. Esclangon A. Maffrand JP. Le Fur G. Oury-Donat F. Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 148.Cota D. CB1 receptors: emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes Metab Res Rev. 2007;23:507–517. doi: 10.1002/dmrr.764. [DOI] [PubMed] [Google Scholar]
- 149.Despres JP. Golay A. Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 150.Qiao L. Schaack J. Shao J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology. 2006;147:865–874. doi: 10.1210/en.2005-1030. [DOI] [PubMed] [Google Scholar]
- 151.Corman CL. Leung NM. Guberman AH. Weight gain in epileptic patients during treatment with valproic acid: a retrospective study. Can J Neurol Sci. 1997;24:240–244. doi: 10.1017/s0317167100021879. [DOI] [PubMed] [Google Scholar]
- 152.Tan H. Orbak Z. Kantarci M. Kocak N. Karaca L. Valproate-induced insulin resistance in prepubertal girls with epilepsy. J Pediatr Endocrinol Metab. 2005;18:985–989. doi: 10.1515/JPEM.2005.18.10.985. [DOI] [PubMed] [Google Scholar]
- 153.Park SK. Oh SY. Lee MY. Yoon S. Kim KS. Kim JW. CCAAT/enhancer binding protein and nuclear factor-Y regulate adiponectin gene expression in adipose tissue. Diabetes. 2004;53:2757–2766. doi: 10.2337/diabetes.53.11.2757. [DOI] [PubMed] [Google Scholar]
- 154.Bottner A. Kratzsch J. Muller G. Kapellen TM. Bluher S. Keller E. Bluher M. Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 155.Xu A. Chan KW. Hoo RL. Wang Y. Tan KC. Zhang J. Chen B. Lam MC. Tse C. Cooper GJ. Lam KS. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 156.Lanfranco F. Zitzmann M. Simoni M. Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–507. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 157.Kleiblova P. Springer D. Haluzik M. The influence of hormonal changes during menstrual cycle on serum adiponectin concentrations in healthy women. Physiol Res. 2006;55:661–666. doi: 10.33549/physiolres.930977. [DOI] [PubMed] [Google Scholar]
- 158.Catalano PM. Hoegh M. Minium J. Huston-Presley L. Bernard S. Kalhan S. Hauguel-De Mouzon S. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 159.Asai-Sato M. Okamoto M. Endo M. Yoshida H. Murase M. Ikeda M. Sakakibara H. Takahashi T. Hirahara F. Hypoadiponectinemia in lean lactating women: Prolactin inhibits adiponectin secretion from human adipocytes. Endocr J. 2006;53:555–562. doi: 10.1507/endocrj.k06-026. [DOI] [PubMed] [Google Scholar]
- 160.Catalano PM. Roman-Drago NM. Amini SB. Sims EA. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am J Obstet Gynecol. 1998;179:156–165. doi: 10.1016/s0002-9378(98)70267-4. [DOI] [PubMed] [Google Scholar]
- 161.Nilsson L. Binart N. Bohlooly YM. Bramnert M. Egecioglu E. Kindblom J. Kelly PA. Kopchick JJ. Ormandy CJ. Ling C. Billig H. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun. 2005;331:1120–1126. doi: 10.1016/j.bbrc.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 162.Ling C. Svensson L. Oden B. Weijdegard B. Eden B. Eden S. Billig H. Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J Clin Endocrinol Metab. 2003;88:1804–1808. doi: 10.1210/jc.2002-021137. [DOI] [PubMed] [Google Scholar]
- 163.Landgraf R. Landraf-Leurs MM. Weissmann A. Horl R. von Werder K. Scriba PC. Prolactin: a diabetogenic hormone. Diabetologia. 1977;13:99–104. doi: 10.1007/BF00745135. [DOI] [PubMed] [Google Scholar]
- 164.Degawa-Yamauchi M. Moss KA. Bovenkerk JE. Shankar SS. Morrison CL. Lelliott CJ. Vidal-Puig A. Jones R. Considine RV. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes Res. 2005;13:662–669. doi: 10.1038/oby.2005.74. [DOI] [PubMed] [Google Scholar]
- 165.Fallo F. Scarda A. Sonino N. Paoletta A. Boscaro M. Pagano C. Federspil G. Vettor R. Effect of glucocorticoids on adiponectin: a study in healthy subjects and in Cushing's syndrome. Eur J Endocrinol. 2004;150:339–344. doi: 10.1530/eje.0.1500339. [DOI] [PubMed] [Google Scholar]
- 166.Patel JV. Cummings DE. Girod JP. Mascarenhas AV. Hughes EA. Gupta M. Lip GY. Reddy S. Brotman DJ. Role of metabolically active hormones in the insulin resistance associated with short-term glucocorticoid treatment. J Negat Results Biomed. 2006;5:14. doi: 10.1186/1477-5751-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Libe R. Morpurgo PS. Cappiello V. Maffini A. Bondioni S. Locatelli M. Zavanone M. Beck-Peccoz P. Spada A. Ghrelin and adiponectin in patients with Cushing's disease before and after successful transsphenoidal surgery. Clin Endocrinol (Oxf) 2005;62:30–36. doi: 10.1111/j.1365-2265.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- 168.Rask E. Olsson T. Soderberg S. Andrew R. Livingstone DE. Johnson O. Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 169.Masuzaki H. Paterson J. Shinyama H. Morton NM. Mullins JJ. Seckl JR. Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 170.Morton NM. Paterson JM. Masuzaki H. Holmes MC. Staels B. Fievet C. Walker BR. Flier JS. Mullins JJ. Seckl JR. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2004;53:931–938. doi: 10.2337/diabetes.53.4.931. [DOI] [PubMed] [Google Scholar]
- 171.Olsson B. Bohlooly YM. Fitzgerald SM. Frick F. Ljungberg A. Ahren B. Tornell J. Bergstrom G. Oscarsson J. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146:920–930. doi: 10.1210/en.2004-1232. [DOI] [PubMed] [Google Scholar]
- 172.del Rincon JP. Iida K. Gaylinn BD. McCurdy CE. Leitner JW. Barbour LA. Kopchick JJ. Friedman JE. Draznin B. Thorner MO. Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes. 2007;56:1638–1646. doi: 10.2337/db06-0299. [DOI] [PubMed] [Google Scholar]
- 173.Silha JV. Krsek M. Hana V. Marek J. Jezkova J. Weiss V. Murphy LJ. Perturbations in adiponectin, leptin and resistin levels in acromegaly: lack of correlation with insulin resistance. Clin Endocrinol (Oxf) 2003;58:736–742. doi: 10.1046/j.1365-2265.2003.01789.x. [DOI] [PubMed] [Google Scholar]
- 174.Fukuda I. Hizuka N. Ishikawa Y. Itoh E. Yasumoto K. Murakami Y. Sata A. Tsukada J. Kurimoto M. Okubo Y. Takano K. Serum adiponectin levels in adult growth hormone deficiency and acromegaly. Growth Horm IGF Res. 2004;14:449–454. doi: 10.1016/j.ghir.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 175.Wiesli P. Bernays R. Brandle M. Zwimpfer C. Seiler H. Zapf JGAS. Schmid C. Effect of pituitary surgery in patients with acromegaly on adiponectin serum concentrations and alanine aminotransferase activity. Clin Chim Acta. 2005;352:175–181. doi: 10.1016/j.cccn.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 176.Ronchi CL. Corbetta S. Cappiello V. Morpurgo PS. Giavoli C. Beck-Peccoz P. Arosio M. Spada A. Circulating adiponectin levels and cardiovascular risk factors in acromegalic patients. Eur J Endocrinol. 2004;150:663–669. doi: 10.1530/eje.0.1500663. [DOI] [PubMed] [Google Scholar]
- 177.Lam KS. Xu A. Tan KC. Wong LC. Tiu SC. Tam S. Serum adiponectin is reduced in acromegaly and normalized after correction of growth hormone excess. J Clin Endocrinol Metab. 2004;89:5448–5453. doi: 10.1210/jc.2003-032023. [DOI] [PubMed] [Google Scholar]
- 178.Lo JC. Mulligan K. Havel PJ. Noor MA. Lee GA. Schwarz JM. Grunfeld C. Schambelan M. The effect of recombinant human growth hormone treatment on circulating leptin and adiponectin concentrations in patients with HIV-associated fat accumulation. Antiviral Therapy. 2004;9:L4–L5. [Google Scholar]
- 179.Delporte ML. Funahashi T. Takahashi M. Matsuzawa Y. Brichard SM. Pre- and post-translational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J. 2002;367:677–685. doi: 10.1042/BJ20020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nowak L. Adamczak M. Wiecek A. Blockade of sympathetic nervous system activity by rilmenidine increases plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2005;18:1470–1475. doi: 10.1016/j.amjhyper.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 181.Yoda M. Nakano Y. Tobe T. Shioda S. Choi-Miura NH. Tomita M. Characterization of mouse GBP28 and its induction by exposure to cold. Int J Obes Relat Metab Disord. 2001;25:75–83. doi: 10.1038/sj.ijo.0801482. [DOI] [PubMed] [Google Scholar]
- 182.Imai J. Katagiri H. Yamada T. Ishigaki Y. Ogihara T. Uno K. Hasegawa Y. Gao J. Ishihara H. Sasano H. Oka Y. Cold exposure suppresses serum adiponectin levels through sympathetic nerve activation in mice. Obesity (Silver Spring) 2006;14:1132–1141. doi: 10.1038/oby.2006.130. [DOI] [PubMed] [Google Scholar]
- 183.Puerta M. Abelenda M. Rocha M. Trayhurn P. Effect of acute cold exposure on the expression of the adiponectin, resistin and leptin genes in rat white and brown adipose tissues. Horm Metab Res. 2002;34:629–634. doi: 10.1055/s-2002-38252. [DOI] [PubMed] [Google Scholar]
- 184.Lee NK. Sowa H. Hinoi E. Ferron M. Ahn JD. Confavreux C. Dacquin R. Mee PJ. McKee MD. Jung DY. Zhang Z. Kim JK. Mauvais-Jarvis F. Ducy P. Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Berg AH. Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 186.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 187.Weisberg SP. McCann D. Desai M. Rosenbaum M. Leibel RL. Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hotamisligil GS. Arner P. Caro JF. Atkinson RL. Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kern PA. Saghizadeh M. Ong JM. Bosch RJ. Deem R. Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hak AE. Stehouwer CD. Bots ML. Polderman KH. Schalkwijk CG. Westendorp IC. Hofman A. Witteman JC. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol. 1999;19:1986–1991. doi: 10.1161/01.atv.19.8.1986. [DOI] [PubMed] [Google Scholar]
- 191.Kern PA. Ranganathan S. Li C. Wood L. Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 192.Kim CS. Park HS. Kawada T. Kim JH. Lim D. Hubbard NE. Kwon BS. Erickson KL. Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 193.Van Gaal LF. Mertens IL. De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 194.Engeli S. Feldpausch M. Gorzelniak K. Hartwig F. Heintze U. Janke J. Mohlig M. Pfeiffer AF. Luft FC. Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52:942–947. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 195.Krakoff J. Funahashi T. Stehouwer CD. Schalkwijk CG. Tanaka S. Matsuzawa Y. Kobes S. Tataranni PA. Hanson RL. Knowler WC. Lindsay RS. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26:1745–1751. doi: 10.2337/diacare.26.6.1745. [DOI] [PubMed] [Google Scholar]
- 196.Kappes A. Loffler G. Influences of ionomycin, dibutyryl-cycloAMP and tumour necrosis factor-alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Horm Metab Res. 2000;32:548–554. doi: 10.1055/s-2007-978684. [DOI] [PubMed] [Google Scholar]
- 197.Fasshauer M. Kralisch S. Klier M. Lossner U. Bluher M. Klein J. Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 198.Yuan G. Chen X. Ma Q. Qiao J. Li R. Li X. Li S. Tang J. Zhou L. Song H. Chen M. C-reactive protein inhibits adiponectin gene expression and secretion in 3T3-L1 adipocytes. J Endocrinol. 2007;194:275–281. doi: 10.1677/JOE-07-0133. [DOI] [PubMed] [Google Scholar]
- 199.Hotamisligil GS. Peraldi P. Budavari A. Ellis R. White MF. Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 200.Rotter V. Nagaev I. Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 201.Emanuelli B. Peraldi P. Filloux C. Sawka-Verhelle D. Hilton D. Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- 202.Shi H. Tzameli I. Bjorbaek C. Flier JS. Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem. 2004;279:34733–34740. doi: 10.1074/jbc.M403886200. [DOI] [PubMed] [Google Scholar]
- 203.Quinn LS. Strait-Bodey L. Anderson BG. Argiles JM. Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int. 2005;29:449–457. doi: 10.1016/j.cellbi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 204.Rosen P. Nawroth PP. King G. Moller W. Tritschler HJ. Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 205.Keaney JF., Jr. Larson MG. Vasan RS. Wilson PW. Lipinska I. Corey D. Massaro JM. Sutherland P. Vita JA. Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 206.Furukawa S. Fujita T. Shimabukuro M. Iwaki M. Yamada Y. Nakajima Y. Nakayama O. Makishima M. Matsuda M. Shimomura I. Increased oxidative stress in obesity, its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu B. Ennis D. Lai R. Bogdanovic E. Nikolov R. Salamon L. Fantus C. Le-Tien H. Fantus IG. Enhanced sensitivity of insulin-resistant adipocytes to vanadate is associated with oxidative stress and decreased reduction of vanadate (+5) to vanadyl (+4) J Biol Chem. 2001;276:35589–35598. doi: 10.1074/jbc.M106783200. [DOI] [PubMed] [Google Scholar]
- 208.Kamigaki M. Sakaue S. Tsujino I. Ohira H. Ikeda D. Itoh N. Ishimaru S. Ohtsuka Y. Nishimura M. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;339:624–632. doi: 10.1016/j.bbrc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 209.Chevillotte E. Giralt M. Miroux B. Ricquier D. Villarroya F. Uncoupling protein-2 controls adiponectin gene expression in adipose tissue through the modulation of reactive oxygen species production. Diabetes. 2007;56:1042–1050. doi: 10.2337/db06-1300. [DOI] [PubMed] [Google Scholar]
- 210.Miyazaki T. Shimada K. Mokuno H. Daida H. Adipocyte derived plasma protein, adiponectin, is associated with smoking status in patients with coronary artery disease. Heart. 2003;89:663. doi: 10.1136/heart.89.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Iwashima Y. Katsuya T. Ishikawa K. Kida I. Ohishi M. Horio T. Ouchi N. Ohashi K. Kihara S. Funahashi T. Rakugi H. Ogihara T. Association of hypoadiponectinemia with smoking habit in men. Hypertension. 2005;45:1094–1100. doi: 10.1161/01.HYP.0000169444.05588.4c. [DOI] [PubMed] [Google Scholar]
- 212.Thamer C. Stefan N. Stumvoll M. Haring H. Fritsche A. Reduced adiponectin serum levels in smokers. Atherosclerosis. 2005;179:421–422. doi: 10.1016/j.atherosclerosis.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 213.Das I. Raised C-reactive protein levels in serum from smokers. Clin Chim Acta. 1985;153:9–13. doi: 10.1016/0009-8981(85)90133-0. [DOI] [PubMed] [Google Scholar]
- 214.Chen B. Lam KS. Wang Y. Wu D. Lam MC. Shen J. Wong L. Hoo RL. Zhang J. Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549–556. doi: 10.1016/j.bbrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 215.Sierksma A. Patel H. Ouchi N. Kihara S. Funahashi T. Heine RJ. Grobbee DE. Kluft C. Hendriks HF. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, insulin sensitivity. Diabetes Care. 2004;27:184–189. doi: 10.2337/diacare.27.1.184. [DOI] [PubMed] [Google Scholar]
- 216.Pischon T. Girman CJ. Rifai N. Hotamisligil GS. Rimm EB. Association between dietary factors and plasma adiponectin concentrations in men. Am J Clin Nutr. 2005;81:780–786. doi: 10.1093/ajcn/81.4.780. [DOI] [PubMed] [Google Scholar]
- 217.Englund Ogge L. Brohall G. Behre CJ. Schmidt C. Fagerberg B. Alcohol consumption in relation to metabolic regulation, inflammation, and adiponectin in 64-year-old Caucasian women: a population-based study with a focus on impaired glucose regulation. Diabetes Care. 2006;29:908–913. doi: 10.2337/diacare.29.04.06.dc05-1782. [DOI] [PubMed] [Google Scholar]
- 218.Behre CJ. Adiponectin: Saving the starved and the overfed. Med Hypotheses. 2007;69:1290–1292. doi: 10.1016/j.mehy.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 219.Kim JY. van de Wall E. Laplante M. Azzara A. Trujillo ME. Hofmann SM. Schraw T. Durand JL. Li H. Li G. Jelicks LA. Mehler MF. Hui DY. Deshaies Y. Shulman GI. Schwartz GJ. Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marchesini G. Brizi M. Morselli-Labate AM. Bianchi G. Bugianesi E. McCullough AJ. Forlani G. Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 221.Kraegen EW. Cooney GJ. Ye JM. Thompson AL. Furler SM. The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S189–201. doi: 10.1055/s-2001-18581. [DOI] [PubMed] [Google Scholar]
- 222.Westphal S. Borucki K. Taneva E. Makarova R. Luley C. Extended-release niacin raises adiponectin and leptin. Atherosclerosis. 2007;193:361–365. doi: 10.1016/j.atherosclerosis.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 223.Heliovaara MK. Remitz A. Reitamo S. Teppo AM. Karonen SL. Ebeling P. 13-cis-Retinoic acid therapy induces insulin resistance, regulates inflammatory parameters, and paradoxically increases serum adiponectin concentration. Metabolism. 2007;56:786–791. doi: 10.1016/j.metabol.2007.02.002. [DOI] [PubMed] [Google Scholar]