Abstract
Acute kidney injury (AKI) is a major clinical problem associated with high morbidity and mortality. Likely due to its complex pathophysiology, therapies with a single pharmacological agent have generally failed to improve outcomes. In contrast, stem cell-based interventions utilize these cells' ability to simultaneously target multiple pathophysiological components of AKI and thus represent a promising new tool for the treatment of AKI. The aims of the this study were to investigate the long-term outcome and safety of treatment with autologous and allogeneic mesenchymal stem cells (MSCs) after AKI and the role of vascular endothelial growth factor (VEGF) as one of the principal paracrine mediators of renoprotection of MSCs. MSC administration after AKI was not associated with adverse events and proved to be renoprotective in animals with severe renal failure. Identical doses of autologous MSC were more effective than allogeneic. At 3 months, MSCs were not engrafted in any tissues except in the bone marrow in 50% of animals given the highest allogeneic cell dose. There was no long-term fibrotic response in the kidneys attributable to MSC therapy, and animals with severe AKI were protected from development of fibrotic lesions after AKI. Furthermore, this study establishes VEGF as a critical factor mediating renal recovery. VEGF knockdown by small-interfering RNA reduced effectiveness of MSCs significantly and decreased survival. In summary, our results show that both autologous and allogeneic MSC are safe and effective in AKI, and importantly, reduce late renal fibrosis and loss of renal function in surviving animals and that VEGF is a critical factor in renoprotection by MSCs. Together, we posit that these data provide further justification for the conduct of clinical trails in which AKI is treated with MSC.
Introduction
The treatment of acute kidney injury (AKI) has remained a daunting task. Its incidence is on the rise and it is increasingly recognized as a significant cause of end-stage renal disease [1]. Due to AKI's complex pathophysiology, it would be expected that therapies with the ability to simultaneously target multiple components of its pathophysiology should afford the highest degree of protection. Adult, bone marrow–derived stem cells (BMDSCs) were found to be very effective in the treatment of organ injuries in animal studies [2]. Importantly, BMDSCs have already found their way into the clinic and proven to be safe and effective in the treatment of humans with various disorders [2].
Despite intensive research, the mechanisms of action of cell therapy in the various models of AKI remain to be defined. Although replacement of damaged parenchymal cells by differentiated hematopoietic stem cells and multipotent marrow stromal or mesenchymal stem cells (MSCs) has been shown to occur in principle, this process takes considerable time and occurs only under rare circumstances thereby making this a subordinate mechanism whereby organ repair is carried out. We and others have shown that administered MSC secrete a number of factors that are known to be renoprotective, thereby making paracrine or endocrine mechanisms the most likely modus operandi whereby administered cells exert their organ protective and repair stimulating effects [3–5]. MSC are currently the cell type with the highest potential for cell-based therapies because they can be rapidly generated from bone marrow, both in an autologous and allogeneic setting, because large-scale culture expansion can be achieved easily, and because they secrete a large number of growth factors and have immunomodulatory properties [6]. MSCs have been shown to be renoprotective in cisplatin- and ischemia/reperfusion-induced AKI [7–9] in a glomerulonephritis model [10] and are potentially useful in other renal diseases like Alport's syndrome [11–13].
Vascular endothelial growth factor (VEGF) is not only a growth and survival factor primarily acting on endothelial cells, but is also involved in matrix remodeling, monocyte chemotaxis, and adhesion molecule expression [14]. VEGF has been shown to be a critical factor for glomerular function and stimulates proliferation of peritubular capillaries, which is essential to tubular regeneration [14]. Furthermore, VEGF directly protects renal epithelial cells [15,16]. MSCs secrete considerable quantities of VEGF and have vasculoprotective properties [4,17]. For these reason we hypothesized that VEGF is a major mediator of renoprotection exerted by MSCs. Molecular techniques such as small-interfering RNA (siRNA) allow the specific knockdown of a molecule in a cell population and are thereby ideal tools to test this hypothesis.
Because there are insufficient dose response and long-term outcomes data from animals whose AKI was treated with MSC, this study was designed to assess the effectiveness of different doses of autologous and allogeneic MSC, as well as the long-term outcomes of animals with ischemia/reperfusion AKI. Besides monitoring of physiological variables, animals were screened for the development of renal fibrosis or other potential serious side effects such as ectopic differentiation of MSCs or tumor formation. Treatment with both autologous and allogeneic MSC was found to be safe and effective in the short term and did not lead to increased renal fibrosis 3 months after AKI, and reducing VEGF secretion by siRNA knockdown reduced their effectiveness significantly. Furthermore, MSC therapy ameliorated the development of fibrotic reactions and chronic inflammation in the kidneys in animals with severe initial AKI. We conclude that VEGF is an important mediator of renoprotection by MSCs, and our results further justify the timely conduct of clinical trials in which patients at high risk for or with established AKI are treated with MSC.
Materials and Methods
Animals and cells
All procedures involving animals were approved by the Institutional Animal Use and Care Committees of the University of Utah and Veterans Affairs Medical Center. Heat-resistant human placental alkaline phosphatase (hPAP) transgenic rats were kindly provided by Dr. Eric Sandgren's laboratory [18].
MSCs for all experiments were generated by standard procedures. In brief, femurs of sacrificed rats were flushed with saline, and bone marrow cells were collected in tubes, washed, and placed in plastic Falcon tissue culture flasks (BD Biosciences, San Jose, CA). After 3 days of culture, non-adherent cells were removed and attached MSCs expanded in DMEM/F12 (Sigma) and 10% fetal bovine serum (FBS) (Hyclone, Logan, UT).
For series 1 experiments, MSCs derived from a transgenic hPAP rat were transfected with a G418 resistance gene after expansion over more than 30 passages and selected for transfected cells by G418-medium. MSCs were phenotypically unchanged but had lost their ability to differentiate into mesenchymal lineages (fat-cartilage-bone), most likely due to prolonged culture [19].
For series 2 and 3 experiments, MSCs were derived from a newborn hPAP positive animal, using standard procedures [7], and expanded in culture with DMEM/F12 (Sigma) and 10% FBS (Hyclone, Logan, UT) until sufficient cells for administration were generated. Before administration, batches were tested for mesenchymal differentiation capability and were only used when MSC ISCT criteria were fulfilled [20]. For all experiments, groups (n = 6 per group) of adult male Sprague-Dawley (SD) or Fisher 344 (F344) rats, weighting 200–300 g, were used (Charles River, Wilmington, MA).
MSC characterization
MSCs were generated from hPAP transgenic F344 rats according to standard procedures. Culture expanded MSCs were negative for CD45 and positive for CD59 and CD90 expression, determined by FACS analysis (data not shown). At the time of administration (approximately 30 passages), MSC for series 2 and 3 experiments readily differentiated into adipocytes, chondrocytes, and osteocytes, and exhibited the characteristic panel of MSC surface markers, confirming the ISCT criteria for MSCs [20,21]. MSCs from series 1 experiments were transfected with a G418 resistance plasmid using the Ca-Pho method for selection and tracking, and a resistant clone was selected and expanded. These MSCs were phenotypically unchanged in vitro but failed to differentiate into adipocytes, chondrocytes, and osteocytes, most likely due to prolonged culture and selection [19].
Surgical procedures and MSC treatment
Ischemia/reperfusion AKI was induced as described before [8]. Three series of experiments were performed containing six animals per group: Series 1 late passage experiments (Allo1) were carried out to determine the renoprotective potential of allogeneic MSCs after severe AKI. Accordingly, renal pedicles of SD rats were clamped for 58 min, and animals were infused immediately after reflow via the left carotid artery with 1.5 × 106 hPAP F344 G418 MSCs in 1 mL of phosphate-buffered saline (PBS). All controls with identical AKI were infused, via the left carotid artery, with an identical volume of PBS. Series 2 experiments (Allo2) were conducted to determine if there exists a dose-response relationship between the number of administered MSCs and the degree of renoprotection that is obtained. Therefore, a low (0.5 × 106 per kg bodyweight (BW)), medium (2 × 106 per kg BW), and high dose (5 × 106 per kg BW) of F344-derived hPAP MSCs was administered once to SD rats via the left carotid artery after 40 min of bilateral renal pedicle clamping and confirmed reflow. Finally, to determine the effectiveness of MSCs in the autologous setting, series 3 experiments (Auto1) were conducted. Accordingly, a low (0.5 × 106 per kg BW), medium (2 × 106 per kg BW), and high dose (5 × 106 per kg BW) of F344-derived hPAP MSCs was administered once to syngeneic F344 rats following 35 min of renal clamping and confirmed reflow.
Sham operated animals (n = 3) were treated exactly as controls but renal pedicles were not clamped.
Screening for side effects
At the time of sacrifice, major organs of animals were macroscopically inspected for tumor formation or ectopic differentiation. Tissue sections were prepared for histology and examined by a pathologist in a blinded fashion.
Kidney function
Serum creatinine, BUN, and urinary protein excretion were determined using the Dimension RxL Max Clinical Chemistry System (Dade Behring, Deerfield, IL). Proteinuria and creatinine clearances were determined in 24-h urines collected in metabolic cages. Urine osmolarity was measured by changes in freezing point thermodynamics, using The Advanced™ Osmometer Model 3D3 (Advanced Instruments, Norwood, MA). Blood pressure was determined by tail cuff plethysmography after the animal had been properly accustomed to the utilized procedure.
PCR
All primers were ordered from the University of Utah nucleotide core facility. Tracking of administered cells was performed in tissues by polymerase chain rection (PCR) for the hPAP transgene using primer sequences for the SVpoly40 promoter [18]:
SVpoly-1f: 5′-CTGATGAATGGGAGCAGTGGTGGAATG-3′ and SVpoly-2r: 5′-GCAGACACTCTATGCCCTGTGTGGAG-3′, producing a 360-bp product in transgenic cells.
RT-PCR for fibrotic genes
Real-time PCR with relative quantification of target gene copy numbers in relation to beta-actin transcripts was carried out using the following primers: tumor growth factor-β (TGF-β): 5′-GGACTACTACGCCAAAGAAG-3′ and 5′-TCAAAAGACAGCCACTCAGG-3′; plasminogen activator inhibito-1 (PAI)-1: 5′-GAGCCAGATTCATCATCAACG-3′ and 5′-CTGCAATGAACATGCTGAGG-3′
Histology
Kidneys were histologically evaluated at 3 months after AKI for Allo1, Allo2, and Auto1 groups. Kidney coronary sections were paraffine embedded and 4 μm sections were stained with trichrome and PAS, according to standard protocols. Fibrosis scoring in kidneys was performed by a blinded pathologist, using a scoring system of 1–5, with the following criteria: 0—normal; 1—mild tubular atrophy/interstitial fibrosis; 3—moderate tubular atrophy/interstitial fibrosis; 5—marked tubular atrophy/interstitial fibrosis.
VEGF siRNA knockdown
Cultured MSCs were treated with siRNA targeted at three different exons of the VEGF gene, common to all splice variants (exons 2–6) and NeoFx transfection agent (Ambion, Austin, TX). Silencer® predesigned siRNAs were purchased (Ambion, Austin, TX) and tested at three different concentrations (5, 10, 30 nM) in standard culture medium. Cells were incubated for 24 h with siRNA and washed with PBS. A concentration of 10 nM proved to be most effective and was therefore used for all subsequent experiments. Morphology, viability, and growth rate were not different between VEGF siRNA treated and control MSC preparations. Controls consisted of cells treated with Silencer® negative control siRNA (Ambion, Austin, TX), NeoFx transfection agent only, and untreated cells. Gene expression of VEGF was assessed 24 and 48 h after knockdown by quantitative reverse transcriptase (RT)-PCR with a SmartCycler (Cepheid, Sunnyvale, CA) and VEGF protein secretion measured by enzyme-linked immunosorbent assay (ELISA) (RnD Systems, Minneapolis, MN) in medium conditioned for 24 h. Injection experiments with VEGF knockdown MSCs and wild-type MSCs after AKI (n = 6 per group) were carried out as described above. Animals were followed for 4 weeks and sacrificed thereafter.
Statistical analyses
Data are presented as means ± SD, unless otherwise stated. Statistical analyses were performed using GraphPad Prism 4 for Macintosh (GraphPad Software, San Diego, CA). Analysis of variance and t-tests were used to assess differences between data means as appropriate. A P value of <0.05 was considered significant.
Results
Allogeneic MSCs after AKI
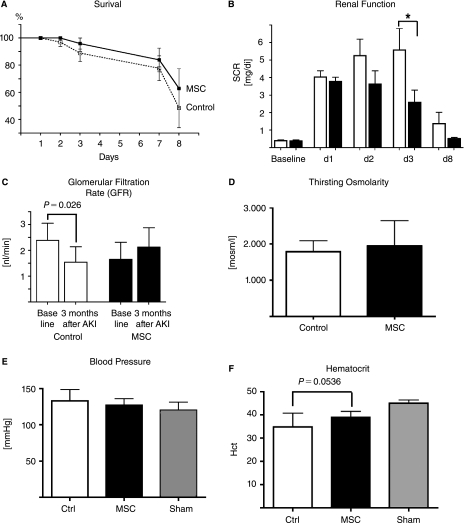
In the Allo1 series, the effectiveness of allogeneic MSCs in AKI was determined. 1.5 × 106 hPAP G418 resistant MSCs (corresponding to ∼5.5 × 106 MSCs per kg BW) were infused into the suprarenal aorta of rats with ischemia/reperfusion AKI. The mortality in the MSC treatment group was lower but did not reach statistical significance using Kaplan-Meier analysis (P = 0.369) (Fig. 1A). Renal function, as determined by serum creatinine levels, recovered faster in MSC-treated animals starting at day 2 and became significant at day 3 (P = 0.028) (Fig. 1B).
FIG. 1.
The effect of allogeneic MSCs after AKI (Allo 1). (A) MSC-treated animals showed a trend towards improved survival after severe AKI. (B) Renal function as determined by serum creatinine. MSC-treated animals had significantly better renal function on day 3 after AKI compared to controls. (C) Creatinine clearance at baseline and 3 months after AKI. Control animals showed a significant decline in renal function compared to baseline demonstrating a loss of function induced by AKI (white bars). In contrast, MSC-treated animals showed preservation of renal function compared to baseline, indicating a protective effect of MSCs in the long-term. (D) Urine concentration ability as demonstrated by thirsting urine osmolarity was not different between groups. (E) Tail blood pressure showed no difference between groups. (F) Hematocrit, an indicator of renal anemia and potential compromise in renal function, was decreased in control animals and relatively preserved in MSC-treated animals, but this did not reach statistical significance. N = 6 for all groups. Age of the rats at the time of evaluation of the parameters was ∼6 months.
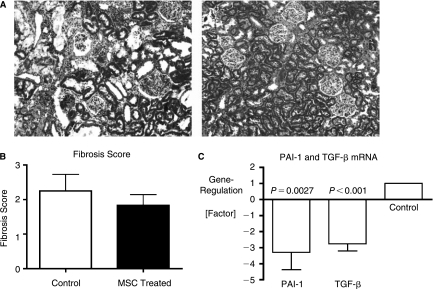
The overall aim of this series was to assess the long-term effects of MSC therapy in rats with AKI compared to controls. Accordingly, animals were maintained on standard living conditions for 3 months after induction of AKI, and glomerular filtration rate (GFR), thirsting urinary concentration ability, blood pressure, body weight and hematocrits were determined. Surviving, vehicle-treated control animals showed a significant decline in creatinine clearance 3 months after AKI (P = 0.026, Fig. 1C) compared to baseline, whereas MSC-treated animals had preserved renal function with a slight increase in GFR. Urinary concentrating ability, determined by urine osmolarity, after 24 h of thirsting was not different between groups (Fig. 1D), as was blood pressure (Fig. 1E). Hematocrit was higher in MSC-treated animals but did not reach statistical significance (P = 0.054, Fig. 1F). To determine the extent of renal fibrosis, trichrome-stained kidney sections were evaluated in blinded fashion by a pathologist. Control animals had more trichrome positive collagenous connective tissue in the renal interstitium as well as greater interstitial inflammatory cell accumulation (Fig. 2A, left panel), reflected by the respective fibrosis score (2.25 vs. 1.83, Fig. 2B) and the higher renal expression of profibrotic genes PAI-1 and TGF-β, respectively (P < 0.05, Fig. 2C).
FIG. 2.
Allogeneic MSCs after AKI (Allo 1). Renal histology at 3 months after AKI. Representative sections of an animal per group are shown. (A) Renal cortex, trichrome staining. There is marked fibrosis and interstitial inflammation in control animals (left panel), whereas MSC-treated animals have only minimal signs of interstitial fibrosis (right panel). Magnification 40×. (B) Renal fibrosis scoring by a pathologist in a blinded fashion reflects lower interstitial fibrosis in MSC-treated animals at 3 months after AKI. (C) Gene expression of PAI-1 and TGF-β in control compared to MSC-treated animals. PAI-1 and TGF-β mRNA are significantly downregulated in MSC-treated animals compared to controls. N = 6 for all groups.
We showed previously that administered MSC, can only be detected for approximately up to 72 h after infusion. We utilized, therefore, the transgenic marker hPAP to detect infused cells in major organs by a highly sensitive RT-PCR assay. At 3 months, brain, liver, lung, spleen, and kidneys were negative for hPAP, whereas three of six animals had a positive hPAP signal in the bone marrow, indicating that some infused cells had engrafted at this site.
Allogeneic MSC dose-response study after AKI
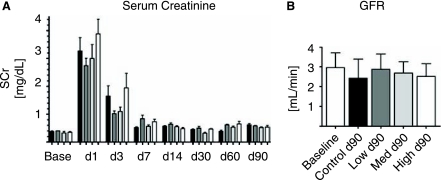
To further define the relationship between the number of administered MSCs and recovery from renal failure, we undertook a dose-response study using the same rat model. MSC in this series (Allo2) were expanded for ∼30 passages and not transfected with the G418 resistance plasmid. There was no statistically significant renoprotection of MSC-treated animals at any administered dose although there was a trend toward better renal function in low- and medium-dose groups (Fig. 3A). Creatinine clearance at 3 months was not different between groups (Fig. 3B), as were hematocrit, thirsting osmolarity, proteinuria, and blood pressure (data not shown). No animal had a positive signal for the transgene hPAP in heart, lung, liver, spleen, kidney, or bone marrow as determined by RT-PCR (data not shown), suggesting that there was no long-term engraftment of administered MSCs.
FIG. 3.
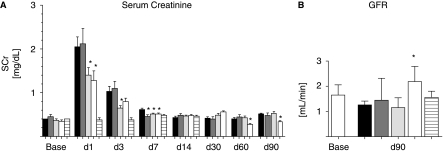
Allogeneic dose-response study after AKI (Allo 2). (A) Renal function as determined by serum creatinine [SCr]. Black bars: Control group; grey bars: low-dose group (0.5 × 106 cells per kg BW; light grey bars: medium-dose group (2 × 106 cells per kg BW); white bars: high dose group (5 × 106 cells per kg BW). N = 6 per group. (B) Glomerular filtration rate (GFR) determined by creatinine clearance at 90 days after AKI compared to baseline. N = 6 for all groups.
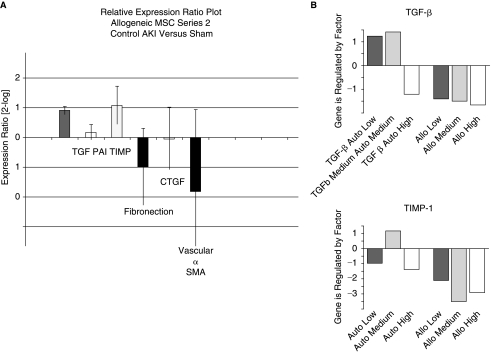
Testing a panel of fibrosis-related genes [TGF-β, PAI-1, tissue inhibitor of matrix metalloprotease-1 (TIMP-1), Fibronectin, connective tissue growth factor, α-smooth muscle actin], real-time quantitative PCR from renal cortical tissue showed a significant upregulation of TGF-β and TIMP-1 in control animals compared with a sham group (Fig. 4, left panel). Both genes were significantly downregulated in MSC-treated groups with no significant differences between dosing groups (Fig. 4, right panels).
FIG. 4.
Gene expression analysis by real-time quantitative PCR in kidneys 3 months after AKI. (A) TGF-β and TIMP are significantly upregulated in control AKI compared to sham animals. PAI-1, fibronectin, CTGF, and vascular-α-SMA are not expressed differently. (B) TGF-β and TIMP expression levels are lower in the allogeneic and high-dose autologous MSC groups compared to control animals.
Histological scores of kidneys correlated with these data, showing a lower fibosis score in MSC-treated animals compared with control groups (1.3 vs. 1.5). Although all control animals showed chronic interstitial inflammation, MSC-treated animals only had mild tubular atrophy and interstitial fibrosis without inflammatory reaction.
Autologous dose-response study after AKI
To determine the effectiveness and a potential dose response effect of MSC in the autologous setting, a study with hPAP MSC in syngeneic F344 rats was undertaken with the same ischemia/reperfusion model (Auto1). MSC-treated animals were significantly renoprotected in the medium- and high-dose groups (2 × 106 and 5 × 106 MSC per kg BW, respectively) but not in the low-dose group (Fig. 5A). Creatinine clearance at 3 months after AKI was significantly better only in the high-dose MSC treatment group (Fig. 5B). There were no differences in hematocrit, blood pressure, thirsting urinary osmolarity, and proteinuria between groups. One animal had a positive signal for hPAP in bone marrow and one in the heart, whereas all other animals had no positive PCR signals in heart, lung, liver, spleen, kidney, or bone marrow, suggesting the absence of long-term engraftment of administered MSCs (data not shown).
FIG. 5.
Autologous dose-response study after AKI (Auto 1). (A) Renal function as determined by serum creatinine [SCr]. Black bars: Control group; grey bars: low-dose group (0.5 × 106 cells per kg BW; light grey bars: medium-dose group (2 × 106 cells per kg BW); white bars: high-dose group (5 × 106 cells per kg BW); horizontal stripes: sham-operated group. N = 6 per group. (B) GFR determined by creatinine clearance at d90 after AKI compared to baseline. Control group, low- and medium-dose groups had a decline in GFR after AKI compared to baseline (not significant). High-dose treatment group had a significant better GFR compared to controls (P < 0.05). There is no change in GFR in sham-operated animals.
There was no difference in gene expression for fibrotic genes and fibrosis scores, which is most likely a reflection of the milder renal injury that was induced in this group.
VEGF knockdown in MSCs reduces renoprotective activity
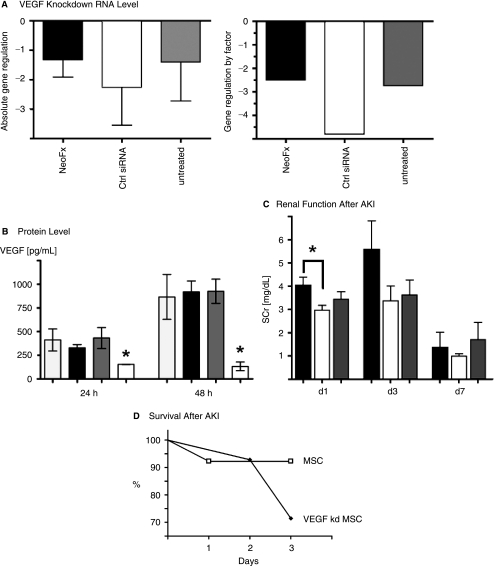
To determine if VEGF is a significant mediator of renoprotection after AKI, we tested wild-type MSCs and MSCs in which VEGF expression had been knocked down using a siRNA approach. Treatment with three different siRNAs at a concentration of 10 nM targeted against VEGF exons 2–6 resulted in absolute gene downregulation [2-log] of VEGF messenger RNA (mRNA) by −1 to −3, as determined by quantitative real-time RT-PCR and REST-analysis (Fig. 6A). mRNA downregulation resulted in a significant decrease in VEGF secretion measured by ELISA (Fig. 6B). When compared to animals treated with unmodified MSCs, animals with AKI that were treated with VEGF knockdown MSC had higher mortality rates and reduced functional recovery (Fig. 6C and D).
FIG. 6.
VEGF knockdown validation in MSCs and treatment of AKI. (A) Absolute gene regulation is significantly decreased in VEGF siRNA-treated MSCs compared to controls (2-log scale, left panel). VEGF gene regulation by factor (right panel). The graphs show VEGF mRNA levels of VEGF siRNA treated MSCs compared to: MSCs treated with NeoFX transfection agent only, MSCs treated with control siRNA, and untreated MSCs. (B) VEGF secretion into culture medium as measured by ELISA is significantly reduced in 24 and 48 h following VEGF siRNA treatment compared to controls. *P < 0.05. (C) Renal function (SCr) recovery is delayed in VEGF knockdown MSC-treated animals compared to MSC-treated animals. Because animals with severe AKI were dead before day 3 (see panel D), the graph is biased towards lower AKI in the VEGF siRNA group. *P < 0.05. N = 6 per group. (D) Survival is decreased in VEGF knockdown MSC-treated animals compared to animals treated with regular MSCs.
Discussion
This study was designed to identify possible adverse effects of MSC treatment after AKI over a 3 months period, the human equivalent of 3–4 years of follow-up, to define the effectiveness and dose-dependency of MSC treatment of AKI and to test the hypothesis that VEGF is involved in renoprotection. We show that both autologous and allogeneic MSCs are effective in the immediate treatment for AKI, and furthermore, that there are no significant, MSC-induced adverse effects late after AKI, especially no increase in interstitial renal fibrosis and loss of function. VEGF is identified as mediator of survival and renoprotection by MSCs, because its knockdown increased mortality and led to a delayed recovery of renal function. MSC are able to protect from chronic renal damage, which may be related to the severity of the initial lesion, because it was only observed in rats with severe AKI. Whether this long-term protection is the direct result of MSCs' actions per se or due to the organ protection and enhanced recovery elicited by MSC in the period immediately after injury, cannot be determined directly from our data.
We show here for the first time in an in vivo setting that VEGF is an important mediator of the renoprotective effects of MSCs. Effective knockdown of VEGF by siRNA in administered MSCs increased mortality and led to delayed recovery of renal function. The reason that renal function at day 3 after injury seems not different between MSC and VEGF-knockdown MSC-treated animals is due to the mortality bias in the latter, because animals with most severe renal failure died and so their renal function could not be included into the analysis in Fig. 6C. VEGF is an important trophic factor for the kidney, and mediates a number of different responses such as cellular survival, vasodilation, angiogenesis, matrix remodeling, monocyte chemotaxis, and expression of adhesion molecules [14]. Intriguingly, renal VEGF expression is diminished in vivo after ischemia/reperfusion injury [22], and treatment with VEGF in the immediate period after ischemic injury ameliorates resulting damage [23]. Thereby, MSC-delivered VEGF appears to improve both short- and long-term function, including late decrease in renal function and fibrosis. Although VEGF is an important factor, as demonstrated by our data, it is likely not the only factor involved in renoprotection as was recently demonstrated by Imberti et al. [24]). Together, we posit that it is the ability of MSCs to target, through a complex set of mechanisms, several pathophysiological components of AKI, which appears to make cell therapy superior to single-agent pharmacological interventions.
There is concern that MSC administration might lead to or increase interstitial, renal fibrosis, because MSCs are mesodermal, fibroblast-like cells, and TGF-β, which is increased after renal damage and AKI, can promote proliferation and myofibroblast differentiation of MSC [25–28]. Kunter et al. have shown that the infusion of MSCs into the renal artery of rats with anti-Thy-1 glomerulonephritis ameliorates chronic renal injury (10), while they also observed that high numbers (up to 20%) of glomeruli contained MSCs differentiated into fat cells, potentially obstructing affected nephrons that was associated with periglomerular fibrotic response [29]. When MSCs are infused into the suprarenal aorta, as was done in all studies, no adipogenic differentiation of MSCs is observed, in part due to the fact that cells remain no longer than 3 days in the kidney. Endogenous bone marrow–derived cells have also been shown to contribute to interstitial fibrosis after AKI [25]. Finally, late-passage MSCs have been shown to support tumor growth in animal models [30].
It has been reported that MSC can reduce organ fibrosis in various injury models, e.g., in lung and liver fibrosis as well as in Alport's syndrome [11,31–34]. There are significant differences between endogenous bone marrow cells and exogenously administered cells regarding their respective contribution to organ fibrosis. Although endogenous bone marrow derived cells have been shown to contribute to renal fibrosis [25], this has not been documented for exogenously administered MSC. MSCs also have immunomodulatory, mainly antiinflammatory properties, and inflammatory conditions are linked to fibrosis [28,35,36]. It seems therefore plausible that MSCs might be protective against the development of fibrosis by means of their robust antiinflammatory actions. We show here that exogenously administered MSCs do not increase renal fibrosis at 3 months after AKI but rather decreased renal fibrotic reactions in two of the three series of experiments. Protection was obtained with MSC therapy in animals that would develop marked fibrotic responses because of the higher initial severity of the AKI, and profibrotic genes TGF-β and TIMP-1 in these kidneys were expressed at significantly lower levels. Interestingly, only TGF-β and TIMP-1 were elevated in the dose-response series (Allo2) and only PAI-1 in the first allogeneic series (Allo1). The reason for this is unclear. MSC administration immediately after AKI prevented this late upregulation and led to a better outcome at the tissue level. Functional variables such as blood pressures and urinary concentration ability were not different between MSC- and vehicle-treated groups. The lack of differences in blood pressure might be partly explained by the fact that we did not sodium-challenge the animals, a maneuver that is required to induce hypertension after AKI. In addition, residual renal function was obviously adequate to allow for maintained urinary concentration capacity despite histological evidence for tissue injury/fibrosis.
The origin of fibrotic cells in the kidney in this setting is still debated [27,28,36,37]. Possible cellular candidates that have been suggested to contribute to the fibrotic process include tubular epithelial cells via epithelial-mesenchymal transition, renal fibroblasts, renal perivascular smooth muscle cells and extra-renal cell sources. In our hands, administration of exogenous MSCs of either autologous or allogeneic origin does not increase renal fibrosis and appears to have long-term benefits. This might be because of the transient nature of engraftment of administered MSCs. MSCs are only found in the kidney for a short-time window after administration, disappearing after 1–3 days. Although this window is sufficient to elicit a protective response in animal models, MSCs do not per se contribute to subsequent renal remodeling. Improved long-term outcome of treated animals seems to be primarily a function of the early protective effect rather than the presence of MSCs in the long term.
It is currently unknown which MSC population is the best for the treatment of AKI. Duffield et al. reported a protective response following MSC administration in ischemic AKI only when MSC were cultured on matrigel, and not when cultured on plastic [38]. The outcome in terms of renoprotection was also different between our series of experiments, showing that autologous MSC were more potent than allogeneic MSC. This could be attributed to differences in culture conditions, passage number (resulting in selection of dominant MSC clones or aging [19,39,40]), or other currently unknown factors. MSCs are a heterogeneous population of cells and there are marked species and strain differences [41–43], which might also be determinants contributing to different outcomes.
In summary, we have shown that MSC are safe and effective in the treatment of experimental ischemia/reperfusion AKI in rats, and that VEGF plays a major role mediating their renoprotection. MSC treatment does not result in late adverse effects such as increased renal fibrosis, loss of function, hypertension, ectopic differentiation, or tumor formation. These results further justify, in our opinion, the timely conduct of clinical trails in which patients at high risk for or with established AKI are treated with MSC. We have initiated a Phase I clinical trial in which MSC therapy of AKI is investigated.
Acknowledgments
This work was supported in part by funds from the National Kidney Foundation (Utah, Idaho), the Merit Review Program of the Dept. of Veterans Affairs, Washington, DC, the National Institute of Diabetes and Digestive and Kidney Diseases, and Nephrogen, LLC. Parts of this work were presented at the 2006 meeting of the American Society of Nephrology in San Diego, CA, and published in abstract form (J Am Soc Nephrol 17: F-SA-DS411, 2006).
References
- 1.Block CA. Schoolwerth AC. The epidemiology and outcome of acute renal failure and the impact on chronic kidney disease. Semin Dial. 2006;19:450–454. doi: 10.1111/j.1525-139X.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 2.Togel F. Westenfelder C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev Dyn. 2007;236:3321–3331. doi: 10.1002/dvdy.21258. [DOI] [PubMed] [Google Scholar]
- 3.Bi B. Schmitt R. Israilova M. Nishio H. Cantley LG. Stromal Cells Protect against Acute Tubular Injury via an Endocrine Effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 4.Togel F. Weiss K. Yang Y. Hu Z. Zhang P. Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 5.Humphreys BD. Duffield JD. Bonventre JV. Renal stem cells in recovery from acute kidney injury. Minerva Urologica e Nefrologica. 2006;58:13–21. [PubMed] [Google Scholar]
- 6.Giordano A. Galderisi U. Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 7.Lange C. Togel F. Ittrich H. Clayton F. Nolte-Ernsting C. Zander AR. Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 8.Togel F. Hu Z. Weiss K. Isaac J. Lange C. Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 9.Morigi M. Imberti B. Zoja C. Corna D. Tomasoni S. Abbate M. Rottoli D. Angioletti S. Benigni A. Perico N. Alison M. Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 10.Kunter U. Rong S. Djuric Z. Boor P. Muller-Newen G. Yu D. Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–2212. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 11.Ninichuk V. Gross O. Segerer S. Hoffmann R. Radomska E. Buchstaller A. Huss R. Akis N. Schlondorff D. Anders HJ. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto H. Mundel TM. Sund M. Xie L. Cosgrove D. Kalluri R. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci USA. 2006;103:7321–7326. doi: 10.1073/pnas.0601436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McTaggart SJ. Atkinson K. Mesenchymal stem cells: immunobiology and therapeutic potential in kidney disease. Nephrology (Carlton) 2007;12:44–52. doi: 10.1111/j.1440-1797.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 14.Schrijvers BF. Flyvbjerg A. De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 15.Villegas G. Lange-Sperandio B. Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int. 2005;67:449–457. doi: 10.1111/j.1523-1755.2005.67101.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanellis J. Fraser S. Katerelos M. Power DA. Vascular endothelial growth factor is a survival factor for renal tubular epithelial cells. Am J Physiol Renal Physiol. 2000;278:F905–F915. doi: 10.1152/ajprenal.2000.278.6.F905. [DOI] [PubMed] [Google Scholar]
- 17.Al-Khaldi A. Eliopoulos N. Martineau D. Lejeune L. Lachapelle K. Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003;10:621–629. doi: 10.1038/sj.gt.3301934. [DOI] [PubMed] [Google Scholar]
- 18.Kisseberth WC. Brettingen NT. Lohse JK. Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 19.Vacanti V. Kong E. Suzuki G. Sato K. Canty JM. Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop D. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz EM. Le Blanc K. Dominici M. Mueller I. Slaper-Cortenbach I. Marini FC. Deans RJ. Krause DS. Keating A. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 22.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 23.Basile DP. Schimmoller F. Leonard E.C. VEGF-121 ameliorates secondary damage following recovery from renal ischemia reperfusion injury. J Am Soc Nephrol. 2006;17:5A. [Google Scholar]
- 24.Imberti B. Morigi M. Tomasoni S. Rota C. Corna D. Longaretti L. Rottoli D. Valsecchi F. Benigni A. Wang J. Abbate M. Zoja C. Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 25.Broekema M. Harmsen MC. van Luyn MJ. Koerts JA. Petersen AH. van Kooten TG. van Goor H. Navis G. Popa ER. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- 26.Mishra L. Derynck R. Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 27.Neilson EG. Mechanisms of disease: Fibroblasts—a new look at an old problem. Nat Clin Pract Nephrol. 2006;2:101–108. doi: 10.1038/ncpneph0093. [DOI] [PubMed] [Google Scholar]
- 28.Strutz F. Neilson EG. New insights into mechanisms of fibrosis in immune renal injury. Springer Semi Immunopathol. 2003;24:459–476. doi: 10.1007/s00281-003-0123-5. [DOI] [PubMed] [Google Scholar]
- 29.Kunter U. Rong S. Boor P. Eitner F. Muller-Ewen G. Djurik Z. Konieczny A. Ostendorf T. Villa L. Kerjaschki D. Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2006;17:783A. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- 30.Zhu W. Xu W. Jiang R. Qian H. Chen M. Hu J. Cao W. Han C. Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Prodromidi EI. Poulsom R. Jeffery R. Roufosse CA. Pollard PJ. Pusey CD. Cook HT. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006;24:2448–2455. doi: 10.1634/stemcells.2006-0201. [DOI] [PubMed] [Google Scholar]
- 32.Roufosse C. Bou-Gharios G. Prodromidi E. Alexakis C. Jeffery R. Khan S. Otto WR. Alter J. Poulsom R. Cook HT. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J Am Soc Nephrol. 2006;17:775–782. doi: 10.1681/ASN.2005080795. [DOI] [PubMed] [Google Scholar]
- 33.Zhao DC. Lei JX. Chen R. Yu WH. Zhang XM. Li SN. Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz LA. Gambelli F. McBride C. Gaupp D. Baddoo M. Kaminski N. Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uccelli A. Moretta L. Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 36.Okada H. Strutz F. Danoff TM. Kalluri R. Neilson EG. Possible mechanisms of renal fibrosis. Contrib Nephrol. 1996;118:147–154. doi: 10.1159/000425088. [DOI] [PubMed] [Google Scholar]
- 37.Iwano M. Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–284. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Duffield JS. Park KM. Hsiao LL. Kelley VR. Scadden DT. Ichimura T. Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stolzing A. Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 40.Stolzing A. Sethe S. Scutt AM. Stressed stem cells: temperature response in aged mesenchymal stem cells. Stem Cells Dev. 2006;15:478–487. doi: 10.1089/scd.2006.15.478. [DOI] [PubMed] [Google Scholar]
- 41.Phinney DG. Kopen G. Isaacson RL. Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 42.Phinney DG. Kopen G. Righter W. Webster S. Tremain N. Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–436. [PubMed] [Google Scholar]
- 43.Peister A. Mellad JA. Larson BL. Hall BM. Gibson LF. Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]