Abstract
Horizontal gene transfer is an important mechanism for generating genetic diversity. As the number of sequenced genomes continues to increase, so do the examples of horizontal genetic exchange between both related and divergent organisms. Here we discuss the recent finding that certain strains of the human pathogen Neisseria gonorrhoeae have incorporated a small fragment of human DNA sequence into their genomes. The horizontally acquired sequence exhibits 98–100% nucleotide identity to a 685 bp portion of the highly repetitive retrotransposable element L1 and its presence in the gonococcal genome has been confirmed by multiple molecular techniques. The possibility of similar L1 horizontal gene transfer events having occurred in other bacteria based on genomic sequence evidence is explored. Potential mechanisms of how N. gonorrhoeae was able to acquire and maintain this human sequence are also discussed in addition to the evolutionary implications of such an event.
Key words: Neisseria gonorrhoeae, horizontal gene transfer, LINE, L1
Neisseria gonorrhoeae, or the gonococcus, is the causative agent of the human-specific sexually transmitted infection gonorrhea that occurs frequently in both developed and under-developed nations. It is well recognized that N. gonorrhoeae lacks reservoirs outside of the human genitourinary tract and is therefore uniquely and highly adapted to its host niche. Given this exclusive relationship, and the ability of N. gonorrhoeae to readily take up foreign DNA, it is conceivable that N. gonorrhoeae would encounter and be capable of incorporating host DNA into its genome. In support of this notion, recent examination of publically available gonococcal genome sequences (www.broadinstitute.org/, Genbank) in addition to molecular genotyping, has provided strong evidence that certain gonococcal isolates carry a small fragment of DNA in their genomes that match sequences in the human genome.1
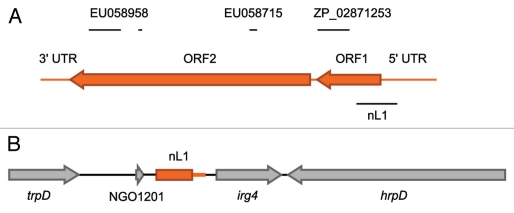
The horizontally acquired fragment of human DNA present in the gonococcal genome corresponds to a 685 bp region of the Long Interspersed Nuclear Element (LINE) L1 (Fig. 1). Despite the vast differences in genetic composition and selective pressures between humans and N. gonorrhoeae, the L1 sequence contained within Neisseria (nL1) retains a remarkable degree of similarity to human copies (≥98% identity), with at least one sequenced L1 being identical to nL1 across the entire 685 bases (Genbank, AC013546). Although other mammalian genomes harbor LINE DNA with high identity to the nL1 sequence (e.g., Pan troglodytes ≤98%), the exclusive relationship between humans and N. gonorrhoeae suggests that humans are the most likely source of donor DNA. Since sequence contamination could easily result in the appearance of a horizontal gene transfer (HGT) event between humans and bacteria, it was necessary to conclusively demonstrate the authenticity of nL1 insertion in the gonococcal genome. We accomplished this using a combination of PCR amplifications specific for the nL1 sequence and flanking gonococcal genetic region, DNA hybridization, and independent sequencing. The results of these experiments verified the composition and location of nL1 as reported in the N. gonorrhoeae genome sequences. The number of gonococcal isolates that have been subjected to whole genome sequencing thus far (16) represents a small sample size and it was necessary to screen additional isolates to estimate the frequency of nL1 occurrence in the population. Seven total nL1-positive strains were identified among 62 tested isolates. The majority of N. gonorrhoeae infections result in an uncomplicated genitourinary disease, but in rare cases can take the form of disseminated gonococcal infection or pelvic inflammatory disease. Interestingly, the presence of nL1 in gonococcal genomes did not correlate with any particular disease phenotype and representative isolates from each disease manifestation were found to carry nL1. Several related Neisseria species were also screened for the presence of nL1, though this sequence was not detected outside of N. gonorrhoeae. Significantly, nL1 was completely absent from the 212 Neisseria meningitidis isolates that were screened, implying that the acquisition of nL1 occurred relatively recently in evolutionary history, after the divergence of N. gonorrhoeae and N. meningitidis.
Figure 1.
Schematic of the N. gonorrhoeae nL1 HGT event. (A) Gene organization of a full-length L1 retrotransposable element (Genbank, U09116.1). The sequence corresponding the nL1 fragment found in N. gonorrhoeae is indicated by the black line below the L1 element. The horizontal lines above the L1 element indicate approximate locations of sequences that were identified by BLAST analysis as having a high degree of similarity to other bacterial genome or protein sequences, as described in the text. The NCBI DNA and protein accession numbers given above the lines indicate the specific bacterial sequences that exhibit identity to L1. (B) The nL1 fragment (orange) containing portions of L1 ORF1 and the 5′ UTR is shown in relation to neighboring gonococcal genes (gray arrows).
All seven of the nL1 inserts were identical in sequence and genomic location. This absolute conservation was striking and several factors may contribute to this observation. It is possible that the presence of nL1 in independent isolates was the result of multiple targeted translocation events. However, this scenario seems unlikely given that a seemingly random fragment of the L1 element is present in gonococci, the insert sequences and locations are identical, and there are no sequence signatures indicative of a targeted event. A more likely possibility is that the presence of nL1 in multiple strains is the result of a single HGT event that was propagated within the gonococcal population. Vertical transmission of nL1 by way of clonal expansion or horizontal transmission of nL1-containing genomic DNA between N. gonorrhoeae isolates are both plausible scenarios given the nature of bacterial replication and that N. gonorrhoeae are naturally competent and readily exchange DNA within the species. Our results using multilocus sequence typing2 favors the latter hypothesis since multiple sequence types were represented among the different nL1-positive isolates. The sequence conservation of nL1 among gonococcal isolates might also imply that there is a selective advantage conferred to N. gonorrhoeae by nL1. While this possibility is remote, given that nL1 is present in only ∼11% of the strains examined in this study and only a portion of the L1 ORF1 gene was acquired, our study was able to demonstrate that transcripts containing the nL1 sequence were detectable in N. gonorrhoeae RNA preparations. As a result, it is possible that a product is produced from the nL1 sequence and/or that the nL1 insertion could alter the transcriptional activity of nearby genes, both of which may influence the physiology of N. gonorrhoeae.
The horizontally acquired fragment of human DNA present in the gonococcal genome corresponds to a 5′ portion of the ORF1 gene, which provides L1 nucleic acid chaperone activity3 in addition to a portion of the 5′ untranslated region (Fig. 1). The mobility of L1 elements through a target primed reverse transcription mechanism is dependent on the ORF1 protein product and the endonuclease and reverse transcriptase functions provided by the ORF2 encoded protein.4,5 Most of the L1 copies in the human genome are no longer competent for retrotransposition but a small portion of the sequences are capable of mobilization to new genomic locations,6 although it is very unlikely that the mobilization of nL1 to the gonococcal genome was accomplished via retrotransposition.
Other examples of eukaryote-to-bacteria HGT events have been described in the literature;7–9 however, in these cases the degree of identity between donor and recipient sequences is considerably lower that what we have observed with nL1. In fact, the degree of identity between nL1 and the corresponding human sequence may be problematic in terms of identifying other similar HGT events since they may simply be regarded as the result of sequencing contamination. A recent survey of non-primate genomic sequence databases using the primate-specific repetitive element AluY as a search query revealed that of 2,749 public databases, 492 contained this specific human sequence, including bacterial species.11 The vast majority of these examples are likely the result of sequence contamination, but our findings establish the possibility that apparent contamination events may actually be the result of authentic HGT. There may even be related L1 HGT events present in other bacterial genomes. BLAST analysis using a full-length L1 DNA sequence (Genbank, U09116.1) and restricted to bacterial genomes results in the identification of genomic sequence fragments with a high degree of identity to L1. Two of these entries (NCBI nucleotide, EU058715.1 and EU058958.1) belong to uncultured bacteria originating from a metagenomics library created from human fecal material.11 Both sequences contain fragments that exhibit ≥94% identity to L1 for fragments of up to 660 nucleotides (Fig. 1). Similarly, if the L1 ORF1 protein sequence is used as the query more potential examples are identified. A hypothetical protein from a single-cell human oral cavity bacterial isolate TM7a12 exhibits 84% identity to L1 ORF1 encoded protein over 109 amino acids (NCBI protein, ZP_02871253.1). Although experimental evidence to determine the authenticity of these potential human-to-bacteria HGT events is currently lacking, these results raise the possibility that the acquisition of nL1 by N. gonorrhoeae is not unique and that any likely candidates will need to be experimentally investigated on a case by case basis. It must also be considered that similar HGT events may be more frequent than anticipated since reads with a high likelihood of sequence contamination are often identified by bioinformatic screening and automatically removed.
Our findings have firmly established the possibility that genetic exchange can occur between humans and resident microbes, yet there are several aspects of this finding that remain unresolved. One important question is whether or not the specific sequence that was acquired has biological significance. It is possible that nL1 was acquired simply because it is statistically favored due to its highly repetitive nature in the human genome.13 Alternatively, other undetected HGT events between humans and N. gonorrhoeae may have occurred but were not maintained due to a lack of selective advantage or the disruption of critical gonococcal sequences. Since nL1 was inserted into a non-coding region of a disrupted gonococcal prophage copy,14 this insertion site may have contributed to the maintenance of nL1. The mechanism by which the nL1 insertion occurred is also not known. N. gonorrhoeae is naturally competent and the most likely mechanism of acquisition would be via the standard transformation machinery. However, the specific mechanism of recombination remains unclear. Comparison of the human L1 sequence and the nL1 flanking region reveals no sequence homology that would facilitate homologous recombination. Nonhomologous DNA end joining, though possible, is likely to be inefficient in N. gonorrhoeae. Finally, the mechanism by which the host DNA became available is also a matter of speculation. N. gonorrhoeae associates with both epithelial and innate immune cells during the course of infection and can survive both intracellularly and extracellularly. However, there is no evidence for nuclear localization of gonococci in infected cells. Therefore, exposure to human DNA may have occurred following host cell death and subsequent release of genetic material.
HGT can be simply defined as a genetic exchange between organisms that is not the result of mating and it is an important source of genetic diversity during evolutionary adaptation. One would predict that the frequency of HGT is inversely proportional to the genetic distance between two organisms. However, this work shows that genetic exchange between two highly divergent, yet intimately associated organisms is possible. Though there is currently no physiological consequence associated with this event, this finding has broad evolutionary implications for both host and pathogen.
Acknowledgments
This work was supported by NIH grants RO1 AI044239 and R37 AI033493 to H.S.S. M.T.A. was partially supported by NIH fellowship F32 AI080083. We would also like to acknowledge A. Chen for contributions to the manuscript.
Abbreviations
- LINE
long interspersed nuclear element
- HGT
horizontal gene transfer
- nL1
neisseria L1
References
- 1.Anderson MT, Seifert HS. Opportunity and means: horizontal gene transfer from the human host to a bacterial pathogen. mBio. 2011;2:5–11. doi: 10.1128/mBio.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett JS, Jolley KA, Sparling PF, Saunders NJ, Hart CA, Feavers IM, et al. Species status of Neisseria gonorrhoeae: evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol. 2007;5:35–45. doi: 10.1128/mBio.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin SL, Bushman FD. Nucleic acid chaperone activity of the ORF1 protein from the mouse LINE-1 retrotransposon. Mol Cell Biol. 2001;21:467–475. doi: 10.1128/MCB.21.2.467-75.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cost GJ, Feng Q, Jacquier A, Boeke JD. Human L1 element target-primed reverse transcription in vitro. EMBO J. 2002;21:5899–5910. doi: 10.1093/emboj/cdf592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin SL, Cruceanu M, Branciforte D, Wai-Lun Li P, Kwok SC, Hodges RS, et al. LINE-1 retrotransposition requires the nucleic acid chaperone activity of the ORF1 protein. J Mol Biol. 2005;348:549–561. doi: 10.1016/j.jmb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, et al. Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci USA. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guljamow A, Jenke-Kodama H, Saumweber H, Quillardet P, Frangeul L, Castets AM, et al. Horizontal gene transfer of two cytoskeletal elements from a eukaryote to a cyanobacterium. Curr Biol. 2007;17:757–759. doi: 10.1016/j.cub.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins C, Samudrala R, Anderson I, Hedlund BP, Petroni G, Michailova N, et al. Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter. Proc Natl Acad Sci USA. 2002;99:17049–17054. doi: 10.1073/pnas.012516899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lurie-Weinberger MN, Gomez-Valero L, Merault N, Glockner G, Buchrieser C, Gophna U. The origins of eukaryotic-like proteins in Legionella pneumophila. Int J Med Microbiol. 300:470–481. doi: 10.1016/j.ijmm.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Longo MS, O'Neill MJ, O'Neill RJ. Abundant human DNA contamination identified in non-primate genome databases. PloS One. 6:16410. doi: 10.1371/journal.pone.0016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manichanh C, Chapple CE, Frangeul L, Gloux K, Guigo R, Dore J. A comparison of random sequence reads versus 16S rDNA sequences for estimating the biodiversity of a metagenomic library. Nucleic Acids Res. 2008;36:5180–5188. doi: 10.1093/nar/gkn496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcy Y, Ouverney C, Bik EM, Losekann T, Ivanova N, Martin HG, et al. Dissecting biological “dark matter” with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc Natl Acad Sci USA. 2007;104:11889–11894. doi: 10.1073/pnas.0704662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 14.Kawai M, Uchiyama I, Kobayashi I. Genome comparison in silico in Neisseria suggests integration of filamentous bacteriophages by their own transposase. DNA Res. 2005;12:389–401. doi: 10.1093/dnares/dsi021. [DOI] [PubMed] [Google Scholar]