Dear Sir,
A crucial step in melanoma initiation is the progressive loss of E-cadherin expression, followed by an upregulation of N-cadherin in melanoma cells (Hsu et al., 1996; Tang et al., 1994; Hsu et al., 2000). This process is essentially accomplished by epithelial-mesenchymal transition regulators (EMTRs). Representatives of EMTRs are Snail and Slug of the Snail family of zinc finger transcription factors and Twist of the basic helix-loop-helix (bHLH) family (Cano et al., 2000; Yang et al., 2004). Growth factors govern the continuous repression of E-cadherin via EMTRs to facilitate tumor progression (Thiery and Chopin, 1999; Fuxe et al., 2010). Grotegut et al. (2006) demonstrated that HGF induced scattering and motility in epithelial cells is related to Snail upregulation. We have shown previously that overexpression of HGF downregulates E-cadherin in melanocytic cells (Li et al., 2001). Here we report that HGF leads to stage dependent changes in EMTR expression.
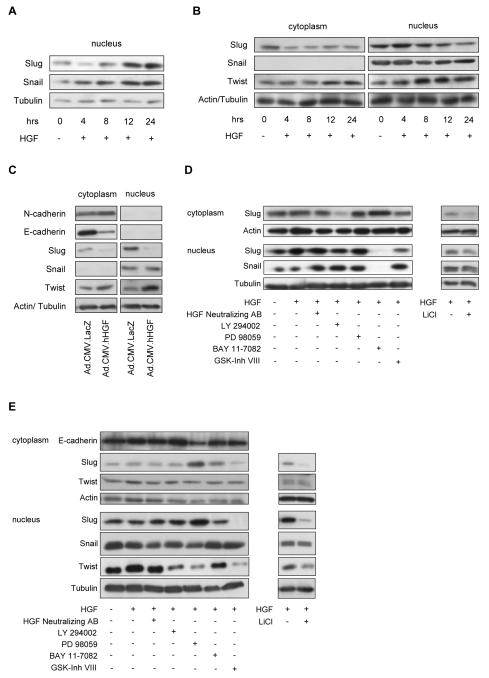
Protein expression levels of Slug, Snail and Twist were determined over a period of 24h after stimulation with 50ng/ml of rhHGF in three representative melanocytic cell lines. In melanocytes (FOM101) an increase in Slug and Snail expression was observed (Fig. 1A), in WM35 (Fig. S1A) and WM164 (Fig. 1B) melanoma cells, Slug was profoundly downregulated, whereas Twist expression increased in the nucleus and the cytoplasm (Fig. 1B). To simulate the cadherin switch in melanoma, WM164 cells, which express E- and N-cadherin, were transduced with an adenoviral vector encoding the cDNA of HGF (Ad.CMV.rhHGF) or a mock vector control (Ad.CMV.LacZ) at 20pfu/cell. A profound decrease of E-cadherin and an increase of N-cadherin expression was observed (Fig. 1C). Further, to investigate on signaling pathways involved in EMTR regulation after HGF exposure, we used specific inhibitors of MAPK (PD 98059), PI3K (LY 294002), GSK3β (GSK3β-inhibitor VIII and lithium chloride [LiCl]) or NF-κB (BAY 11-7082). In FOM101, addition of BAY 11-7082 completely abolished protein levels of Slug and Snail in the nucleus (Fig. 1D, left panel) and inhibition of GSK3β led to decreased Slug levels (Fig.1D, right panel). In melanoma cells exposure to PD 98059 led to a significant increase in Slug expression, which resulted in a downregulation of E-cadherin in WM164 (Fig. 1E, left panel and S1B, left panel). Like in melanocytes, inhibition of GSK3β decreased Slug levels (Fig. 1E, right panel and S1B, right panel). Inhibition of MAPK, PI3K or GSK3β leads to downregulation of Twist in the nucleus (Fig. 1E, left panel).
Figure 1.
HGF mediated stage specific changes of EMTR expression in melanocytic cells are dependent on NF-κB, MAPK, PI3K, and GSK3β. Melanocytic cells were either stimulated with rhHGF (50ng/ml) up to 24h (A, B) or transduced with Ad.CMV.hHGF (20pfu/cell) or vector control (LacZ; 20pfu/cell) for 48h (C). Equal aliquots (10μg) of nuclear or cytoplasmic extracts were separated on a 10% SDS-polyacrylamide gel and then transferred to a PVDF membrane. β-Actin and Tubulin were used as a loading control. (A) Snail and Slug expression in melanocytes. An increase in Snail and Slug was observed after exposure to rhHGF. (B) Snail, Slug and Twist expression in a metastatic melanoma cell line (WM164). An increase in Twist expression and a decrease of Slug was observed after exposure to HGF. (C) Transduced WM164 exhibited decreased E-cadherin and Slug, but increased Twist and N-cadherin levels. For elucidating the signaling pathways involved in EMTR regulation by HGF, melanocytes (D) or serum starved WM164 (E) were pre-treated for 30min without and with the inhibitors LY 294002 (PI3K/Akt; 25μM), PD 98059 (MAPK; 25μM), BAY 11-7082 (NF-κB; 10μM), GSK3β-inhibitor VIII (GSK3β; 25μM) and LiCl (GSK3β; 10mM). Cells were then exposed to HGF (50ng/ml) for 8h, harvested, and subjected to immunoblotting. Levels of E-cadherin, Slug, Snail and Twist were determined. (D) In melanocytes the addition of BAY 11-7082 completely abolished protein levels of Slug and Snail. (E) In WM164, treatment with PD 98059 resulted in an increase of cytoplasmic and nuclear Slug, leading to a decrease of E-cadherin. Inhibition of GSK3β (GSK3β-inhibitor VIII and LiCl) led to a decrease of Slug in melanocytes (D) and WM164 (E), Twist was significantly downregulated by inhibition of PI3K, MAPK and GSK3β (E).
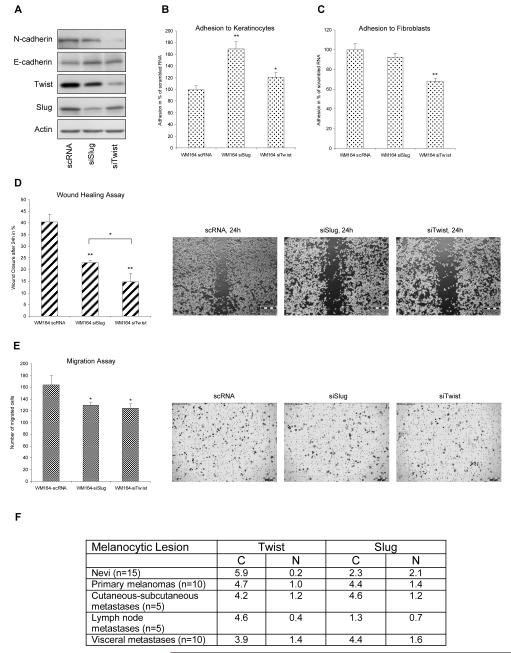
Since HGF led to differential expression levels of Slug and Twist in melanoma cell lines, we determined changes in cadherin levels of both EMTRs after silencing in WM164 cells with consequences for adhesion and migration. Silencing of Slug led to a doubling of E-cadherin levels, whereas expression of N-cadherin was significantly downregulated after Twist silencing with minor changes in E-cadherin levels (Fig. 2A). Correspondingly, we observed a highly significant increase in adhesion of WM164 cells to keratinocytes after silencing of Slug (Fig. 2B) and a profound reduction of adhesion to fibroblasts after silencing of Twist, but not Slug (Fig. 2C). Silencing of both EMTRs resulted in a significant reduction of migration, although silencing of Twist showed a more pronounced effect in scratch assays (Fig. 2D,E). Slug overexpression in WM9 and WM164 cells led to a downregulation of E-cadherin but no changes in N-cadherin levels (Fig. S2A), adhesion to keratinocytes, but not fibroblasts, was clearly reduced (Fig. S2B,C) and migration was enhanced (Fig. S2D,E). Taken together, these results demonstrate Slug to be important for the loss of epithelial properties and a predominant effect of Twist on N-cadherin regulation and adhesion to fibroblasts.
Figure 2.
Twist promotes adhesion of WM164 cells to fibroblasts. Slug (siSlug) and Twist (siTwist) were silenced and expression levels compared to control siRNA (scRNA). (A) Silencing of Slug led to increased E-cadherin levels whereas silencing of Twist resulted in decreased N-cadherin and a slight increase of E-cadherin. (B) Silencing of Slug (p<0,01) and Twist (p<0,05) led to significantly increased adhesion to keratinocytes. (C) Twist but not Slug silencing resulted in decreased adhesion to fibroblasts. (D) Scratch assay showed a highly significant reduction in random migration after Slug and Twist silencing. Wound closure was significantly delayed in siTwist melanoma cells compared to siSlug cells. (E) A significant decrease in site-directed migration was observed after silencing of Slug and Twist. *, p<0.05 significant; **, p<0.01 highly significant. (F) Immunohistochemical analyses of Slug and Twist in paraffin-embedded sections of nevi, primary cutaneous melanoma and metastases for Twist and Slug. The immunoreactive score was obtained by multiplying the percentage of positive cells with staining intensity divided by 10.
Based on the differential regulation of Slug and Twist we examined staining patterns in melanocytic tissue by immunohistochemistry. Examination revealed a highly positive staining for Twist in the cytoplasm of nevi but nearly absence in the nucleus (Fig. 2F and S3A,B). In primary as well as in metastatic lesions cytoplasmatic and nuclear staining of Twist was observed (Fig. 2F and S3A). In contrast, Slug was already found in the nucleus and cytoplasm of nevi (Fig. 2F and S3A). These data suggest an inverse nuclear expression of Slug and Twist in the progression of melanoma. Indeed, probing tissue microarrays with anti-Twist antibodies indicated an association with worse patient survival (Hoek et al., 2004).
Together we show that the cadherin switch mediated by HGF is accomplished through stage specific changes in expression levels of Snail, Slug and Twist, suggesting a hierarchical activation of Slug and Twist in melanoma progression. Our data further suggest that Twist is driving melanoma conversion after the initial de-coupling from keratinocytes initiating binding to fibroblasts.
Supplementary Material
Acknowledgements
We would like to thank the staff of the Center for Medical Research (ZMF), Graz, for technical assistance and Dr. M. Otte, Oridis Biomed GmbH, Graz, for helpful discussions. This work was supported by the Austrian Science Fund (grants Nr. P18630-B05, P21156-B18; PK, CW, SD), S. Joshi is funded by the PhD program Molecular Medicine of the Medical University of Graz, Austria, by the Jubiläumsfond der Österreichischen Nationalbank (12552) and by the Austrian Science Fund (grant Nr. P21156-B18).
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Fuxe J, Vicent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Wheelock MJ, Johnson KR, Herlyn M. Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc. 1996;1:188–1894. [PubMed] [Google Scholar]
- Hsu M, Andl T, Li G, Meinkoth JL, Herlyn M. Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. J Cell Sci. 2000;113:1535–1542. doi: 10.1242/jcs.113.9.1535. [DOI] [PubMed] [Google Scholar]
- Li G, Schaider H, Satyamoorthy K, Hanakawa Y, Hashimoto K, Herlyn M. Downregulation of E-cadherin and Desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene. 2001;20:8125–8135. doi: 10.1038/sj.onc.1205034. [DOI] [PubMed] [Google Scholar]
- Tang A, Eller MS, Hara M, Yaar M, Hirohashi S, Gilchrest BA. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci. 1994;107(Pt 4):983–992. doi: 10.1242/jcs.107.4.983. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999;18:31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.