Abstract
Epidemiological studies have demonstrated higher frequencies of the O blood group and the nonsecretor phenotype of ABH antigens among patients suffering from peptic ulcers. Since Helicobacter pylori has been established as the main etiological factor in this disease, controversies about the associations of the ABO and Lewis blood group phenotypes and secretor and nonsecretor phenotypes in relation to susceptibility towards infection by this bacillus have been presented. The aim of this study was to verify the frequencies of ABO and Rhesus (Rh) blood groups in H. pylori seropositive symptomatic patients. The study included (n = 1108) patients with dyspepsia symptoms referred from an outpatient clinic in Erbil city for investigation. Age, sex, and residency were recorded as a routine laboratory framework. Patients underwent SD Bioline (Standard Diagnostics Inc, Kyonggi-do, South Korea) and enzyme-linked immunosorbent assay serologic tests for H. pylori. ABO blood group phenotypes were determined by a standard hemagglutination test. Results showed that 64.8% of patients (n = 718/1108) were seropositive for H. pylori infection, and (35.2%) (n = 390/1108) were seronegative. Of the seropositive patients, 40.8% (n = 293/718) were male and 59.2% (n = 425/718) were female; while of the seronegative patients, 46.7% (n = 182/390) were male and 53.3% (n = 208/390) were female. The mean age for seropositives and seronegatives was (38.0 ± 14.6) years and (37.6 ± 15.7) years respectively. The frequency of the ABO and Rh-positive (Rh+) blood groups among seropositive patients was (A = 32.0%, B = 19.5%, AB = 6.7%, O = 41.8%, and Rh+ = 92.5%) and was (A = 32.3%, B = 28.2%, AB = 8.0%, O = 31.5%, and Rh+ = 92.5%) in seronegatives. The results of this study suggest that ABO blood groups, age, and gender influence seropositivity for H. pylori infection.
Keywords: age, sex, prevalence, seropositive, H. pylori
Introduction
The association of ABO blood groups with some infectious and noninfectious diseases has been described.1,2
Before Helicobacter pylori identification as the main etiology of peptic ulcers, chronic gastritis, and a variety of gastrointestinal symptoms,3–5 many epidemiologic studies had found that nonsecretors of ABO blood group antigens and individuals of blood group O were overrepresented among patients with peptic ulcers.6–8
These studies encouraged many researchers to investigate the relation between ABO blood groups and their secretor status with peptic ulcer. Many authors report an association between blood group O and H. pylori infection,9–13 while others failed to find such an association.14–16
Many methods are used in clinical practice to diagnose H. pylori infection, including measurement of serum immunoglobulin G (IgG) antibodies by enzyme-linked immunosorbent assay (ELISA)17 and Helisal rapid blood (HRB) test, which is a reliable, rapid, and inexpensive screening test of H. pylori used in epidemiological studies with greatest usefulness as a primary office diagnostic device.18
There were no local data on the epidemiology of H. pylori infection in the Kurdistan region of Iraq; therefore, the aim of this study was to verify the incidence of seropositive H. pylori infection among patients with dyspepsia symptoms and to verify the frequencies of ABO blood groups in H. pylori seropositive symptomatic patients.
Subjects and methods
Subjects
From February 2010 to March 2011, a total of 1108 patients with the symptoms of dyspepsia or other symptoms referable to the proximal alimentary tract, from an enterology outpatient clinic, were referred for serologic diagnosis of H. pylori infection. The study was performed according to the local Ethical Committee of Medical Sciences.
From each patient, a sample of 3 mL of peripheral blood was collected and centrifuged, and the sera were separated for use.
Methods
This study was a prospective study of patients attending the outpatient clinic for symptoms of dyspepsia for the first time, with no previous history of H. pylori infection and treatment. The study population was screened for H. pylori infection by SD Bioline H. pylori, a rapid HRB kit (MT Promedt Consulting GmbH, Ingbert, Germany) to receive treatment. For this research purpose, the positive SD Bioline H. pylori screening test results were confirmed, by estimating the serum levels of anti-H. pylori IgG, using the commercial ELISA (Trinity Biotech, Wicklow, Ireland).17 The results by this method were obtained as immune status ratio (ISR), and values of ≥1.1 were considered positive. Those patients who were positive for H. pylori infection by both methods were included in the seropositives, those who were negative by both methods were regarded as seronegative, and the rest (n = 38) were not included within the total study population (n = 1108).
ABO and Rhesus (Rh) blood groups were determined for seropositive and seronegative patients, using standardized hemagglutination methods.
The results of this study (seropositives) were compared with the seronegative patient group and with the author’s previous study on the ABO blood group frequency in the region,19 as controls for both age and sex, and blood groups.
Statistical analysis
Data generated from this study were analyzed using Statistical Package for Social Sciences (SPSS) (Chicago, IL). Chi square test was used to detect statistically significant differences among variables. P-values <0.05 were considered significant.
Results
The seropositivity for H. pylori infection was present in 718/1108 (64.8%) and absent in 390/1108 (35.2%) of these patients (by both methods). Only in 38 patients, there was disagreement between two tests (ie, an agreement of 95%), and these patients were not included in the study population.
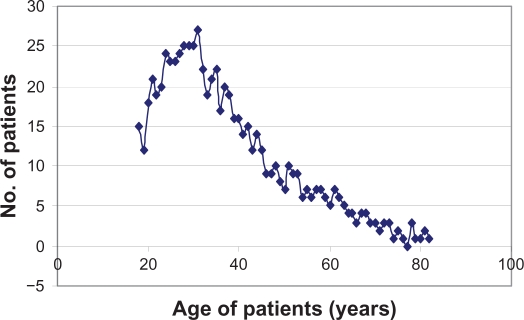
The mean age of seropositive patients was (37.99 ± 14.6) years (range, 18–82 years), with a median age of 38.4 years and no significant difference from the mean age of seronegatives (37.6 ± 15.7) years (range, 18–70 years). There was a significant increase in the incidence of seropositivity up to the age of 31 years (r = 0.91, P = 0) (Figure 1) then a significant decline in the incidence above the age of 31 years (−r = −0.94, P = 0).
Figure 1.
Helicobacter pylori seropositivity in patients of different ages.
Of these 718 seropositive patients, 37.7% (271/718) were male (M) and 62.3% (447/718) were female (F) (M/F ratio 0.61:1.0). A significant difference was observed when comparing gender in H. pylori infection (P < 0.0001) with that of the general population (M/F ratio, 1.14:1.0) (Table 1) and with seronegative patients (M/F ratio, 1.2:1.0) (Table 2) but to a lesser degree (P = 0.0148).
Table 1.
Gender and blood group relation to Helicobacter pylori infected patients compared with the general population
| Characteristic | Seropositive N = 718/1108 (64.8%) | General population14 N = 53,234 | P-value | ||
|---|---|---|---|---|---|
| Sex (M/F) | 293/425 | 0.69:1.00 | 28379/24855 | 1.14/1.00 | 0.0001 |
| M: 40.8% | M: 53.3% | ||||
| F: 59.2% | F: 46.7% | ||||
| Blood group | |||||
| A | 230 | 32.0% | 17,283 | 32.47% | 0.811 |
| B | 140 | 19.5% | 12,693 | 23.84% | 0.007 |
| AB | 48 | 6.7% | 3475 | 6.53% | 0.880 |
| O | 300 | 41.8% | 19,783 | 37.16% | 0.011 |
| Rhesus positive | 664 | 92.5% | 48,833 | 91.73% | 0.497 |
| Rhesus negative | 54 | 7.5% | 4401 | 8.27 | 0.497 |
| Total | 718 | 53,234 |
Abbreviations: F, female; M, male.
Table 2.
Gender and blood group relation to Helicobacter pylori infection in seropositive patients compared with seronegative patients
| Characteristic | Seropositives N = 718/1108 (64.8%) | Seronegatives N = 390/1108 (35.2%) | P-value | ||
|---|---|---|---|---|---|
| Sex (M/F) | 293/425 | 0.69:1.00 | 213/177 | 1.2/1.0 | 0.0148 |
| M: 40.8% | M: 54.6% | ||||
| F: 59.2% | F: 45.4% | ||||
| Blood group | |||||
| A | 230 | 32.0% | 126 | 32.3% | 0.936 |
| B | 140 | 19.5% | 110 | 28.2% | 0.0495 |
| AB | 48 | 6.7% | 31 | 8.0% | 0.663 |
| O | 300 | 41.8% | 19,783 | 31.5% | 0.0397 |
| Rhesus positive | 664 | 92.5% | 349 | 89.5% | 0.288 |
| Rhesus negative | 54 | 7.5% | 41 | 10.5% | 0.288 |
| Total | 718 | 390 |
Abbreviations: F, female; M, male.
When the frequencies of blood group phenotypes were analyzed separately in the seropositive patients, seronegative patients, and the general population, it was possible to verify that the frequency of blood group O in seropositive patients was higher and blood group B was lower than in the general population and to a lesser degree in seronegative patients.
ABO and Rh blood group frequencies in seropositive and seronegative patients are shown in Tables 1 and 2.
These differences between the higher prevalence of type O and the lower prevalence of blood group B in the seropositive patients compared with that in the general population were statistically significant, with P-values of 0.01 and 0.007 respectively, and also when compared with that in seronegative patients with P-values of 0.0397 and 0.0495 respectively.
No significant differences in the frequency of ABO and Rh blood groups were observed in different ages in seropositive patients. There was also no significant difference in Rh+ frequency between seropositive patients, seronegative patients, and the general population (Tables 1 and 2).
Discussion
Results of this study showed a significant association between the O blood group and infection caused by H. pylori (P = 0.01), a finding which is reinforced by data obtained from many other studies.9–13
Blood group B patients in this study were less prone to H. pylori infection than other blood groups (P = 0.007) – a finding not observed in other studies, to the best of the author’s knowledge. In another study, AB blood group individuals were less prone to H. pylori infection.9
The findings of this present study suppor t the epidemiological view of the greater susceptibility of blood group O to infection by H. pylori, as well as support the conclusions of Alkout et al,20 who demonstrated that the H antigen represents an important receptor expressed in the gastroduodenal mucosal cells to which H. pylori adheres. The findings of this present study disagree with some previous studies which demonstrated that the O blood group did not represent a risk factor for H. pylori infection.15–17
In the author’s view, this discrepancy could be the result mainly of the type of the control population they used and/or to the surveys which were done in asymptomatic individuals16,20,21 rather than symptomatic patients.
This present study used two methods to diagnose H. pylori infection serologically to avoid, at least to some extent, some other authors’ claims that this discrepancy could be due to different methods used to detect H. pylori infection.15–17,22
For example, some authors who have used polymerase chain reaction (PCR) stress that other tests employed in the diagnosis of H. pylori infection differ in specificity and sensitivity from the PCR method, and may have an influence on the different infection frequencies observed within distinct populations.23,24
It is the present author’s view that the higher susceptibility of O blood group individuals to H. pylori infection is most probably due to the higher frequency of secretor status in O blood group individuals.25 This view is supported by a previous demonstration, by Alkout et al, that H antigen represents an important receptor expressed in the gastroduodenal mucosal cells to which H. pylori adheres,20 which also enhances colonization of H. pylori bacteria.26
The support to the above view is that the ABH and Lewis (Le) antigens on the gastric and duodenal mucosa are synthesized through a specific glycosyltransferase from the basic H substance, and the O and Le (a− b+) phenotypes (secretors) express a greater quantity of these basic fucosylated antigens,27 in comparison with other groups, as Borén et al speculated later on.8
Blood group O individuals express a higher inflammatory responses to H. pylori with higher levels of lymphocyte infiltration in the gastrointestinal mucusa,20,26,28 a lower level of Von Willebrand’s factor,29,30 and a higher frequency of secretor status;25 all these together, in the view of the present author, explain these individuals’ increased susceptibility to peptic ulceration.
Regarding Rh status, this present study showed no significant differences between the seropositive patients, seronegative patients, and the general population, which is in an agreement with previous studies.31
The prevalence of seropositivity will change between countries and within the same country, according to the socioeconomic status, being higher among groups with lower socioeconomic status.32,33 In this study, the prevalence of seropositivity to H. pylori infection was (64.8%) in symptomatic patients in the Kurdistan region of Iraq, which is higher than the average prevalence in the world’s population (50%).34–36 It was in between the high prevalence in some developing countries (80%–90%) and the low prevalence in some developed countries (<40%),37,38 eg, 35%–40% in the United States,39 while it was similar to those in the neighboring countries and some other countries, eg, Turkey40 (68%), Saudi Arabia41 (61%), Kuwait42 (62%), some regions in Iran43,44 (66.7% to >85%), and Brazil23 (61.7%).
In this study, more females than males were seropositive (P = 0.0001), as seen by some other studies,9,39 while some other studies have noticed no such relation to gender31,32,40,42,43 and some have even noticed a higher prevalence of H. pylori in men.45,46 The differences in socioeconomic conditions in the studied population and the type of controls used for comparison can explain this discrepancy.
Regarding acquisition of infection, the results of this study suggest that newly acquired H. pylori infections had happened during childhood and early adolescence, increasing to reach its peak at adulthood at 31 years with a median age of about 38 years (Figure 1). This present author’s observation was in concordance with many other authors’ findings.47–49
From various studies (including this one), genetic predisposition, as well as environmental factors, are suggested as important influencing factors in H. pylori infection, a view supported by the Malaty and colleagues’ study on twins.50
Conclusion
From this study, which to date is the only study of this type in the Kurdistan region of Iraq, it can be concluded that H. pylori infection is an endemic problem, which should be dealt with by improving sanitation and purified water supply and also should be investigated for and eradicated. It can also be concluded that O blood group individuals are more susceptible to H. pylori infection and its symptomatic gastrointestinal complications, and/or they have more cellular and immunological response to it (expressed by seropositivity) than other ABO blood groups (group B in particular), while no significant differences between Rh+ and Rh− patients were seen. Also, it can be concluded that females and adolescents are more prone to H. pylori infection.
Acknowledgments
The author is grateful to Mr Dashty Hadi Hamad (Med Lab Tech of the Microbiology Department, Hawler Medical University), Mr Sa’ady Khalid Kadir, and Dr Kadhim Hasan Kamil at Aynda Medical Laboratories, Erbil, Iraq, for their cooperation during the laboratory work for this study. The author also thanks Professor Hama Najm Jaff for referring patients for investigation.
Footnotes
Disclosure
The author is the principal investigator in this study. He takes primary responsibility for the paper, as he was in charge of the main laboratory works. The author reports no conflicts of interest in this work.
References
- 1.Mourant AE, Kopec AC, Domaniewska-Sobczak K. Blood Groups and Diseases: A Study of Associations of Diseases with Blood Groups and Other Polymorphisms. London: Oxford University Press; 1978. [Google Scholar]
- 2.Jaff MS, O’Brian DS. Excess of blood group B in primary myelofibrosis. Vox Sang. 1987;52:250–253. doi: 10.1111/j.1423-0410.1987.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 3.Marshall BJ. Campylobacter pyloridis and gastritis. J Infect Dis. 1986;153:650–658. doi: 10.1093/infdis/153.4.650. [DOI] [PubMed] [Google Scholar]
- 4.Tytgat GNJ, Axon ATR, Dixon MF, Graham DY, Lee A, Marshall BJ. London: Blackwell Scientific Publications; 1990. Helicobacter pylori, causal agent in peptic ulcer disease. Working party reports 1990; pp. 36–45. [Google Scholar]
- 5.Rosenstock S, Kay L, Rosenstock C, Andersen LP, Bonnevie O, Jørgensen T. Relation between Helicobacter pylori infection and gastrointestinal symptoms and syndromes. Gut. 1997;41:169–176. doi: 10.1136/gut.41.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aird I, Bentall HH, Roberts JAF. A relationship between cancer of stomach and the ABO blood groups. BMJ. 1953;1:799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark CA, Edwards JW, Haddock DRW, et al. ABO blood groups and secretor character in duodenal ulcer. BMJ. 1956;2:725–731. doi: 10.1136/bmj.2.4995.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borén T, Falk P, Roth KA. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 9.Kanbay M, Gur G, Arslan H, et al. The relationship of ABO blood group, age, gender, smoking, and Helicobacter pylori infection. Dig Dis Sci. 2005;50:1214–1217. doi: 10.1007/s10620-005-2762-y. [DOI] [PubMed] [Google Scholar]
- 10.Alkout AM, Blackwell CC, Weir DM, et al. Isolation of cell surface component of Helicobacter pylori that binds H type 2, Lewis A and Lewis B antigens. Gastroenterology. 1997;112:1179–1187. doi: 10.1016/s0016-5085(97)70129-x. [DOI] [PubMed] [Google Scholar]
- 11.Lin CW, Chang YS, Wu SC, Cheng KS. Helicobacter pylori in gastric biopsies of Taiwanese patients with gastroduodenal diseases. Jpn J Med Sci Biol. 1998;51:13–23. doi: 10.7883/yoken1952.51.13. [DOI] [PubMed] [Google Scholar]
- 12.Henriksson K, Uribe A, Sandstedt B, Nord CE. Helicobacter pylori infection, ABO blood group, and effect of misoprostol on gastroduodenal mucosa in NSAID-treated patients with rheumatoid arthritis. Dig Dis Sci. 1993;38:1688–1696. doi: 10.1007/BF01303179. [DOI] [PubMed] [Google Scholar]
- 13.Hein HO, Suadicani P, Gyntelberg F. Genetic markers for peptic ulcer: a study of 3387 men aged 54 to 74 years: the Copenhagen Male Study. Scand J Gastroenterol. 1997;32:6–21. doi: 10.3109/00365529709025057. [DOI] [PubMed] [Google Scholar]
- 14.Niv Y, Fraser G, Delpre G, Neeman A, et al. Helicobacter pylori infection and blood groups. Am J Gastroenterol. 1996;91:101–104. [PubMed] [Google Scholar]
- 15.Dickey W, Collins JSA, Watson RGP, Sloan JM, Porter KG. Secretor status and Helicobacter pylori infection are independent risk factors for gastroduodenal disease. Gut. 1993;34:351–353. doi: 10.1136/gut.34.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loffeld RJF, Stobberingh E. Helicobacter pylori and ABO blood groups. J Clin Pathol. 1991;44:516–517. doi: 10.1136/jcp.44.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans DJ, Jr, Evans DG, Graham DY, Klein PD. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology. 1989;96:1004–1008. doi: 10.1016/0016-5085(89)91616-8. [DOI] [PubMed] [Google Scholar]
- 18.Borody TJ, Andrews P, Shortis NP. Evaluation of whole blood antibody kit to detect active Helicobacter pylori infection. Am J Gastroenterol. 1996;91:2509–2512. [PubMed] [Google Scholar]
- 19.Jaff MS. ABO and rhesus blood group distribution in Kurds. J Blood Med. 2010;1:143–146. doi: 10.2147/JBM.S12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkout AM, Blackwell CC, Weir DM. Increased inflammatory responses of persons of blood group O to Helicobacter pylori. J Infect Dis. 2000;181:1364–1369. doi: 10.1086/315375. [DOI] [PubMed] [Google Scholar]
- 21.Klein PD, Opekun AR, Smith EO, Graham DY, Gaillour A. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet. 1991;337:1503–1506. doi: 10.1016/0140-6736(91)93196-g. [DOI] [PubMed] [Google Scholar]
- 22.Hooke-Nikanne J, Sistonen P, Kosunen TU. Effect of ABO blood group and secretor status on the frequency of Helicobacter pylori antibodies. Scand J Gastroenterol. 1990;25:815–818. doi: 10.3109/00365529008999220. [DOI] [PubMed] [Google Scholar]
- 23.de Mattos LC, Cintra JR, Sanches FE, et al. ABO, Lewis, secretor and non-secretor phenotypes in patients infected or uninfected by the Helicobacter pylori bacillus. Sao Paulo Med J. 2002;120:55–58. doi: 10.1590/S1516-31802002000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valentine JL, Arthur RR, Mobley HLT, Dick JD. Detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1991;29:689–695. doi: 10.1128/jcm.29.4.689-695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaff MS. Higher frequency of secretor phenotype in O blood group – its benefits in prevention and/or treatment of some diseases. Int J Nanomedicine. 2010;5:901–905. doi: 10.2147/IJN.S13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heneghan MA, Moran AP, Feeley KM, et al. Effect of host Lewis and ABO blood group antigens expression on Helicobacter pylori colonisation density and the consequent inflammatory response. FEMS Immunol Med Microbiol. 1998;20:257–266. doi: 10.1111/j.1574-695X.1998.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 27.Watkins WM. Biochemistry and genetics of the ABO, Lewis and P blood group systems. Adv Hum Genet. 1980;10:1–136. doi: 10.1007/978-1-4615-8288-5_1. [DOI] [PubMed] [Google Scholar]
- 28.Abdulhamid M, Alkout C, Blackwell C, Weir DM. Increased inflammatory responses of persons of blood group O to Helicobacter pylori. J Infect Dis. 2000;181:1364–1390. doi: 10.1086/315375. [DOI] [PubMed] [Google Scholar]
- 29.Franchini M, Capra F, Targher G, Montagnana M, Lippi G. Relationship between ABO blood group and von Willebrand factor levels: from biology to clinical implications. Thromb J. 2007;5:14. doi: 10.1186/1477-9560-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003;1:33–40. doi: 10.1046/j.1538-7836.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 31.Petrovic M, Artiko V, Novosel S, et al. Relationship between Helicobacter pylori infection estimated by 14C-urea breath test and gender, blood groups and Rhesus factor. Hell J Nucl Med. 2011;14:21–24. [PubMed] [Google Scholar]
- 32.Murray L, McCrum E, Evans A, Bamford K. Epidemiology of Helicobacter pylori infection among 4742 randomly selected subjects from Northern Ireland. Inter J Epidemiol. 1997;26:880–887. doi: 10.1093/ije/26.4.880. [DOI] [PubMed] [Google Scholar]
- 33.Graham DY, Malaty HM, Evans DG, Evans DJ, Jr, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 34.Parsonnet J. Helicobacter pylori: the size of the problem. Gut. 1998;43:6–9. doi: 10.1136/gut.43.2008.s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DN, Blaser MJ. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- 36.The EUROGAST Study Group Epidemiology of, and risk factors for, Helicobacter pylori infection among 3,154 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–1676. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pounder R, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9:33–39. [PubMed] [Google Scholar]
- 38.Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004;9:1–6. doi: 10.1111/j.1083-4389.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 39.Lacy B, Semore J. Helicobacter pylori: ulcers and more: the beginning of an era. J Nutr. 2001;131:89–93. doi: 10.1093/jn/131.10.2789S. [DOI] [PubMed] [Google Scholar]
- 40.Seyda T, Derya C, Füsun A, Meliha K. The relationship of Helicobacter pylori positivity with age, sex, and ABO/Rhesus blood groups in patients with gastrointestinal complaints in Turkey. Helicobacter. 2007;12:244–250. doi: 10.1111/j.1523-5378.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 41.Khan MA, Ghazi HO. Helicobacter pylori infection in asymptomatic subjects in Makkah, Saudi Arabia. J Pak Med Assoc. 2007;57:114–117. [PubMed] [Google Scholar]
- 42.Alazmi WM, Siddique I, Alateeqi N, Al-Nakib B. Prevalence of Helicobacter pylori infection among new outpatients with dyspepsia in Kuwait. BMC Gastroenterol. 2010;10:14. doi: 10.1186/1471-230X-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farshad SH, Japoni A, Alborzi A-V, Zarenezhad M, Ranjbar R. Changing prevalence of Helicobacter pylori in south of Iran Iranian. J Clin Infect Dis. 2010;5:65–69. [Google Scholar]
- 44.Massarrat S, Saberi-Firoozi M, Soleimani A, Himmelmann GW, Hitzges M, Keshavarz H. Peptic ulcer disease, irritable bowel syndrome and constipation in two populations in Iran. Eur J Gastroenterol Hepatol. 1995;7:427–433. [PubMed] [Google Scholar]
- 45.Lin SK, Lambert JR, Nicholson L, Lukito W, Wahlqvist M. Prevalence of H. pylori in a representative Anglo-Celtic population of urban Melbourne. J Gastroenterol Hepatol. 1998;13:505–510. doi: 10.1111/j.1440-1746.1998.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 46.Jafarzadeh A, Ahmadi-Kahanali J, Bahrami M, Taghipour Z. Seroprevalence of anti-Helicobacter pylori and anti-CagA antibodies among healthy children according to age, sex, ABO blood groups and Rh status in south-east of Iran. Turk J Gastroenterol. 2007;18:165–171. [PubMed] [Google Scholar]
- 47.Bergenzaun P, Kristinsson KG, Thjodleifsson B, et al. Seroprevalence of Helicobacter pylori in south Sweden and Iceland. Scand J Gastroenterol. 1996;31:1157–1161. doi: 10.3109/00365529609036904. [DOI] [PubMed] [Google Scholar]
- 48.Eamranond PP, Torres J, Muñoz O, Pérez-Pérez GI. Age-specific immune response to HspA in Helicobacter pylori-positive persons in Mexico. Clin Diagn Lab Immunol. 2004;11:983–985. doi: 10.1128/CDLI.11.5.983-985.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malaty HM, El-Kasabany A, Graham DY, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931–935. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 50.Malaty HM, Engstrand L, Pedersen NL, Graham DY. Helicobacter pylori infection: genetic and environmental influences (a study of twins) Ann Intern Med. 1994;120:982–986. doi: 10.7326/0003-4819-120-12-199406150-00002. [DOI] [PubMed] [Google Scholar]