Abstract
Mesenchymal stem cells (MSCs) represent a source of pluripotent cells that are already in various phases of clinical application. However, the use of MSCs in tissue engineering has been hampered largely due to their limitations, including low proliferation, finite life span, and gradual loss of their stem cell properties during ex vivo expansion. Nanog and Oct4 are key transcription factors essential to the pluripotent and self-renewing phenotypes of undifferentiated embryonic stem cells (ESCs). To determine whether Nanog and Oct4 improve human bone marrow-MSC quality, we therefore established stable Nanog and Oct4 overexpressing MSCs using a lentiviral system and showed that this promoted cell proliferation and enhanced colony formation of MSCs. In differentiating MSCs, Nanog, and Oct4, overexpression had converse effects on adipogenesis of MSCs and Nanog overexpression slowed down adipogenesis, whereas Oct4 overexpression improved adipogenesis. Nanog and Oct4 overexpression both improved chondrogenesis. Microarray data showed many differences in transcriptional targets in undifferentiated MSCs overexpressing Nanog and Oct4. These results provide insight into the improvement of the stemness of MSCs by genetic modification with stemness-related genes.
Introduction
Mesenchymal stem cells (MSCs) represent a potential source of pluripotent cells for tissue engineering. MSCs show multilineage differentiation capacity including osteogenic, chondrogenic, adipogenic, and myogenic lineages [1–3]. Human MSCs derived from the bone marrow (hBMSCs) represent a source of pluripotent cells that are already in various phases of clinical application [4–6]. Their most immediate use is in the orthopedic context because of the clear demonstration of their ability to differentiate into bone and cartilage [1,7]. However, the use of MSCs in tissue engineering has been hampered largely due to their disadvantages including lower proliferation, limited lifespan and progressive loss of their stem cell properties during ex vivo expansion etc.
The pluripotent cell-specific gene Nanog encodes a homeodomain-bearing transcription factor required for maintaining the undifferentiated state and self-renewal of stem cells [8–9]. Nanog was expressed in pluripotent cells of preimplantation and early postimplantation embryos, embryonic stem cells (ESCs) and embryonic germ (EG) cells. Downregulation of Nanog induces differentiation of human ESCs to extraembryonic lineages [10], overexpression of Nanog in human embryonic stem cells (hESCs) cells enables feeder-free growth while inducing primitive ectoderm features [11], indicating that Nanog functions as a key player in maintaining the pluripotency of stem cells. It was shown that Nanog overexpressing MSCs had much higher capabilities for expansion and osteogenesis [12]. Ectopic expression of Nanog in NIH3T3 increases growth rate and transformed phenotype [13–14], suggesting Nanog might function in a similar fashion in mature cells as in ESCs.
Oct4 encoded by Pou5f1 belongs to the family of Pou-domain transcription factors, Oct4 expression is normally confined to pluripotent cells of the developing embryos [15]. Oct4 is also transiently expressed in the developing endoderm [16] and neurectoderm [17] of the embryos. Downregulation of Oct4 in ESCs induces trophectoderm differentiation, whereas overexpression induces differentiation into extraembryonic mesoderm and endoderm [18], showing that Oct4 is a crucial and dose-dependent determinant of pluripotency in ESCs. In mice, a loss-of-function mutation for Oct4 results in early embryonic lethality because of the inappropriate differentiation of pluripotent epiblast cells into trophectoderm [19]. Ectopic expression of Oct4 in certain somatic cells has been associated with active dedifferentiation [20].
Oct4 is expressed at very low levels in early passage MSCs and disappears in MSCs at late passage whereas Nanog is almost undetected in MSCs even at early passage (data not shown). To determine whether ectopic expression of Nanog and Oct4 will improve bone marrow derived MSC quality, we examined the effects of Nanog and Oct4 overexpression on MSCs and their targets in undifferentiated MSCs in this study.
Materials and Methods
MSC culture, chondrocyte, and adipocyte differentiation
Human bone marrow-derived mesenchymal stem cells (hBMSCs) were harvested from the iliac crest and cultured as described [21] after informed consent according to guidelines of the IRB of the National University Hospital, Singapore. To prevent spontaneous differentiation, cells were maintained at subconfluent levels. MSCs were induced to differentiate towards adipocytes as described [2], 2 × 105 MSCs in W6 plate were induced to differentiate into adipocytes for 14 days in adipogenic medium. Adipogenic medium contained 0.5 mM isobutyl-methylxanthine (IBMX), 1 μM dexamethasone (Sigma Chemical, St. Louis, MO), 10 μM insulin, 200 μM indomethacin, and 1% antibiotic/antimycotic. Pellet culture system described [22] was used for chondrocyte differentiation. Briefly, 2 × 105 MSCs were placed in a 15 mL polypropylene tube (Falcon) and centrifuged to a pellet. The pellet was cultured at 37°C with 5% CO2 in 500 μL of chondrogenic medium that contained 10 ng/mL transforming growth factor (TGF)-β3, 10−7 M dexamethasone, 50 μg/mL ascorbate-2-phosphate, 40 μg/mL proline, 100 μg/mL pyruvate, and 50mg/mL ITS + Premix (Becton Dickinson, Franklin Lakes, NJ; 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25μg/mL selenious acid, 1.25 mg/mL BSA, and 5.35 mg/mL linoleic acid). The medium was replaced every 3–4 days for 28 days. Differentiation of MSCs was evaluated by real time polymerase chain reaction (PCR) and stain. Oil red O stain for lipoid deposits in adipogenesis, and immunostaining against collagen type 2 (COL2A1) and alcian blue stain for cartilage proteoglycans in chondrogenesis was used in this study.
Construction of expression plasmids and infection of MSCs
Nanog and Oct4 was amplified from cDNA of hESCs H9 and then cloned into pENTR™3C (Invitrogen). Via LR recombination between pENTR™3C and pLenti6/V5 (Invitrogen), pLentiviral vectors for overexpressing Nanog or Oct4 were created (Fig. 1A). Lentivirus was generated by cotransfecting pLentiviral vector for overexpressing Nanog or Oct4 and packaging mix (Invitrogen) into 293FT cells, then MSCs were infected with viral supernatant to achieve Nanog or Oct4 overexpression and were selected with 5 μg/mL blasticidin for 7 days. The empty pLenti6/V5 with no insert was used as control (Dest).
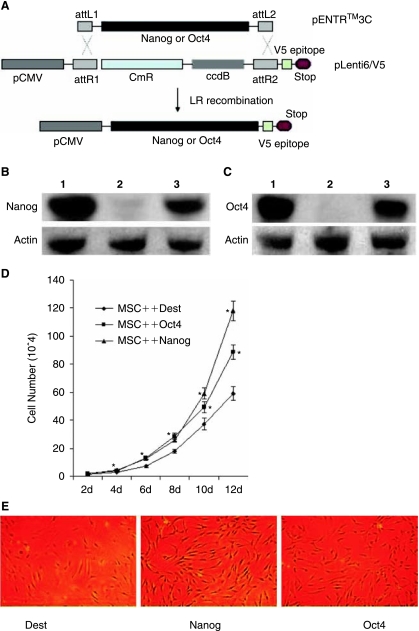
FIG. 1.
Characterization of Nanog and Oct4 overexpressing mesenchymal stem cells (MSCs) and effects of their overexpression on proliferation of MSCs, showing Nanog and Oct4 promoted proliferation of MSCs by an average 1.67-fold and 1.51-fold, respectively. (A) Schematic representation of transgenes used to produce Nanog or Oct4 overexpressing MSCs. The Nanog or Oct4 from human embryonic stem cells (ESCs) was cloned into pENTR™3C under the control of CMV promoter. By LR recombination, the lentiviral vector for Nanog or Oct4 overexpression was created. (B) Nanog detection in Nanog overexpressing MSCs by Western blot analysis 2 months after infection with lentivirus for Nanog overexpression. 1 = Human ESCs with MEF (H9); 2 = Dest (no insert)-infected MSCs; and 3 = Nanog overexpressing MSCs. (C) Oct4 detection in Oct4 overexpressing MSCs by Western blot analysis 2 months after infection with lentivirus for Oct4 overexpression. 1 = Human ESCs with MEF (H9); 2 = Dest (no insert)-infected MSCs; and 3 = Oct4 overexpressing MSCs. (D) Proliferation curves of Nanog or Oct4 overexpressing MSCs compared with Dest control MSCs. Time points represent average values from duplicate measurements and their standard deviation. Nanog or Oct4 overexpression promoted cell growth of MSCs. Asterisks indicates a significant difference compared to no insert control (t-test: P < 0.05). (E) Nanog or Oct4 overexpression maintained MSCs morphology. At passage 10, no insert control (Dest) MSCs became flat and gradually lost MSC morphology whereas Nanog and Oct4 overexpressing MSCs retained MSC morphology.
Cell proliferation analysis
To determine the effects of Nanog or Oct4 overexpression on MSC proliferation rate, 1 × 104 cells were plated in 6-well plates in duplicate, and Nanog or Oct4 overexpression on cell proliferation were determined by counting cells with a hematometer in duplicate at day 2, 4, 6, 8, 10, and 12 after seeding compared with no insert control MSCs. MSCs that were confluent were subcultured to a bigger flask.
Colony forming unit–fibroblast assay
To determine the effects of Nanog or Oct4 overexpression on colony formation of MSCs, 250, 500, and 1000 Nanog or Oct4 overexpressing and control MSCs were seeded into 10 cm dishes in triplicate. The colonies were counted after Giemsa stain 18 days after seeding.
Quantitative real time PCR
To quantify effect of Nanog and Oct4 overexpression on differentiation of MSCs, quantitative real time PCR was performed with Taqman expression assay according to the manufacturer and an ABI 7700 Prism (Applied Biosystems, Foster City, CA). Briefly, 0.3 μg of total RNA was converted to cDNA using high capacity cDNA archive kit in 30 μL and then diluted to 300 μL. Quantitative reverse transcriptase (RT)-PCR was done as follows: initial denaturation for 2 min at 50°C, 10 min at 95°C, following 40 cycles of PCR (95°C for 15 s, 60°C for 1 min) by using 5 μL of 2× Master mix, 0.5 μL of Taqman probe and 4.5 μL of cDNA. All probes were designed with a 5′ fluoregenic 6-carboxylfluorescein, and a 3′ quencher, tetramethyl-6-carboxyrhodamine. The expression of human GAPDH was used to normalize gene expression levels.
Western blot analysis
Cells were collected by centrifugation, cell pellet was resuspended in lysis buffer (25 mM Tris, pH7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing proteinase inhibitors and incubated on ice for 30 min. Following centrifugation at 16,000g for 15 min at 4°C, the supernatant containing total cell extract was collected and kept at −80°C. Protein from cell extracts in the gel was electrophoretically transferred onto a Hybond-PVDF membrane (Amersham Biosciences, Piscataway, NJ). The membrane was incubated for 1 h at room temperature in blocking buffer (TBS-T containing 5% skim milk) to block nonspecific protein binding and then incubated at room temperature for 1 h with the primary antibody against Nanog (eBioscience, San Diego, CA) or Oct4 (Santa Cruz, Biotechnology Inc., Santa Cruz, CA) diluted (1:300) in blocking buffer for 1 h. Following four washes with TBS-T, the membrane was incubated for 1 h with the HPR-conjugated secondary antibody diluted (1:3000) in blocking buffer for 1 h. Antibody binding was visualized with an ECL Western blotting detection system (Amersham Biosciences).
cDNA microarray analysis
To determine the targets of Nanog or Oct4 in MSCs, we overexpressed Nanog or Oct4 in MSCs and analyzed their gene expression profiles in undifferentiated MSCs using microarrays. Total RNA was isolated from Nanog-, Oct4-overexpressing and no insert control MSCs using RNeasy mini-kit (Qiagen, Chatsworth, CA) per the manufacturer's protocol. In brief, 3.5 μg total RNA was used to synthesize double-strand DNA using one cycle cDNA synthesis kit. cDNA was purified by using Sample Cleanup Module. In vitro transcription was performed to produce biotin-labeled cRNA using GeneChip IVT Labeling Kit. Biotinylated cRNA was cleaned and fragmented to 50–200 nucleotides with Sample Cleanup Module and hybridized 16 h at 45°C to Affymetrix HG U133 plus 2 containing more than 54,675 human genes. After washing, the array was stained with streptavidin-phycoerythrin (Molecular Probes, Eugene, OR). The staining signal was amplified by biotinylated anti-streptavidin (Vector Laboratories, Burlingame, CA), followed by streptavidin-phycoerythrin stain, and then scanned on GCOS 3000 (Affymetrix). The data were analyzed using Software Genespring V7.3. A t-test on normalized intensity followed by ratio change (ratio of normalized intensity ≥1.5 or ≤−1.5) was used to generate the gene list with significant change in gene expression profile. In this study, MSCs from two patients in duplicate were used. Functional classification of Nanog or Oct4 targets in MSCs was performed by the Panther Classification System. Statistically significant overrepresented annotation categories were determined using the observed number of genes versus the numbers expected by chance within a certain annotation group.
Statistical Analysis
All experiments were performed at least twice (n ≥ 4). Values are expressed as average and standard deviations. The probability associated with a Student's test was performed for comparisons of the parameters between two groups. P-values less than 0.05 were considered statistically significant.
Results
Stable expression of Nanog and Oct4 in MSCs by lentiviral vector
Endogenous Oct4 was only expressed at low levels in MSCs at early passage (data not shown). This is consistent with studies by Tai et al. [23]. The expression of Nanog was almost undetectable in MSCs (data not shown). It was very difficult to transfect MSCs with conventional transfection methods including lipofectamine 2,000 etc. To mediate highly efficient and stable expression of Nanog or Oct4 in the MSCs, lentiviral vectors carrying a CMV promoter and blasticidin selectable marker for overexpression of Nanog or Oct4 were constructed by cloning Nanog or Oct4 from cDNA of hESCs H9 into pENTR™3C and then LR recombination between pENTR™3C and pLenti6/V5 (Fig. 1A). MSCs were infected with lentivirus and selected with 5 μg/mL blasticidin for 7 days. Blasticidin-resistant MSCs were generated and Western blot analysis showed that Nanog or Oct4 was expressed at high levels in Nanog or Oct4 overexpressing MSCs and not detected in control MSCs for even as long as 2 months after infection with lentivirus (Fig. 1B and 1C). This showed also that stable expression of Nanog or Oct4 can be efficiently mediated by lentivirus system. Compared with expression levels in ESCs, expression of Nanog or Oct4 in MSCs was a little lower in Nanog or Oct4 overexpressing MSCs (Fig. 1B and 1C). Overexpressed Nanog and Oct4 in MSCs were localized to the nuclei as shown by immunostain (Supplementary Fig. 1A and 1B; Supplementary materials are available online at http://www.liebertpub.com).
Effect of Nanog and Oct4 overexpression on cell proliferation of MSCs
Nanog and Oct4 are transcribed specifically in pluripotent ESCs and EG cells and both play a crucial role in the maintenance of both undifferentiated states. It was shown that Oct4-expressing MSCs displayed high proliferative capacity [24], and ectopic expression of Nanog increased growth rate of MSCs [12] and NIH3T3 [13–14]. It was expected that Nanog and Oct4 promoted cell proliferation of MSCs by acting as a transcriptional regulator. Compared with control MSCs, overexpression of Nanog enhanced the proliferation rate of MSCs by average 1.67-fold. This was consistent with increased proliferation rate of MSCs [12] and NIH3T3 [13–14] by Nanog overexpression. Effect of Oct4 overexpression on proliferation rate of MSCs was similar to that of Nanog overexpression. Oct4 overexpression increased proliferation rate of MSCs by average 1.51-fold (Fig. 1D). During passage, control MSCs became flat at passage 10 and began to lose MSC morphology, whereas Nanog or Oct4 overexpressing MSCs still retained the morphology of MSCs (Fig. 1E), showing Nanog or Oct4 overexpression not only promoted proliferation rate of MSCs, but also maintained the morphology of MSCs.
Effect of Nanog and Oct4 overexpression on colony formation of MSCs
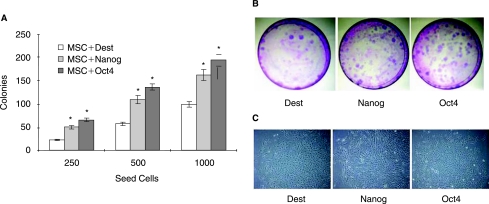
Formation of colony-forming units was used to test stemness of Nanog or Oct4 overexpressing MSCs. As Nanog or Oct4 overexpressing MSCs enhanced the proliferation rate of MSCs, we examined whether the Nanog or Oct4 overexpression improved formation of colony-forming units. Nanog and Oct4 overexpressing and control MSCs were trypsinized to single cells, 250, 500, and 1,000 MSCs were seeded in φ10 cm dish in triplicate, and the ability to form colonies was assayed. Nanog overexpression enhanced colony formation of MSCs by an average 1.94-fold (Fig. 2A and 2B). Compared with Nanog overexpression, Oct4 overexpression enhanced colony formation of MSCs a little higher by an average 2.43-fold (Fig. 2A and 2B). MSCs from colonies retained MSC morphology (Fig. 2C). These findings suggested Nanog or Oct4 overexpression was capable of maintaining MSCs undifferentiated state.
FIG. 2.
Overexpression of Nanog and Oct4 enhanced colony formation of mesenchymal stem cells (MSCs) compared with no insert control (Dest) MSCs. (A) Nanog and Oct4 overexpressing MSCs were seeded at 250, 500, and 1,000 cells in 10 cm dishes, and colonies were counted after Giemsa stain 18 days after seeding, showing Nanog and Oct4 overexpression enhanced colony formation of MSCs compared with no insert control. Asterisks indicate a significant difference compared to no insert control (t-test: P < 0.05). (B) Colony formation from no insert control MSCs, Nanog overexpressing MSCs, and Oct4 overexpressing MSCs seeded at 500 cells in 10 cm dishes. (C) Single colony from no insert control MSCs, Nanog overexpressing MSCs, and Oct4 overexpressing MSCs.
Effect of Nanog and Oct4 overexpression on adipogenesis of MSCs
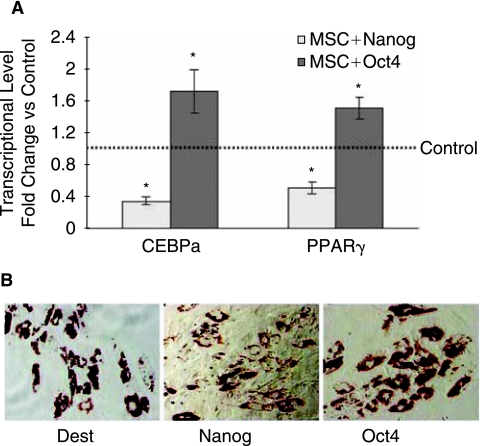
MSCs can differentiate into adipocytes, osteoblasts and chondrocytes, so we examined the effect of Nanog or Oct4 overexpression on differentiation of MSCs. We showed that Nanog overexpression decreased adipogenic marker CEBPα by 0.34-fold and PPARγ by 0.51-fold compared with no insert control (Fig. 3A). Oil red stain for lipid deposits in adipogenesis showed that adipogenesis of Nanog-infected MSCs was not incomplete and showed weaker oil red stain than control MSCs (Fig. 3B), suggesting Nanog overexpression slowed down adipogenesis of MSCs. In contrast to Nanog overexpression, adipogenic marker CEBPα was upregulated by 1.73-fold and PPARγ by 1.52-fold by Oct4 overexpression (Fig. 3A). Different from Nanog overexpression, oil red stain of Oct4 overexpressing MSCs showed complete differentiation towards adipogenesis (Fig. 3B). These showed that Oct4 overexpression improved adipogenesis of MSCs.
FIG. 3.
Effect of Nanog and Oct4 overexpression on adipogenesis of mesenchymal stem cells (MSCs) evaluated by QPCR and oil red stain compared to no insert control (Dest) MSCs under adipogenic medium at Day 14, showing Nanog overexpression slowed down adipogenesis of MSCs, whereas Oct4 overexpression improved adipogenesis of MSCs. (A) Transcriptional level of adigogenic markers regulated by Nanog and Oct4 overexpression by quantitative real time PCR. Asterisks indicate a significant difference compared to no insert control (t-test: P < 0.05). (B) Oil red stain for lipid deposits showing Nanog and Oct4 overexpression had converse effects on adipogenesis of MSCs.
Effect of Nanog and Oct4 overexpression on chondrogenesis of MSCs
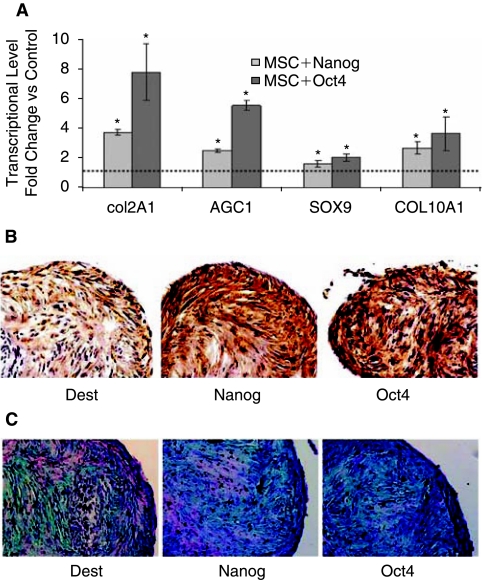
The rapidly emerging field of tissue engineering holds great promise for the generation of cartilage tissue from chondrogenesis of MSCs. We examined the effects of Nanog or Oct4 overexpression on chondrogenesis using pellet culture compared with control MSCs, Nanog overexpression increased the expression level of COL2A1 by 3.71-fold and aggrecan by 2.49-fold. Chondrogenenic master regulator Sox9 and hypertrophic marker COL10A1 was upregulated by Nanog overexpression to 1.63-fold and 2.7-fold, respectively (Fig. 4A). Consistent with transcriptional level, Nanog overexpression enhanced COL2 immunostaining and alcian blue stain for cartilage specific proteoglycan (Fig. 4B and 4C). Similar to Nanog overexpression, Oct4 overexpression increased the expression level of COL2A1 by 7.79-fold and aggrecan by 5.53-fold, Sox9 by 2.04-fold and COL10A1 by 3.65-fold (Fig. 4A). These were consistent with immunostain against COL2A1 and alcian blue stain, showing enhanced expression of collagen type 2 and cartilage specific proteoglycan by Oct4 overexpression (Fig. 4B and 4C). Upregulation of chondrogenic master regulator Sox9 by both Nanog and Oct4 might be responsible for improved cartilage differentiation potential.
FIG. 4.
Effect of Nanog and Oct4 overexpression on chondrogenesis of mesenchymal stem cells (MSCs) evaluated by QPCR, COL2A1 immunostaining, and Alcian blue stain compared to no insert control (Dest) MSCs under chondrogenic medium at Day 28. (A) Transcriptional level of chondrogenic markers was upregulated by Nanog and Oct4 overexpression by quantitative real time PCR. Asterisks indicate a significant difference compared to no insert control (t-test: P < 0.05). (B) Immunostaining against COL2A1. (C) Alcian blue stain for cartilage specific proteoglycan. These data showing Nanog and Oct4 overexpression improved chondrogenesis of MSCs by stain.
Targets of Nanog and Oct4 in undifferentiated MSCs
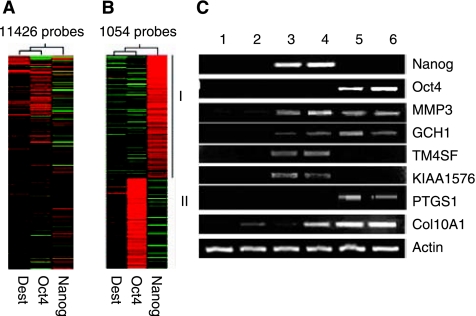
To understand the molecular mechanisms underlying the effects of Nanog or Oct4 on MSCs, we used microarray analysis to determine gene expression profiles of Nanog or Oct4 overexpressing MSCs in comparison to control MSCs, we focused on targets of Nanog or Oct4 in MSCs. A fold change threshold above 1.5 and below −1.5 was considered as upregulated or downregulated genes. Our data showed that 606 genes were upregulated by Nanog overexpresison and 480 genes by Oct4 overexpresion in undifferentiated MSCs (Supplementary Fig. 2, Supplementary Table 1), while 638 genes were downregulated by Nanog overexpresison and 333 genes by Oct4 overexpresion in undifferentiated MSCs. Nanog and Oct4 showed many differences in targets in MSCs (Fig. 5A and 5B), among upregulated genes by Nanog and Oct4, only 32 genes were common genes (Supplementary Fig. 2). Comparing these lists with the lists of 1,687 genes whose promoters were found to be occupied by Nanog in ESCs and 623 genes whose promoters were found to be occupied by Oct4 in ESCs [25], 56 upregulated genes by Nanog and 21 upregulated genes by Oct4 were common between MSCs and ESCs (Supplementary Table 2), while 31 downregulated genes by Nanog and 13 downregulated genes by Oct4 were common (Supplementary Table 3).Targets of Nanog or Oct4 in MSCs were much less than those of Nanog or Oct4 in ESCs. This maybe a result of reprogramming of methylation and other chromatin modifications in adult stem cells MSCs, which blocked the accessibility of promoters in genes that were not be accessible for Nanog or Oct4 regulation.
FIG. 5.
Global gene expression analyses by microarrays. (A) Pearson correlation analysis of 11,426 probes was performed to cluster no insert control (Dest) mesenchymal stem cells (MSCs), Nanog, and Oct4 overexpressing MSCs. Red indicates increased expression, whereas green indicates decreased expression. (B) Genes upregulated in Nanog overexpressing MSCs and/or Oct4 overexpressing MSCs. Genes in group I are upregulated in Nanog overexpressing MSCs, genes in group II are upregulated in Oct4 overexpressing MSCs. (C) Verification of microarray data by RT-PCR. 1. Patient YLJ derived Dest MSCs; 2. Patient DT derived Dest MSCs; 3. Patient YLJ derived Nanog-overexpressing MSCs; 4. Patient DT derived Nanog-overexpressing MSCs; 5. Patient YLJ derived Oct4-overexpressing MSCs; 6. Patient DT derived Oct4-overexpressing MSCs. YLJ and DT are different patients.
The expression patterns of selected genes from parallel samples analyzed by microarrays were subsequently compared by RT-PCR for validation. RT-PCR assays were consistent with the microarray data (Fig. 5C). MMP3, regulator of matrix remodeling and involved in cell migration and enhanced collagen affinity, was expressed at very low level in control MSCs, but were both upregulated to as high as 8.85-fold by Nanog and to 12.66-fold by Oct4. GCH1, the only gene currently known to be associated with GTPCH1-deficient dopa-responsive dystonia (DRD) [26–27], was commonly upregulated to 3.72-fold by Oct4 and 2.11-fold by Nanog. Interestingly, KIAA1576, one of the 25 most positively significant genes in human ESCs defined by SAM [28] was upregulated to 7.2-fold by Nanog compared with no insert control and compared to control-differentiated cells by 12.38-fold. TM4SF4, involved in X-linked mental retardation [29] was also upregulated to 6.2-fold by Nanog. PTGS1, which plays an important role in regulating or promoting cell proliferation in some normal and neoplastically transformed cells [30–31], was specially upregulated to 5.1-fold by Oct4. Chondrogenic hytrophic marker COL10A1 [32] was upregulated by both Oct4 and Nanog in MSCs, compared with Nanog, expression of COL10A1 was upregulated higher by Oct4 than by Nanog.
Among matrix extracellular genes, matrix extracellular genes upregulated by Nanog included MMP3, MMP11, MFAP3, GPC3, SPP1, TIMP3, and EMILIN2. Compared to matrix extracellular genes regulated by Nanog, besides common gene MMP3, matrix extracellular genes upregulated by Oct4 also included MMP1, MMP12, and TFPI2. In cell proliferation genes, besides common genes CXCL2 by Nanog and Oct4, cell proliferation genes by Nanog included TACSTD2, PRL, TGFB2, KITLG, PDCD1LG1, IL6, MAPRE2, and FZD3. Cell proliferation genes upregulated by Oct4 included ChGn, IL11, NDP, INSIG1, PGF, EDNRA, ISG20, and VEGF. In genes related to extracellular matrix structural constituent, hypertrophic marker collagen type X was upregulated by Oct4 to 2.8-fold. TFPI2, involved in inhibition of matrix degradation [33], was upregulated by Oct4 to 3.12-fold. Collagen type 27A1 was upregulated by Oct4 to 1.9-fold (Supplementary Tables 4 and 5). Among statistically significant overrepresented categories, receptor, signaling molecule and cell adhesion molecule were highly overrepresented in unregulated targets of Nanog whereas transcription factor, signaling molecule and extracellular matrix were highly overrepresented in unregulated targets of Oct4 (Supplementary Table 6).The upregulation of cell proliferation and matrix extracellular related genes might be related to their effects in promoted cell proliferation and improved chondrogenetic differentiation potential of MSCs.
Discussion
Mesenchymal stem cells (MSCs) provide an excellent source of pluripotent progenitor cells for tissue-engineering applications due to their proliferation capacity and differentiation potential. Genetic modification of MSCs with genes encoding for tissue-specific growth factors and cytokines can induce and maintain lineage-specific differentiation. It was shown that lentiviral and retroviral gene delivery resulted in long-term transgene expression to effectively genetically modify MSCs [34–35] which provided an efficient tool for ex vivo modification of MSC that does not interfere with differentiation [36–37]. In addition, lentiviral vectors will enable rapid analysis of gene function in MSCs and permit the generation of knock-in / knock-out models of human disease in the rapidly developing field of gene therapy. In this study, by lentiviral system human Nanog or Oct4 was stably and efficiently transduced into human MSCs.
Nanog and Oct4 are transcription factors required to maintain the pluripotency and self-renewal of ESCs. The pluripotent cell-specific gene Nanog is required for maintaining the undifferentiated state and self-renewal of stem cells [8–9]. In ESCs, knockout or knockdown of Nanog abolishes both self-renewal and pluripotency and results in differentiation to extraembryonic endoderm whereas Nanog overexpression enables their propagation for multiple passages during which the cells remain pluripotent. Nanog overexpressing cells form colonies efficiently even at a very low density and acquire expression of a marker specific to the primitive ectoderm (the consecutive pluripotent population in the embryo) [11]. Ectopic expression of Nanog increased growth rate of MSCs [12] and NIH3T3 [13–14]. Our data showed that ectopic expression of Nanog promoted cell proliferation and enhanced colony formation of MSCs, suggesting Nanog ovexexpression maintained stemness of MSCs and might function in a similar fashion in progenitor MSCs as in ESCs.
Oct4, a POU domain-containing transcription factor encoded by Pou5f1, is expressed in totipotent ESCs and germ cells, and has a unique role in development and in the determination of pluripotency. The absence of Oct4 in vivo and vitro reverts ESCs to the trophoblast lineage. Increasing the expression of Oct4 above the endogenous levels in ESCs leads to differentiation toward the extraembryonic mesoderm lineage [18]. It was shown that Oct4-expressing MSCs displayed high proliferative capacity [24] whereas Oct4 knockdown significantly lowered growth rates of MSCs [38]. Studies by Hochedlinger et al. [39] showed that only progenitor cells responded to ectopic expression of Oct4 with increased proliferation. Conversely, ectopic expression of Oct4 in differentiated fibroblasts had adverse effects on cell proliferation and resulted in a 2-fold reduction in cell proliferation of fibroblast, showing that Oct4 overexpression had converse effects on proliferation of progenitor cells and differentiated cells. In addition, Oct4 overexpression expanded the proportion of immature cells in teratomas [40] as well as neural-cell populations derived from ESCs [41]. In agreement with the findings that Oct4 increased progenitor and/or stem cells rather than differentiated cells, our findings demonstrated that Oct4 overexpression promoted proliferative rate and enhanced colony formation of MSCs, suggesting Oct4 ovexexpression played a role in maintaining stemness of MSCs. Thus, the specific effects of Oct4 overexpression on adult MSCs may reflect similarities between ESCs and adult stem cells. However, the presence of Oct4 in adult human peripheral blood mononuclear cells, genetically stable and mainly terminally differentiated cells with well defined functions and a limited lifespan challenges its role as a pure stem cell marker [42].
Very interestingly, our findings showed Nanog and Oct4 overexpression had different effects on adipogenesis of MSCs, Nanog slowed down adipogenesis whereas Oct4 improved adipogenesis, suggesting that Nanog and Oct4 functioned in adipogenesis of MSCs in a different manner. During development, although both Nanog and Oct4 are required for the maintenance of pluripotency, Nanog function is required at a later point than the initial requirement for Oct4, showing a difference between Nanog and Oct4. In chondrogenesis, our findings showed that Nanog and Oct4 both improved chondrogenesis, which may have resulted from maintenance of stemness of MSCs by Nanog or Oct4 overexpression. These results suggest that Oct4 could be a useful transcription factor to engineer high quality MSCs.
Genome-wide gene expression profiles provide help in understanding the molecular and genetic basis underlying effects or function of Nanog and Oct4 in MSCs. It was shown that Nanog regulated a unique set of genes in different cell lines [14], suggesting targets of Nanog were accessible in a tissue specific manner. There are many differences in targets of Nanog or Oct4 between MSC and ESCs explained in part by the different epigenetic status between the two types of cells. In ESCs where the genome tends to be hypomethylated, the chromatin is accessible for Nanog or Oct4 to occupy their target sites in many promoters [25]. Methylation and other chromatin modifications occurring during development lead to blocking of the accessibility of promoters in genes that will not be accessible for Nanog or Oct4 regulation. In our study, it was striking that similar target genes were reproducibly up- or down-regulated by Nanog or Oct4 in MSCs derived from two different individuals. In addition, there is clearly a subset of genes in MSCs that are commonly responsive to Nanog and Oct4 overexpression, such as MMP3. Nanog and Oct4 targets in MSCs by microarray also showed many differences between Nanog and Oct4, which may be responsible for their different effects on differentiations of MSCs.
The data in this study also raised the pertinent issue of how and why the genome of a specific cell type (in this instance MSCs) responds to the enforced ectopic expression of transcriptional factors (in this instance Nanog and Oct4) that are normally not expressed. We observed clearly Nanog- or Oct4-responsive genes in MSCs indicating that some degree of promiscuous transcriptional activation by the same transcriptional factor in different cell types existed. More importantly, we showed here that genes bound and activated by Nanog and Oct4 in ESCs can also be activated, therefore presumably bound, by Nanog and Oct4.
Recently, it has been shown that the introduction of defined factors into human somatic cells resulted in generation of induced pluripotent stem (iPS) cells similar to hESCs in morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity [43,44]. Thus Oct4, Sox2 and Nanog are key reprogramming factors for human ESCs. However, introduction of single Nanog or Oct4 did not generate iPS cells from mouse fibroblast [45]. This was consistent with our data in human MSCs. In addition, no synergistic effects of combination of Nanog and Oct4 in MSCs were observed (data not shown). However, our data showed that single Nanog or Oct4 overexpression in human MSCs promoted cell proliferation and enhanced colony formation of MSCs, suggesting Nanog or Oct4 overexpression maintained stemness of MSCs. Knowledge gained through these study systems may shed more light on the kinetics and mechanism of reprogramming. The study also demonstrates the possible use of stemness-related genes to engineer high quality MSCs for the treatment of cartilage injury and physeal (growth plate) defects in orthopedics and other clinical applications.
Supplementary Material
Acknowledgments
We would like to thank Soh Boon Seng, Zheng Yang, Wai Leong Tam, and Jin Qiu Zhang for their assistance in this study. This research was funded by BMRC grant (R-175-000-085-305) and A*STAR. B.L. is also partially supported by grants (DK04763 and AI54973) from the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA. Zhu M. Ashjian P. De Ugarte DA. Huang JI. Mizuno H. Alfonso ZC. Fraser JK. Benhaim P. Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y. Jahagirdar BN. Reinhardt RL. Schwartz RE. Keene CD. Ortiz-Gonzalez XR. Reyes M. Lenvik T. Lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low WC. Largaespada DA. Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz EM. Gordon PL. Koo WK. Marx JC. Neel MD. McNall RY. Muul L. Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain JR. Schwarze U. Wang PR. Hirata RK. Hankenson KD. Pace JM. Underwood RA. Song KM. Sussman M. Byers PH. Russell DW. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 6.Arinzeh TL. Peter SJ. Archambault MP. van den Bos C. Gordon S. Kraus K. Smith A. Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85-A:1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Pountos I. Jones E. Tzioupis C. McGonagle D. Giannoudis PV. Growing bone and cartilage: The role of mesenchymal stem cells. J Bone Joint Surg Br. 2006;88:421–426. doi: 10.1302/0301-620X.88B4.17060. [DOI] [PubMed] [Google Scholar]
- 8.Mitsui K. Tokuzawa Y. Itoh H. Segawa K. Murakami M. Takahashi K. Maruyama M. Maeda M. Yamanaka S. The Homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 9.Chambers I. Colby D. Robertson M. Nichols J. Lee S. Tweedie S. Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 10.Hyslop L. Stojkovic M. Armstrong L. Walter T. Stojkovic P. Przyborski S. Herbert M. Murdoch A. Strachan T. Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- 11.Dar H. Mayshar Y. Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2006;133:1193–1201. doi: 10.1242/dev.02286. [DOI] [PubMed] [Google Scholar]
- 12.Go MJ. Takenaka C. Ohgushi H. Forced expression of Sox2 or Nanog in human bone marrow derived mesenchymal stem cells maintains their expansion and differentiation capabilities. Exp Cell Res. 2008;314:1147–1154. doi: 10.1016/j.yexcr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J. Wang X. Chen B. Suo G. Zhao Y. Duan Z. Dai J. Expression of Nanog gene promotes NIH3T3 cell proliferation. Biochem Biophys Res Commun. 2005;338:1098–1102. doi: 10.1016/j.bbrc.2005.10.071. [DOI] [PubMed] [Google Scholar]
- 14.Piestun D. Kochupurakkal BS. Jacob-Hirsch J. Zeligson S. Koudritsky M. Domany E. Amariglio N. Rechavi G. Givol D. Nanog transforms NIH3T3 cells and targets cell-type restricted genes. Biochem Biophys Res Commun. 2006;343:279–285. doi: 10.1016/j.bbrc.2006.02.152. [DOI] [PubMed] [Google Scholar]
- 15.Pesce M. Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri SL. Peter W. Hess H. Schöler HR. Oct-4 transcription factor is differentially expressed in the mouse embryos during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 17.Reim G. Brand M. Spiel-ohne-grenzen/pou2 mediates regional competence to respond to Fgf8 during zebrafish early neural development. Development. 2002;129:917–933. doi: 10.1242/dev.129.4.917. [DOI] [PubMed] [Google Scholar]
- 18.Niwa H. Miyazaki J. Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation, or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 19.Nichols J. Zevnik B. Anastassiadis K. Niwa H. Klewe-Nebenius D. Chambers I. Schöler H. Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 20.Shimazaki T. Okazawa H. Fujii H. Ikeda M. Tamai K. McKay RD. Muramatsu M. Hamada H. Hybrid cell extinction and re-expression of Oct-3 function correlates with differentiation potential. EMBO J. 1993;12:4489–4498. doi: 10.1002/j.1460-2075.1993.tb06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekiya I. Vuoristo JT. Larson BL. Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone B. Hering TM. Caplan AI. Goldberg VM. Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 23.Tai MH. Chang CC. Kiupel M. Webster JD. Olson LK. Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26(2):495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 24.Tondreau T. Meuleman N. Delforge A. Dejeneffe M. Leroy R. Massy M. Mortier C. Bron D. Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 25.Boyer LA. Lee TI. Cole MF. Johnstone SE. Levine SS. Zucker JP. Guenther MG. Kumar RM. Murray HL. Jenner RG. Gifford DK. Melton DA. Jaenisch R. Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;23(122):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichinose H. Ohye T. Takahashi E. Seki N. Hori T. Segawa M. Nomura Y. Endo K. Tanaka H. Tsuji S. Fujita K. Nagatsu T. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa Y. Update on dopa-responsive dystonia: locus heterogeneity and biochemical features. Adv Neurol. 2004;94:127–138. [PubMed] [Google Scholar]
- 28.Sperger JM. Chen X. Draper JS. Antosiewicz JE. Chon CH. Jones SB. Brooks JD. Andrews PW. Brown PO. Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci USA. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemni R. Bienvenu T. Vinet MC. Sefiani A. Carrié A. Billuart P. McDonell N. Couvert P. Francis F. Chafey P. Fauchereau F. Friocourt G. des Portes V. Cardona A. Frints S. Meindl A. Brandau O. Ronce N. Moraine C. van Bokhoven H. Ropers HH. Sudbrak R. Kahn A. Fryns JP. Beldjord C. Chelly J. A new gene involved in X-linked mental retardation identified by analysis of an X; 2 balanced translocation. Nat Genet. 2000;24(2):167–170. doi: 10.1038/72829. [DOI] [PubMed] [Google Scholar]
- 30.Narko K. Ristimäki A. MacPhee M. Smith E. Haudenschild CC. Hla T. Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-1 overexpression. J Biol Chem. 1997;272(34):21455–21460. doi: 10.1074/jbc.272.34.21455. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita T. Takahashi Y. Sakashita T. Inoue H. Tanabe T. Yoshimoto T. Growth stimulation and induction of epidermal growth factor receptor by overexpression of cyclooxygenases 1 and 2 in human colon carcinoma cells. Biochim Biophys Acta. 1999;1438(1):120–130. doi: 10.1016/s1388-1981(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 32.Zheng Q. Zhou G. Morello R. Chen Y. Garcia-Rojas X. Lee B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol. 2003;162:833–842. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman MP. Sukhova GK. Kisiel W. Foster D. Kehry MR. Libby P. Schönbeck U. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107:1117–1126. doi: 10.1172/JCI10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XY. La Russa VF. Reiser J. Transduction of bone-marrow-derived mesenchymal stem cells by using lentivirus vectors pseudotyped with modified RD114 envelope glycoproteins. J Virol. 2004;78(3):1219–1229. doi: 10.1128/JVI.78.3.1219-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stiehler M. Duch M. Mygind T. Li H. Ulrich-Vinther M. Modin C. Baatrup A. Lind M. Pedersen FS. Bünger CE. Optimizing viral and non-viral gene transfer methods for genetic modification of porcine mesenchymal stem cells. Adv Exp Med Biol. 2006;585:31–48. doi: 10.1007/978-0-387-34133-0_3. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XY. La Russa VF. Bao L. Kolls J. Schwarzenberger P. Reiser J. Lentiviral vectors for sustained transgene expression in human bone marrow-derived stromal cells. Mol Ther. 2002;5:555–565. doi: 10.1006/mthe.2002.0585. [DOI] [PubMed] [Google Scholar]
- 37.Clements MO. Godfrey A. Crossley J. Wilson SJ. Takeuchi Y. Boshoff C. Lentiviral manipulation of gene expression in human adult and embryonic stem cells. Tissue Eng. 2006;12:1741–1751. doi: 10.1089/ten.2006.12.1741. [DOI] [PubMed] [Google Scholar]
- 38.Greco SJ. Liu K. Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem cells. 2007;25(12):3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- 39.Hochedlinger K. Yamada Y. Beard C. Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Gidekel S. Pizov G. Bergman Y. Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 41.Shimozaki K. Nakashima K. Niwa H. Taga T. Involvement of Oct3/4 in the enhancement of neuronal differentiation of ES cells in neurogenesis-inducing cultures. Development. 2003;130:2505–2512. doi: 10.1242/dev.00476. [DOI] [PubMed] [Google Scholar]
- 42.Zangrossi S. Marabese M. Broggini M. Giordano R. D'Erasmo M. Montelatici E. Intini D. Neri A. Pesce M. Rebulla P. Lazzari L. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells. 2007;25:1675–1680. doi: 10.1634/stemcells.2006-0611. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:1–12. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V. Stewart R. Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.