Abstract
Background
Metabolic syndrome occurs commonly in the United States. The purpose of this study was to measure the prevalence of metabolic syndrome among American Indian and Alaska Native people.
Methods
We measured the prevalence rates of metabolic syndrome, as defined by the National Cholesterol Education Program, among four groups of American Indian and Alaska Native people aged 20 years and older. One group was from the southwestern United States (Navajo Nation), and three groups resided within Alaska. Prevalence rates were age-adjusted to the U.S. adult 2000 population and compared to rates for U.S. whites (National Health and Nutrition Examination Survey [NHANES] 1988–1994).
Results
Among participants from the southwestern United States, metabolic syndrome was found among 43.2% of men and 47.3% of women. Among Alaska Native people, metabolic syndrome was found among 26.5% of men and 31.2% of women. In Alaska, the prevalence rate varied by region, ranging among men from 18.9% (western Alaska) to 35.1% (southeast), and among women from 22.0% (western Alaska) to 38.4 % (southeast). Compared to U.S. whites, American Indian/Alaska Native men and women from all regions except western Alaska were more likely to have metabolic syndrome; men in western Alaska were less likely to have metabolic syndrome than U.S. whites, and the prevalence among women in western Alaska was similar to that of U.S. whites.
Conclusion
The prevalence rate of metabolic syndrome varies widely among different American Indian and Alaska Native populations. Differences paralleled differences in the prevalence rates of diabetes.
Introduction
Metabolic syndrome, a cluster of frequently associated cardiovascular disease risk factors, occurs commonly in the U.S. population.1,2 Using the definition of the Third Report of the National Cholesterol Education Program (NCEP), Ford et al. reported the prevalence of metabolic syndrome to be 21.8% among U.S. adults (23.7% age-adjusted to the U.S. 2000 population), using the 1988–1994 National Health and Nutrition Examination Survey (NHANES) data.2 The NHANES data included whites, African-Americans, Mexican-Americans, and “other,” but did not have a separate category for American Indian/Alaska Native people. Few studies have been published on the prevalence of metabolic syndrome among American Indian/Alaska Native people. The Strong Heart Study, which included 4549 American Indian participants aged 45–74 years at baseline (1988–1992), reported a prevalence of metabolic syndrome of 55.2%. Among men and women aged 45–49 years of age, prevalence rates of metabolic syndrome were over twice as high as rates reported for the general population of U.S. men and women.3 Studies in the Canadian population have found the prevalence of metabolic syndrome to vary by ethnicity; the overall crude rate of metabolic syndrome was 33.3% among Native Indian people, 13.5% among Inuit people, and 30% among non-Aboriginal people.4
The Education and Research Towards Health (EARTH) Study has been collecting data related to risk factors for chronic diseases among American Indian and Alaska Native people since 2004. The study provides the opportunity to measure the prevalence of metabolic syndrome among several different groups of American Indian and Alaska Native people who have a variety of lifestyles and to compare the results to those reported for other populations.
Methods
Study population
Detailed study methods have been described.5 Participants were recruited in several areas of Alaska and on the Navajo Nation in Arizona and New Mexico. Regional, local, and village tribal health boards and chapters within local health boards approved and supported the study. The study protocol was approved by the following institutional review boards: University of Utah, Navajo Nation, the Alaska Area, and National Indian Health Service.
Participants in Alaska were recruited from 26 villages and communities in three distinct regions: southcentral Alaska, an area that contains Anchorage, a large city of over 280,000 people; southeastern Alaska, an area of small towns and villages in a largely coastal setting; and western Alaska, which includes over 50 small villages accessible only by air or river travel. Participants in the southwestern United States were recruited on the Navajo Reservation in two separate areas. In addition to the two clinic sites, a mobile van was used on the Navajo reservation to increase access to the study.
Eligibility criteria included: at least 18 years of age; American Indian or Alaska Native beneficiary of Indian Health Service services; not pregnant; not actively undergoing cancer chemotherapy treatment; and physically and mentally able to read and understand the consent form and to complete survey instruments and medical tests. In participating communities, all community members were invited to participate. Staff disseminated information about the project through brochures and posters, presentations at formal and informal gatherings, advertisements in newspapers and announcements on local radio and television. For this effort, only participants aged 20 years and older were included in analysis to compare to previously published studies. Potential biases of the open recruitment method include reaching a nonrepresentative group of participants, which might be healthier, younger, and more motivated to participate. To examine the representativeness of our study population, we compared demographic characteristics of the study participants to those reported in the 2000 U.S. Census for American Indian/Alaska Native people in each of the regions included in the study.
Data collection
Medical tests at the baseline study visit were seated blood pressure, fasting serum lipid and glucose levels, height, weight, and waist/hip circumferences. Participants were asked to fast for at least 9 hours.
Blood pressure was measured using the Omron HEM-907 automatic blood pressure device.6 The arm circumference was measured to determine proper cuff size. After participants were seated for 5 minutes in a quiet environment, three blood pressure measurements were taken at 1-minute intervals, and the average blood pressure was calculated from the last two measurements. Fasting glucose and lipid panel were measured on a blood sample obtained by fingerstick using the Cholestech LDX® System (Cholestech, Hayward, Ca).7
Tanita® digital scales (BWP800/BWP627A, Tanita Corporation of America Inc., Arlington Hills, IL) and the Road Rod Stadiometer® (Seca, Hamburg, Germany) were used for weight and height, respectively. For both weight and height, two measurements were taken, and the average of the two was used for analysis.
Waist and hip circumferences were measured to the nearest 0.5 inch while the participant was standing using either the Novel Products Figure Finder® tape (Novel Products Inc., Rockton, IL) or the Gulick II Plus® tape (Country Technology Inc., Gays Mills, WI). Waist circumference was measured at the smallest point between the tenth rib and the iliac crest; hip circumference was measured at the level of maximum protrusion of the gluteal muscles. Measurements were taken in duplicate, and the average of the two used for analysis.
Participants completed questionnaires related to lifestyle and usual diet using a touch screen computer. At the conclusion of the study visit, participants were provided feedback regarding the results of their medical tests and responses to questions about health risk behaviors. Quality control procedures were in place to assure that data were collected in a standardized way across study centers.
Metabolic syndrome
Metabolic syndrome was defined according to the NCEP criteria as the presence of three or more of the following criteria1:
Waist circumference > 102 cm (40.2 inches) in men and > 88 cm (34.6 inches) in women;
Triglycerides ≥ 150 mg/dL (1.69 mmol/L);
High-density lipoprotein cholesterol (HDL-C) < 40 mg/dL (1.04 mmol/L) in men and < 50 mg/dL (1.29 mmol/L) in women;
Blood pressure ≥ 130/85 mmHg (either systolic or diastolic blood pressure elevated) or self-reported physician diagnosed hypertension;
Fasting glucose ≥ 110 mg/dL (6.1 mmol/L) or self-reported physician diagnosed diabetes.
Although newer definitions suggest that the glucose cutpoint be decreased to 100 mg/dL (5.5 mmol/L) to meet American Diabetes Association recommendations, we used the 110 mg/dL cut point to be consistent with the published prevalence rates of metabolic syndrome that used the original NCEP criteria.8
Data analysis
Data were analyzed using EpiInfo (version 3.3.2). Prevalence rates of each of the individual criteria for metabolic syndrome and prevalence rates of metabolic syndrome based on the criteria were calculated and age-adjusted to the U.S. 2000 adult population using the direct method.
Results
Study populations
A total of 9696 participants aged 20 years and older enrolled in the EARTH Study from March 1, 2004, through September of 2006; 3576 were from Alaska and 6120 were from the southwestern United States. Participants with missing values for any of the metabolic syndrome components, predominantly those who were not fasting at the time of the study visit, were excluded from the analysis (78 from Alaska and 1586 from the southwestern United States). Data are presented for 8032 participants (3498 from Alaska and 4534 from the southwestern United States).
The two populations showed similar distributions for age and sex (Table 1).Overall the study population was predominately young (46.2% less than 40 years of age) and included more women than men (64.3% vs. 35.7%). Although the sample was one of convenience, the age distribution of the two populations closely resembled the distribution of age, employment, and marital status reported by the 2000 U.S. census for American Indian Alaska Native people in the respective regions (data not shown).9
Table 1.
Characteristics of the Study Populationa
| Characteristic | Alaska (%) | Southwest (%) |
|---|---|---|
| Total | 3498 | 4534 |
| Age (years) | ||
| 20–29 | 812 (23.2) | 1102 (24.3) |
| 30–39 | 779 (22.2) | 1024 (22.6) |
| 40–49 | 927 (26.5) | 1163 (25.7) |
| 50–59 | 556 (15.9) | 817 (18.0) |
| 60–69 | 270 (7.7) | 352 (7.8) |
| 70+ | 154 (4.4) | 76 (1.7) |
| Sex | ||
| Male | 1345 (38.5) | 1519 (33.5) |
| Female | 2153 (61.5) | 3015 (66.5) |
| Area in Alaska | ||
| Southcentral | 1318 (37.7) | N.A. |
| Southeast | 785 (22.4) | N.A. |
| Western | 1395 (39.9) | N.A. |
Includes study participants aged 20 years and higher, and for whom all data were available to evaluate presence of metabolic syndrome.
Notes: N.A., not applicable.
Of the Alaskan participants, 37.7% lived in southcentral Alaska, 22.4% lived in southeastern Alaska, and 39.9% resided in western Alaska. Those living in the southcentral area were diverse in terms of Alaska Native ethnic origins; most from southeastern Alaska were Tlingit, whereas the vast majority of participants from western Alaska were Yupik.
Prevalence of metabolic syndrome and components
Because women were overrepresented in the EARTH Study population, data are presented by sex. The overall age-adjusted prevalence of metabolic syndrome was 34.9% among men and 40.0% among women (Table 2).Among American Indian men and women from the southwestern United States, metabolic syndrome was found among 43.2% of men and 47.3% of women. Among Alaska Native people overall, metabolic syndrome was found among 26.5% of men and 31.2% of women. In Alaska, the prevalence rate of metabolic syndrome among Alaska Native people varied by region, ranging from 18.9% among western Alaska men to 38.4 % among southeastern women.
Table 2.
Age-Adjusted Prevalence of Individual Metabolic Abnormalities of the Metabolic Syndrome and Metabolic Syndrome Among 8032 American Indian and Alaska Native People Aged 20 Years and Older Compared to Reported U.S. White Prevalence
| |
|
Abdominal obesitya |
Elevated triglyceridesb |
Low HDL-Cc |
High blood pressure or diagnosed hypertensiond |
Elevated glucose or diagnosed diabetese |
Metabolic syndromef |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants | Percent | SE | Percent | SE | Percent | SE | Percent | SE | Percent | SE | Percent | SE | |
| Men | |||||||||||||
| AIAN | 2864 | 43.7 | 0.9↑ | 37.3 | 0.9 | 33.2 | 0.9 | 46.4 | 0.9↑ | 30.9 | 0.9↑ | 34.9 | 0.9↑ |
| Southwestern | 1519 | 48.7 | 1.3↑ | 48.0 | 1.3↑ | 43.0 | 1.3↑ | 44.8 | 1.3↑ | 40.1 | 1.3↑ | 43.2 | 1.3↑ |
| United States | |||||||||||||
| Alaska | 1345 | 37.7 | 1.3↑ | 26.9 | 1.2↓ | 23.6 | 1.2↓ | 47.9 | 1.4↑ | 21.6 | 1.1↑ | 26.5 | 1.2 |
| Southcentral | 428 | 41.7 | 2.4↑ | 33.0 | 2.2 | 25.0 | 2.0↓ | 55.1 | 2.4↑ | 20.8 | 2.0 | 30.7 | 2.2↑ |
| Southeastern | 281 | 49.2 | 3.0↑ | 35.1 | 2.8 | 34.8 | 2.8 | 48.1 | 3.0↑ | 22.6 | 2.5↑ | 35.1 | 2.8↑ |
| Western | 636 | 28.8 | 1.8 | 18.2 | 1.5↓ | 17.2 | 1.5↓ | 43.6 | 2.0 | 20.2 | 1.6 | 18.9 | 1.6↓ |
| U.S. white Men | 1712 | 30.5 | 1.2 | 36.9 | 2.0 | 36.8 | 1.6 | 37.2 | 1.8 | 15.6 | 1 | 24.8 | 1.4 |
| Women | |||||||||||||
| AIAN Women | 5168 | 79.9 | 0.6↑ | 38.7 | 0.7↑ | 41.1 | 0.7 | 36.2 | 0.7↑ | 25.1 | 0.6↑ | 40.0 | 0.7↑ |
| Southwestern | 3015 | 84.9 | 0.7↑ | 46.2 | 0.9↑ | 50.0 | 0.9↑ | 33.4 | 0.9↑ | 31.0 | 0.8↑ | 47.3 | 0.9↑ |
| United States | |||||||||||||
| Alaska | 2153 | 73.3 | 1.0↑ | 30.0 | 1.0↑ | 29.3 | 1.0↓ | 40.2 | 1.1↑ | 18.7 | 0.8↑ | 31.2 | 1.0↑ |
| Southcentral | 890 | 75.0 | 1.5↑ | 41.1 | 1.6↑ | 31.1 | 1.6↓ | 40.6 | 1.6↑ | 22.3 | 1.4↑ | 38.4 | 1.6↑ |
| Southeastern | 504 | 70.3 | 2.0↑ | 31.9 | 2.1↑ | 35.9 | 2.1 | 34.4 | 2.1↑ | 18.4 | 1.7↑ | 32.7 | 2.1↑ |
| Western | 759 | 73.5 | 1.6↑ | 17.8 | 1.4↓ | 21.8 | 1.5↓ | 42.2 | 1.8↑ | 14.3 | 1.3↑ | 22.0 | 1.5 |
| U.S. white Women | 1887 | 43.5 | 1.4 | 25.0 | 1.1 | 39.3 | 1.9 | 27.8 | 0.9 | 8.5 | 0.6 | 22.8 | 1.1 |
All prevalence rates age-adjusted to US 2000 adult population aWaist circumference >102 cm (40.2 inches) in men and >88 cm (34.6 inches) in women;
Triglycerides ≥ 150 mg/dl (1.69 mmol/L).
High-density lipoprotein (HDL) cholesterol < 40 mg/dL (1.04 mmol/L) in men and < 50 mg/dL (1.29 mmol/L) in women.
Blood pressure ≥ 130/85 mmHg (either systolic or diastolic blood pressure elevated) or self-reported physician diagnosed hypertension.
Fasting glucose ≥ 110 mg/dL (6.1 mmol/L) or self-reported physician diagnosed diabetes.
Any three of the criteria.
Notes: HDL-C, high-density lipoprotein cholesterol; SE, standard error; AIAN, American Indian/Alaskan Native; Highlighted, most common risk factor; ↑, higher than U.S. white prevalence; ↓, lower than U.S. white prevalence (non-overlapping 95% confidence intervals, ± 1.96*SE); U.S. white rates from Ford et al. 2002.2
Compared to U.S. whites, American Indian and Alaska Native men and women from all regions, with the exception of western Alaska, were more likely to have metabolic syndrome; men in western Alaska were less likely to have metabolic syndrome than U.S. whites, and the prevalence among women in western Alaska was similar to that of U.S. whites. Men and women from all regions of Alaska had a lower prevalence of metabolic syndrome than American Indian men and women from the southwestern United States.
The most common component of the metabolic syndrome among U.S. white men and Alaska Native men from south-central and western Alaska was high blood pressure; among Alaska Native men from southeastern Alaska and American Indian men from the southwestern United States, the most common component was high waist circumference. Among all women, the most common component was high waist circumference. Among participants with metabolic syndrome, 22.9% had self-reported diabetes (16.6% for Alaska and 26.0% for southwestern United States).
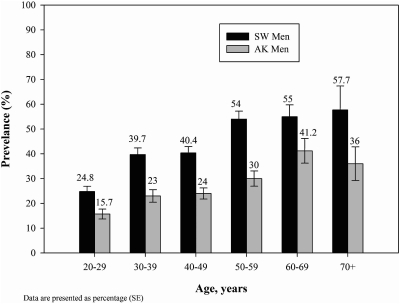
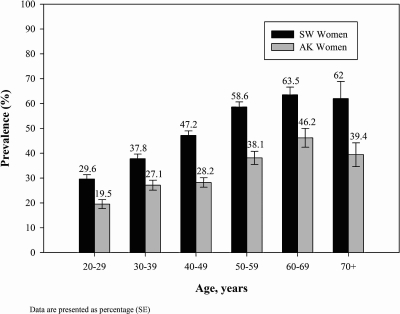
The prevalence of metabolic syndrome increased with age among American Indian southwestern U.S. men and women until age group 50–59, and then appeared to level at approximately 55% among men and 60% among women (Figs. 1 and 2). Among Alaska Native people, the age-specific rates increased until age group 60–69, and then decreased. Age-specific rates were lower at every age group for Alaska men and women compared to American Indian people from the southwestern United States.
FIG. 1.
Age-specific prevalence of metabolic syndrome among American Indian and Alaska Native men.
FIG. 2.
Age-specific prevalence of metabolic syndrome among American Indian and Alaska Native women.
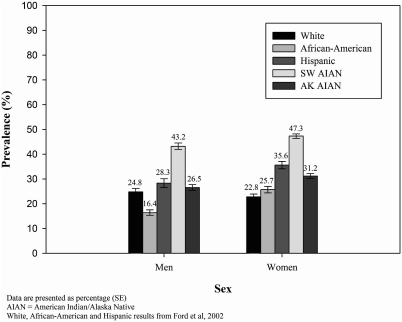
Southwestern American Indian people had higher prevalence rates of metabolic syndrome than rates reported for U.S. white, African-American, and Hispanic peoples (Fig. 3). The rate for Alaska Native men was similar to the rate for U.S. white men, and the rate for Alaska Native women was higher than the rate for U.S. white women. Both Alaska Native men and women had rates that were higher than rates for African-American men and women, and similar to rates for Hispanic men and women.
FIG. 3.
Age-adjusted prevalence of metabolic syndrome by race and sex.
Discussion
This study is the first to measure the prevalence of metabolic syndrome among American Indians and Alaska Native adults of all ages living in a variety of settings. The vast majority of American Indian participants from the southwestern United States lived on the Navajo reservation; participants from Southcentral Alaska lived in or near Alaska's largest city and were from a wide variety of Alaska Native ethnic backgrounds; those from southeastern Alaska lived in small communities and were largely Tlingit in ethnicity, and those from Western Alaska also lived in small communities and were Yupik in ethnicity.
Metabolic syndrome was more common among southwestern United States American Indian people than among Alaska Native people. Although within Alaska, rates varied by region, the southwestern United States rates for both men and women were higher than the rates for each of the Alaska regions. The lowest prevalence rates of metabolic syndrome were found in western Alaska, where the vast majority of the participants were Yupik. The variation in prevalence was similar to that reported by Canadian studies, where rates were lowest among the Inuit (both Yupik and Inuit are peoples of the North American Arctic region and are culturally related) and highest among First Nations people.4,10–14
Not only did the overall prevalence rates of metabolic syndrome vary by region, but the distribution of the individual risk factors varied as well. Western Alaska EARTH Study men and women had the lowest prevalence rates of elevated triglycerides, low HDL and elevated glucose; this pattern was also similar to the pattern described in the Canadian population for the Inuit compared to the Oji-Cree Indians and to non-aboriginal populations.4 Previous studies in Alaska communities have also documented lower prevalence rates of elevated triglycerides/low HDL levels among the Yupik people.15,16
The age-adjusted prevalence rate of metabolic syndrome among southwestern EARTH Study men (43.2%) was almost twice as high as that reported for U.S. whites (24.8%), and the rate for women (47.3%) was over twice as high as that for U.S. whites (22.8%). In addition, the prevalence rates of each of the risk factors were higher than those for U.S. whites. The prevalence rates for metabolic syndrome found for southwestern EARTH Study participants were similar to or higher than those reported by the Strong Heart Study for American Indian people aged 45–74 years of age. Among southwestern United States EARTH Study participants living on the Navajo Nation aged 45–74, the overall prevalence of metabolic syndrome was 55.9%, and among those without diabetes or heart disease, the rate was 45.3%; the Strong Heart Study reported an overall prevalence of 55.2% and a prevalence of 35% among nondiabetic participants without cardiovascular disease.17
Differences in lifestyle and genetics likely contribute to the variation in the prevalence rates of metabolic syndrome found in this study. Differences in lifestyle among the groups included in this study include dietary differences, with the Alaska groups eating much more fish and sea mammals, for example. Daily physical activity level and obesity prevalence may also differ. Distinguishing which of the lifestyle and genetic factors most contributes is beyond the scope of this cross-sectional analysis. The Strong Heart Family Study examined genetic and environmental contributions to the insulin resistance syndrome, and found three clusters of risk factors: glucose/insulin/obesity; blood pressure; and dyslipidemia. Significant heritabilities were found for each of the three clusters, leading researchers to conclude that heredity may explain a large proportion of the variability of factors that underlie the insulin resistance syndrome among American Indians.18,19 Studies in the Canadian populations have found similar groupings and ethnic variation in the distribution of the individual risk factors.20
Although metabolic syndrome is a constellation of cardiovascular disease risk factors, it has been found to be a better predictor of type 2 diabetes (among people without diabetes) than elevated fasting glucose alone.21–23 Similar findings have been reported for American Indian and Alaska Native people.16 The prevalence of type 2 diabetes among American Indian and Alaska Native people is over twice as high as the prevalence among the U.S. general population; similarly, diabetes mortality rates are higher.24,25 In addition, regional variations exist for the prevalence rate of diabetes that parallel the differences in the prevalence of metabolic syndrome. Among American Indian people in the southwestern region included in the Navajo EARTH Study, the prevalence rate for type 2 diabetes is over twice that of Alaska, and mortality rates from diabetes are approximately four-fold higher.23–26 Within Alaska, the lowest prevalence rates of diabetes have been found among the Yupik people of Western Alaska.27,28 However, the rates of diabetes have been increasing in all American Indian and Alaska Native populations, and the rate of increase has been largest for Alaska.25 The Alaska Area Diabetes program also has found that Western Alaska has one of the highest rates of increase in the prevalence of diabetes in the state.29,30 Within Alaska, the prevalence of metabolic syndrome in two regions was higher than rates for U.S. whites. The rate may currently be low in western Alaska, but given the historical trend toward increasing diabetes and increasing obesity, once might expect the rate to increase in the future.
One limitation of this analysis is that we compared our data to those for U.S. whites measured during the time period 1988–1994. Ford et al. measured the change in prevalence of metabolic syndrome among participants from NHANES from 1988 to 1994 and 1999 to 2000 and found that the prevalence of metabolic syndrome increased by 2.2% among men and 23.5% among women.31 The report did not present results specifically by race.
Another limitation is that we measured lipids and glucose from a fingerstick blood sample using the Cholestech LDX, which may not be directly comparable to the NHANES laboratory methodology. However, a number of studies have shown good correlation of lipid values measured on the Cholestech LDX and those from serum obtained from venous blood samples.7,32 The Cholestech LDX has recently received Cholesterol Reference Method Laboratory Network certification for the LDX total and HDL cholesterol tests. The Cholestech cassette has a filtration device so that the analytes are measured on plasma rather than whole blood. The package insert instructs users to use the normal and recommended values designated by the National Cholesterol Education Program and the American Diabetes Association. Although studies validating the glucose measurement have not been done, the device has received a Clinical Laboratory Improvement Amendments (CLIA) waiver, which indicates that clinical decisions can be made on the basis of study results. Our study used careful quality control measures, including staff training, procedure manuals, site visits, and keeping records as designated by the Cholestech manual to assure consistent and accurate data collection.
We used the glucose cut point of ≥110 mg/dL to compare to previously published studies. Using the glucose cut point of ≥100 mg/dL, as expected, increases the prevalence of metabolic syndrome. The age-adjusted rates using the lower glucose cut point were 32.9% and 39.4% among Alaska men and women, respectively, and 51.6% and 53.5% among southwestern U.S. men and women, respectively.
This study found a wide variation in prevalence of metabolic syndrome among American Indian and Alaska Native populations. The variation in prevalence roughly parallels the differences in diabetes prevalence. Because metabolic syndrome is a good indicator of who may progress on to type 2 diabetes, identification of metabolic syndrome in these populations could help direct prevention efforts, especially with limited resources. This may be particularly true in regions such as western Alaska where rates of obesity are high, but low HDL and high triglyceride levels are less prevalent. Patients with the metabolic syndrome could be targeted as opposed to all individuals with an elevated body mass index (BMI).
Disparities in metabolic syndrome prevalence rates may indicate differences that are either heritable, environmental, or a combination of both. Possible protective environmental factors include traditional/subsistence foods or more active lifestyles. Further exploration of this could expand our understanding of the increasing prevalence of diabetes in these populations and how to prevent it.
Acknowledgments
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute or the Indian Health Service. The primary author has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would like to acknowledge the contributions and support of the Navajo Nation, the Indian Health Service, the Alaska Native Tribal Health Consortium Board of Directors, Southcentral Foundation (SCF), Southeast Alaska Regional Health Consortium (SEARHC), the Yukon-Kuskokwim Health Corporation (YKHC), Ft. Defiance and Shiprock Health Boards, Tribal Advisory Board Members, the staff on the Navajo Nation, the staff in Alaska, and the University of Utah Coordinating Center Staff. We would also like to acknowledge the support from Omron Health Care Inc. who provided the Omron Hem 907 to the study at a reduced cost; and Alaska Scientific, Inc. who assisted with development of the Cholestech protocol and staff training.
This study was funded by grants CA88958 and CA96095 from the National Cancer Institute.
Author Disclosure Statement
None of the authors has any potential conflicts of interest, including specific financial interests and relationships and affiliations (other than those affiliations listed in the title page of the manuscript) relevant to the subject of this manuscript.
References
- 1.Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults: Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES. Giles WH. Dietz WH. Prevalence of the metabolic syndrome among US adults. J Am Med Assoc. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Resnick HE on behalf of the Strong Heart Study Investigators. Metabolic syndrome in American Indians. Diabetes Care. 2002;25:1246–1247. doi: 10.2337/diacare.25.7.1246. [DOI] [PubMed] [Google Scholar]
- 4.Liu J. Hanley AJ. Young TK. Harris SB. Zinman B. Characteristics and prevalence of the metabolic syndrome among three ethnic groups in Canada. Int J Obesity. 2006;30:669–676. doi: 10.1038/sj.ijo.0803179. [DOI] [PubMed] [Google Scholar]
- 5.Slattery M. Schumacher MC. Lanier AP. Edwards S. Edwards R. Murtaugh MA. Sandidge J. Day GE. Kaufman D. Kanekar S. Tom-Orme L. Henderson JA. A Prospective Cohort of American Indians and Alaska Natives: Study design, methods, and implementation. Am J Epidemiol. 2007;166:606–615. doi: 10.1093/aje/kwm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White WW. Anwar YA. Evaluation of the overall efficiency of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Pressure Monitoring. 2001;6:107–110. doi: 10.1097/00126097-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Cobbaert C. Boerma GJ. Lindemans J. Evaluation of the Cholestech L.D.X. desktop analyser for cholesterol, HDL-cholesterol, and triacylglycerols in heparinized venous blood. Eur J Clin Chem Clin Biochem. 1994;32:391–394. [PubMed] [Google Scholar]
- 8.Grundy SM. Brewer HB. Cleeman JI. Smith SC. Lenfant C for the Conference Participants. Definition of metabolic syndrome: report of the National Heart, Lung and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 9.>Census 2000 American Indian Alaska Native Summary File. http://factfinder.census.gov/servlet/Dataset-MainPageServlet. [Apr 28;2006 ]. http://factfinder.census.gov/servlet/Dataset-MainPageServlet
- 10.Arctic. In Encyclopedia Britannica. [Nov 28;2007 ]. http://www.britannica.com/eb/article-57869/ http://www.britannica.com/eb/article-57869/ from Encyclopedia Britannica Online.
- 11.Pollex RL. Hanley AJ. Zinman B. Harris SB. Khan HM. Hegele RA. Metabolic syndrome in aboriginal Canadians: Prevalence and genetic associations. Atherosclerosis. 2006;184:121–129. doi: 10.1016/j.atherosclerosis.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Liu J. Young TK. Zinman B. Harris SB. Connelly PW. Hanley AJ. Lifestyle variables, nontraditional cardiovascular risk factors, and the metabolic syndrome in an Aboriginal Canadian population. Obesity. 2006;14:500–508. doi: 10.1038/oby.2006.65. [DOI] [PubMed] [Google Scholar]
- 13.Pollex RL. Khan HM. Connelly PW. Young TK. Hegele RA. The metabolic syndrome in the Inuit. Diabetes Care. 2004;27:1517–1518. doi: 10.2337/diacare.27.6.1517-a. [DOI] [PubMed] [Google Scholar]
- 14.Kaler SN. Ralph-Campbell K. Pohar S. King M. Laboucan CR. Toth EL. High rates of metabolic syndrome in a First Nations Community in Western Canada: Prevalence and determinants in adults and children. Int J Circum Health. 2006;65:389–402. doi: 10.3402/ijch.v65i5.18139. [DOI] [PubMed] [Google Scholar]
- 15.Schraer CD. Ebbesson SO. Adler AI. Cohen JS. Boyko EJ. Nobmann ED. Glucose tolerance and insulin-resistance among St. Lawrence Island Eskimos. Int J Circumpolar Health. 1998;57(suppl 1):348–354. [PubMed] [Google Scholar]
- 16.Schraer CD. Risica PM. Ebbesson OE. Go OT. Howard BV. Mayer AM. Low fasting insulin levels in Eskimos compared to American Indians: are Eskimos less insulin resistant? Int J Circumpolar Health. 1999;58:272–281. [PubMed] [Google Scholar]
- 17.Resnick HE. Jones K. Ruotolo G. Jain AK. Henderson J. Lu W. Howard BV. Insulin resistance, the metabolic syndrome and risk of incident cardiovascular disease in nondiabetic American Indians. Diabetes Care. 2003;26:861–867. doi: 10.2337/diacare.26.3.861. [DOI] [PubMed] [Google Scholar]
- 18.Gray RS. Fabsitz RR. Cowan LD. Lee ET. Howard BV. Savage PJ. Risk factor clustering in the insulin resistance syndrome. Am J Epidemiol. 1998;148:869–878. doi: 10.1093/oxfordjournals.aje.a009712. [DOI] [PubMed] [Google Scholar]
- 19.North KE. Williams K. Williams JT. Best LG. Lee ET. Fabsitz RR. Howard BV. Gray RS. MacCluer JW. Evidence for genetic factors underlying the insulin resistance syndrome in American Indians. Obesity Research. 2003;11:1444–1448. doi: 10.1038/oby.2003.193. [DOI] [PubMed] [Google Scholar]
- 20.Young TK. Chateau D. Zhang M. Factor analysis of ethnic variation in the multiple metabolic (insulin resistance) syndrome in three Canadian populations. Am J Hum Biol. 2002;14:649–658. doi: 10.1002/ajhb.10083. [DOI] [PubMed] [Google Scholar]
- 21.Garber AJ. The metabolic syndrome. Med Clin N Am. 2004;88:837–846. doi: 10.1016/j.mcna.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome. A summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo C. Williams K. Hunt KJ. Haffner SM. The National Cholesterol Education Program-Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 24.Indian Health Service. Interim Report to Congress. Special Diabetes Programs for Indians. IHS National Diabetes Program. December 2004, pp 115–122. http://www.ihs.gov/MedicalPrograms/Diabetes/resources/r_rtc2004index.asp/ [Sep;2007 ]. http://www.ihs.gov/MedicalPrograms/Diabetes/resources/r_rtc2004index.asp/
- 25.Indian Health Service. US Department of Health and Human Services; Regional Differences in Indian Health 1998–99. [Google Scholar]
- 26.Burrows NR. Geiss LS. Engelau MM. Acton KJ. Prevalence of diabetes among Native Americans and Alaska Natives 1990-1997. An increasing burden. Diabetes Care. 2000;23:1786–1790. doi: 10.2337/diacare.23.12.1786. [DOI] [PubMed] [Google Scholar]
- 27.Schraer CD. Lanier AP. Boyko EJ. Gohdes D. Murphy NJ. Prevalence of diabetes mellitus in Alaska Eskimos, Indians and Aleuts. Diabetes Care. 1988;11:693–700. doi: 10.2337/diacare.11.9.693. [DOI] [PubMed] [Google Scholar]
- 28.Murphy NJ. Schraer CD. Bulkow LR. Boyko EJ. Lanier AP. Diabetes mellitus in Alaskan Yupik Eskimos and Athabascan Indians after 25 years. Diabetes Care. 1992;15:1390–1392. doi: 10.2337/diacare.15.10.1390. [DOI] [PubMed] [Google Scholar]
- 29.Schraer CD. Adler AI. Mayer AM. Halderson KR. Trimble BA. Diabetes complications and mortality among Alaska Natives: 8 years of observation. Diabetes Care. 1997;20:314–321. doi: 10.2337/diacare.20.3.314. [DOI] [PubMed] [Google Scholar]
- 30.Alaska Area Diabetes Program, Alaska Native Medical Center. http://www.anmc.org/services/diabetes/ [Oct 23;2007 ]. http://www.anmc.org/services/diabetes/
- 31.Ford ES. Giles WG. Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 32.Shemesh T. Rowley KG. Shephard M. Piers LS. O'Dea. Agreement between laboratory results and on-site pathology testing using Bayer DCA2000+ and Cholestech LDX point-of-care methods in remote Australian Aboriginal communities. Clin Chim Acta. 2006;367:69–76. doi: 10.1016/j.cca.2005.11.014. [DOI] [PubMed] [Google Scholar]