Abstract
Alzheimer's disease produces a devastating decline in mental function, with profound effects on learning and memory. Early consequences of the disease include the specific loss of cholinergic neurons in brain, diminished cholinergic signaling, and the accumulation of β-amyloid peptide in neuritic plaques. Of the nicotinic acetylcholine receptors at risk, the most critical may be those containing the α7 gene product (α7-nAChRs), because they are widespread, have a high relative permeability to calcium, and regulate numerous cellular events in the nervous system. With the use of whole-cell patch–clamp recording we show here that nanomolar concentrations of β-amyloid peptides specifically and reversibly block α7-nAChRs on rat hippocampal neurons in culture. The block is noncompetitive, voltage-independent, and use-independent and is mediated through the N-terminal extracellular domain of the receptor. It does not appear to require either calcium influx or G protein activation. β-Amyloid blockade is likely to be a common feature of α7-nAChRs because it applies to the receptors at both somato-dendritic and presynaptic locations on rat hippocampal neurons and extends to homologous receptors on chick ciliary ganglion neurons as well. Because α7-nAChRs in the central nervous system are thought to have numerous functions and recently have been implicated in learning and memory, impaired receptor function in this case may contribute to cognitive deficits associated with Alzheimer's disease.
Alzheimer's disease is the most common form of dementia among the elderly, causing severe impairment of learning and memory; death usually occurs within 10 years after the onset of clinical symptoms (1, 2). Early cellular and molecular correlates of the disease include the accumulation of β-amyloid 40- and 42-aa peptides (Aβ1–40 and Aβ1–42, respectively) in neuritic plaques (2, 3), loss of cholinergic neurons, and accompanying degeneration of cholinergic innervation (4–6). Although most studies of cholinergic deficits in Alzheimer's disease have focused on muscarinic aspects, diminished nicotinic transmission may be an important dimension as well because of reduced acetylcholine (ACh) levels and declines in the numbers of nicotinic acetylcholine receptors (nAChRs) in affected tissues (7–10).
One of the most widely expressed nicotinic receptors in the nervous system is a species containing the α7 gene product (11, 12). Such receptors (α7-nAChRs) have an unusually high relative permeability to calcium and regulate numerous calcium-dependent events in the nervous system (13, 14). Examples include transmitter release (15, 16), second messenger cascades (17), neurite extension (18, 19), and both apoptosis (20) and neuronal survival (21). The receptors can also contribute directly to postsynaptic currents (22–24) and are expressed both at somato-dendritic and presynaptic sites on neurons in the hippocampus (16, 25–27), a structure critical for memory formation (28). Activation of α7-nAChRs can promote long-term potentiation at glutamatergic synapses (29). Mice homozygous null for the α7 gene do not show learning deficits in simple behavioral tests (30), but this lack of learning deficits may reflect compensation by the nervous system during development. Intervention with specific α7-nAChR agonists and antagonists in rats has implicated the receptors in a variety of cognitive processes, including spatial memory and avoidance behavior (31), and in working memory formation, as revealed by radial arm maze tests on normal and lesioned animals (32, 33). The levels of α7-nAChR protein are significantly diminished in the cerebral cortex of Alzheimer patients (9, 34).
Recently the β-amyloid peptides Aβ1–40 and Aβ1–42 were reported to bind selectively and with high affinity to α7-nAChRs; the binding was described as competitive with respect to the snake toxin α-bungarotoxin (αBgt), a convenient marker for the receptors (35, 36). Previous studies have supported the hypothesis that Aβ1–40 and Aβ1–42 contribute to the progression of Alzheimer's disease and may directly impair cholinergic signaling and ACh release (37, 38). Blockade of α7-nAChR function by the peptides would further compromise cholinergic signaling and could have significant secondary effects if the receptors broadly modulate transmitter release and influence neuronal survival as proposed. To identify a possible blockade, we examined the effects of β-amyloid peptides on α7-nAChR function in rat hippocampal cultures because of the significance of the hippocampus for memory formation. We also tested α7-nAChRs on chick ciliary ganglion neurons because the neurons express high levels of the receptors and serve as a useful model.
Materials and Methods
Cell Cultures.
Rat dissociated hippocampal cell cultures were prepared by a method described for cortical neurons (39). Briefly, hippocampal tissue was dissected from embryonic day 18–19 Sprague–Dawley rats. The tissue was cut into small pieces and incubated for 30 min at 37°C in a solution equilibrated with 95% air/5% CO2 and containing (in mM) 116 NaCl, 5.4 KCl, 26 NaHCO3, 1 NaH2PO4, 1.5 CaCl2, 1 MgSO4, 0.5 EDTA, 25 glucose, 1 l-cysteine, and 15–20 units/ml papain (Worthington). The cells were dispersed by gentle trituration, and the dissociated suspension was plated on a confluent layer of glial cells on 12-mm glass coverslips (for electrophysiology) or on plastic culture wells (for binding experiments). The glial cell layer was generated by plating a hippocampal cell suspension (after the substratum was coated with 0. 25 mg/ml poly-d-lysine) and allowing the glial cells to settle and proliferate for 1–2 weeks before treating with 5 μM cytosine arabinoside for 1–2 days to halt further cell division (few neurons were present at that point). For hippocampal cultures, the medium contained Eagle's MEM (GIBCO), 5% (vol/vol) heat-inactivated horse serum (HyClone), 2% B27 supplement, 0.5 mM glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin (GIBCO) for the hippocampal cultures. For glial cultures the B27 supplement was omitted and the horse serum concentration was 10%. Two days after hippocampal cells were added to the confluent glial cells, the cultures were treated with 5 μM cytosine arabinoside for 1–2 days. The cytosine arabinoside was diluted by replacing half of the culture medium each week. Cultures were taken for experiments 8–18 days after the hippocampal cells were added to the glial layers.
Chick ciliary ganglion cells were obtained from 13-day embryos as previously described, allowed to attach to the substratum for 1–5 h, and then taken either for whole-cell patch–clamp recording (40) or for binding studies as described (41), with the use of 125I-αBgt and testing for competition with either Aβ1–42 or d-tubocurarine. Competition binding studies were carried out on hippocampal cultures in the same way. HEK293 cells were obtained and transiently transfected either with a chimeric α7-nAChR/5HT3 receptor construct (α7-V201–5HT3; ref. 42) or with the wild-type 5HT3 receptor construct (43) as described (44), and then analyzed 2 days later with whole-cell patch–clamp recording as outlined above for hippocampal neurons.
Electrophysiological Recording.
Amphotericin B-perforated (45) and conventional whole-cell patch–clamp recordings (46) were obtained from hippocampal neurons as described (40). An Axopatch 200A amplifier and pclamp 7 software (Axon Instruments, Foster City, CA) were used for data acquisition and analysis. The bathing solution contained (in mM) 150 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 0.0005 tetrodotoxin, and 10 Hepes adjusted to pH 7.4 with NaOH. Atropine (0.3 μM) was usually included in the bath when ACh was used as the agonist. For perforated whole-cell recording, the pipette solution contained (in mM) 75 Cs2SO4, 55 CsCl, 5 MgCl2, and 10 Hepes, adjusted to pH 7.2 with CsOH. Amphotericin B was back-filled into the patch pipette at 400 μg/ml. For conventional whole-cell recording, the pipette solution contained (in mM) 100 CsCH3SO3, 20 CsCl, 2 MgCl2, 2 Mg-ATP, 10 Hepes, 20 phosphocreatine, and, if specified, either 0.5 mM GDPβS or GTPγS. When intracellular calcium was to be buffered, the pipette solution contained (in mM) 120 CsCH3SO3, 20 CsCl, 10 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate, 2 MgCl2, 2 Mg-ATP, and 10 Hepes, adjusted to pH 7.2 with CsOH. A rapid solution exchange system (<5 ms) was used to perfuse the cells and to deliver the agonists and β-amyloid peptides (47). Unless otherwise indicated, the solutions and applicator barrels were arranged to allow repeated 0.4- to 1-s tests of ACh responses from a neuron at 1-min intervals before and during a 3- to 5-min application of the peptide.
Data Analysis.
Data are shown as the mean ± SEM of the number of determinations indicated in parentheses, and Student's t test was used to evaluate statistical significance unless otherwise indicated. EC50 and IC50 values were determined by least-squares fit of the data. Miniature excitatory postsynaptic currents (mEPSCs) were analyzed as described (48). Synaptic events were detected with an adjustable threshold, often set at 5–8 pA and kept constant for a given group of data. Cumulative distribution plots (16, 49) were used to compare the distributions of amplitude and interevent intervals for mEPSCs, and differences in these cases were determined statistically by the Kolmogorov–Smirnov test, which estimates the probability (P) that two distributions are similar. With the Kolmogorov–Smirnov test, two cumulative sets of data were considered significantly different only when P < 0.01.
Reagents.
The β-amyloid peptides rat Aβ1–40 and Aβ1–42 and human Aβ1–40 were obtained from Calbiochem; human Aβ40–1 was obtained from Sigma. The β-amyloid peptide solutions were prepared by adding the peptide to deionized water and then adding acetic acid to a final concentration of 5% (vol/vol) for complete solubility, as recommended by the supplier; 100 μM aliquots of the peptide were stored at −20°C until use (<4 weeks). Aliquots were thawed as needed, diluted by at least a 1000-fold in recording buffer for a single experiment, and then discarded after use. The final concentration of acetic acid in the recording buffer was ≤0.005%. Tetrodotoxin was obtained from Calbiochem; unless otherwise indicated, all other chemicals were from Sigma. 125I-αBgt was either purchased commercially (Amersham Pharmacia) or prepared as described (41). The α7-nAChR/5HT3 receptor chimeric construct was provided by Dr. William Green (University of Chicago), and the wild-type 5HT3 receptor construct was provided by Dr. David Julius (University of California, San Francisco).
Results
Blockade of Hippocampal α7-nAChRs by β-Amyloid Peptides.
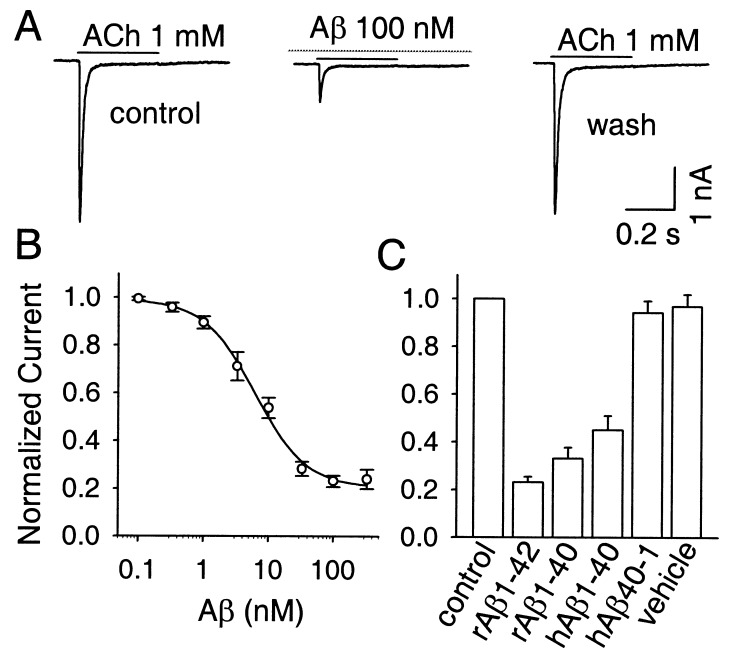
Whole-cell patch–clamp recording from rat hippocampal neurons in dissociated cell culture was used to examine the effects of β-amyloid peptides on α7-nAChR responses. Fast application of 1 mM ACh for 0.4–1 s to neurons voltage-clamped at −60 mV produced rapidly activating and rapidly desensitizing inward currents as reported for α7-nAChRs (47, 50). Peak amplitudes varied greatly among neurons, ranging from a few picoamperes to >10 nA. Cells were discarded if the initial response was below 300 pA. Atropine (0.3 μM) was included in the bath to prevent activation of muscarinic receptors. The ACh-induced responses were blocked by 100 nM αBgt in a pseudoirreversible manner and by 1 nM methyllycaconitine in a rapidly reversible manner (not shown). These features are characteristic of rapidly desensitizing α7-nAChRs (12, 47, 50). Examining the same neurons at 1-min intervals before and during a 3- to 5-min exposure to nanomolar concentrations of rat Aβ1–42 revealed a substantial blockade of the α7-nAChR response caused by the peptide (Fig. 1A). Maximal inhibition approached 80% and occurred within 1 min. Full recovery occurred within 5 min after Aβ1–42 removal. Analysis of the concentration dependence yielded an IC50 for blockade of 7.5 nM (Fig. 1B). Both rat Aβ1–42 and Aβ1–40 produced the blockade, as did human Aβ1–40 (Fig. 1C). No effect was seen when the reverse peptide, human Aβ40–1, was used as a negative control, or when vehicle alone was applied.
Figure 1.
Specific blockade of α7-nAChRs by β-amyloid peptides. (A) ACh responses characteristic of hippocampal α7-nAChRs before (Left), during (Center), and 5 min after (Right) a 3-min exposure to 100 nM rat Aβ1–42. (B) Concentration dependence for the Aβ1–42 blockade of the α7-nAChR response (n = 6–8 for each value). (C) Effects of the rat (r) and human (h) Aβ1–42 and Aβ1–40 peptides (all at 100 nM; n = 8) on the α7-nAChR response. Negative control: hAβ40–1 (100 nM; n = 6); vehicle (n = 6) was extracellular recording solution plus 0.005% acetic acid. Both here and in the other figures, results were normalized to the peak response obtained from the same cell before peptide application (normalized current).
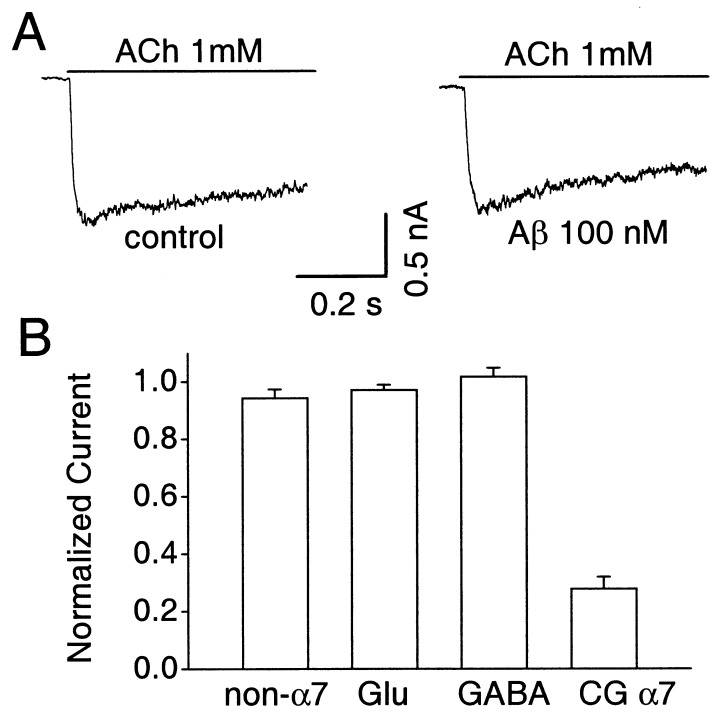
The inhibition of the α7-nAChR response by Aβ1–42 (100 nM) was selective. It did not significantly reduce the peak amplitude response of other nAChRs that could be found on a minor fraction (<10%) of hippocampal neurons in culture (Fig. 2A). Such receptors were distinguished in each case by their slowly decaying ACh responses and their resistance to blockade by 100 nM αBgt (1–2 h at 37°C) and atropine (0.3 μM); in some cases 100 μM d-tubocurarine was also tested and found to block the response completely. Aβ1–42 (100 nM) also had no effect on the response elicited either by 100 μM γ-aminobutyric acid (GABA) or by 100 μM glutamate, indicating that the cognate receptors were spared (Fig. 2B). Blockade by Aβ1–42 may be a feature of all rapidly desensitizing α7-nAChRs, however, because the peptide did produce a substantial blockade of α7-nAChRs on chick ciliary ganglion cells tested with 20 μM nicotine (Fig. 2B).
Figure 2.
Effects of 100 nM Aβ1–42 on other ionotropic receptors. Cells were voltage-clamped at −60 mV. (A) Representative example showing the slowly desensitizing, αBgt-resistant ACh responses of a neuron with non-α7-nAChRs before (Left) and during (Right) application of Aβ1–42. (B) Compiled data showing the absence of significant Aβ1–42 blockade on peak responses from non-α7-nAChRs (non-α7), glutamate receptors (Glu), and GABAA receptors (GABA) on rat hippocampal neurons and the presence of blockade for α7-nAChRs on chick ciliary ganglion neurons (CGα7). Mean initial responses (in nanoamperes) from Left to Right were 0.8 ± 0.2 (n = 6), 3.2 ± 0.7 (7), 4.3 ± 1.2 (4), and 4.5 ± 0.5 (8).
Mechanism of β-Amyloid Blockade.
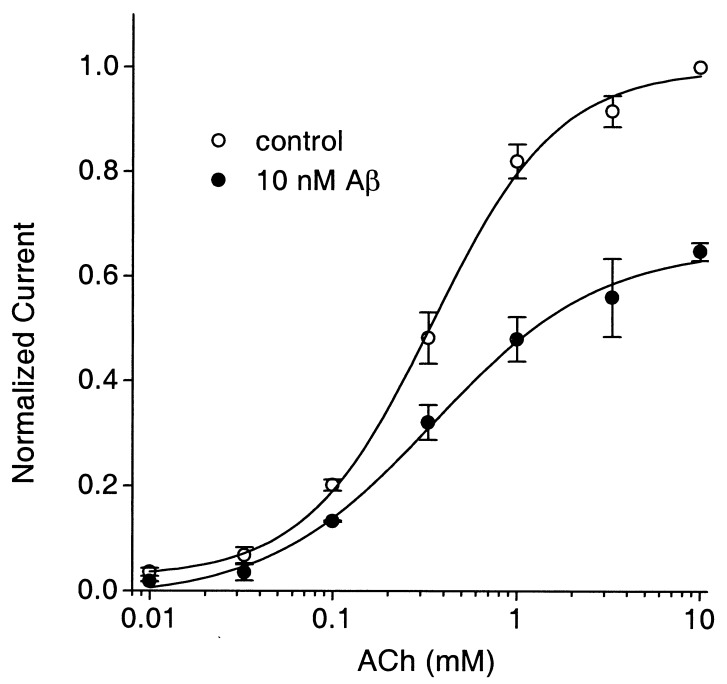
Varying the concentration of agonist in the presence of 10 nM Aβ1–42 indicated that the peptide blockade was noncompetitive (Fig. 3). Thus, the peptide reduced the maximum response without altering the EC50 for agonist (346 μM vs. 351 μM for control and Aβ1–42-treated, respectively). This effect was unexpected because Aβ1–42 was reported to compete with αBgt for binding to α7-nAChRs (35, 36) and αBgt binding is competitive with ACh on the receptors (11, 12). We examined the ability of Aβ1–42 to compete with 125I-αBgt on hippocampal neurons in culture under exactly the same conditions (culture age, cell density, buffer composition) as those used above to demonstrate blockade of function. Aβ1–42 at 200 nM, a concentration representing at least a 25-fold excess over that required for the IC50, produced no significant inhibition of 125I-αBgt binding. Thus, Aβ1–42-treated cells specifically bound 96 ± 18% (n = 5 experiments; three determinations per experiment), as much as control cells did when tested in a 1-h incubation with 10 nM 125I-αBgt. Inclusion of 100 μM d-tubocurarine, in contrast, reduced the level of specific binding to 12 ± 12% (n = 3) of controls, demonstrating that competition by Aβ1–42 could have been seen, had it occurred.
Figure 3.
Noncompetitive blockade of hippocampal α7-nAChRs by Aβ1–42. Individual cells were tested at 10 mM ACh and one or more additional concentrations of ACh before and after application of 10 nM Aβ1–42. Results were normalized to those obtained with 10 mM ACh and then pooled for each data point (n = 5–7). Increasing the concentration of ACh did not overcome the partial block achieved with the concentration of Aβ1–42 used.
Because hippocampal cultures yield variable levels of 125I-αBgt binding among experiments (0.5–5 fmol per culture), similar studies were carried out on chick ciliary ganglion neurons, which consistently yield high levels of receptor. As shown above, ciliary ganglion α7-nAChRs are blocked by Aβ1–42. To increase the chances of detecting competition, the neurons were first incubated with 1 μM Aβ1–42 for 15 min and then with 125I-αBgt under nonsaturating conditions (2 nM, 30 min at 37°) in the continued presence of the peptide. Aβ1–42 caused little, if any, significant reduction in binding compared with untreated controls (91 ± 5% of controls; n = 5 experiments, three determinations per experiment), despite being present in a vast excess over that required for blockade of function. Toxin binding was not saturating under these conditions because 5 nM 125I-αBgt (30 min at 37°C) produced half again as much specific binding on average. The Aβ1–42 peptide was recovered at the end of the experiment (from culture medium lacking 125I-αBgt), diluted 20-fold, and found to produce 51 ± 5% (n = 6 neurons) blockade of the α7-nAChR response. Thus, the peptide retained the activity level expected. The results indicate that blockade of α7-nAChR function by Aβ1–42 is unlikely to involve a competitive interaction with the peptide.
Further studies were conducted to examine the mechanism of Aβ1–42 blockade. Increased desensitization did not seem to play a role. Fitting the decay phase of the response in the presence and absence of 100 nM Aβ1–42 yielded time constants for the decay of 20 ± 3 and 14 ± 2 ms (n = 7; P < 0.05, paired t test), respectively. Thus, if anything, Aβ1–42 slightly decreased the rate of desensitization of the whole-cell response. The Aβ1–42 blockade did not require receptor preactivation: applying the 100 nM Aβ1–42 continuously 1–5 min before, but not along with, agonist yielded substantial blockade (27 ± 2% of control; n = 6). This controlled application was achieved by using separate barrels of the rapid applicator for the Aβ1–42 and ACh. In contrast, when Aβ1–42 was applied with, but not before, agonist (i.e., coapplication only), the mean peak amplitude was 96 ± 3% of control (n = 6). Even repeated trials (three to five) of coapplication for the normal duration (0.4–1 s) at 1-min intervals yielded no significant block. Thus, Aβ1–42 blockade of α7-nAChR function requires preapplication but is not use-dependent, i.e., it does not require activation of the receptors (presence of agonist). Nor is the blockade voltage-dependent, which was shown by comparing the extent of blockade by Aβ1–42 (10 nM) at −60 and +60 mV. Although inward rectification limited the peak amplitude response at +60 mV to 23 ± 3% of that at seen −60 mV, treatment with Aβ1–42 produced the same proportional blockade: 53 ± 4% at +60 mV and 50 ± 5% at −60 mV (mean ± SEM; n = 4). [The inward rectification (11) required us to select neurons with large responses to 1 mM ACh, i.e., ≥2 nA at −60 mV, so that the responses could still be quantified at +60 mV.]
No evidence supported an intracellular mechanism involving either calcium influx or G protein-coupled receptors to mediate the Aβ1–42 inhibition of α7-nAChRs. Thus, dialyzing the neurons with 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate, a calcium chelator, for ≥3 min via the patch pipette during conventional whole-cell recording failed to prevent Aβ1–42 (100 nM) from blocking the response (25 ± 4% of control; n = 7). Dialyzing with either 0.5 mM GDPβS to block G protein-dependent signal transduction pathways or 0.5 mM GTPγS to activate them (≥3 min) also had no significant effect on the blockade: peak amplitudes in the presence of peptide were 26 ± 3% and 25 ± 3% of controls, respectively (n = 6 in each case).
Blockade of Chimeric α7/5HT3 Receptors.
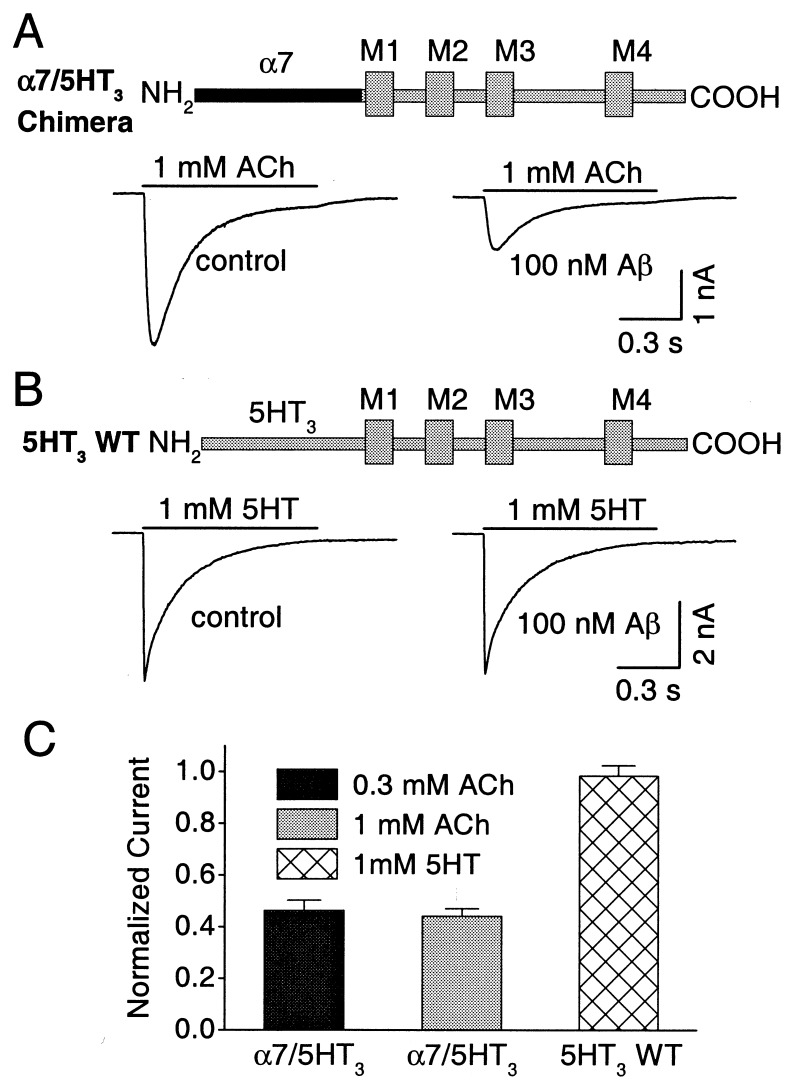
The blockade clearly depends on the extracellular portion of the α7-nAChR, which was shown by comparing the responsiveness of an α7-nAChR/5HT3 chimeric receptor with that of wild-type 5HT3 receptors. The chimeric receptor contained the N-terminal extracellular portion of the α7-nAChR gene (up to valine 201 just before the first putative transmembrane domain) fused to the complementary (remaining) portion of the wild-type 5HT3 receptor, including the four putative transmembrane domains and the C terminus (43). When constructs encoding such chimeras were heterologously expressed in transfected HEK293 cells, the cells became responsive to ACh as reported (43, 51). Aβ1–42 (100 nM) substantially inhibited the response elicited by 1 mM ACh (Fig. 4 A and C). Mean peak amplitudes of 2.5 ± 0.4 and 1.1 ± 0.2 nA (n = 10; P < 0.001) were seen before and after Aβ1–42 application to cells voltage-clamped at −60 mV.
Figure 4.
Aβ1–42 blockade of chimeric α7-nAChR/5HT3 receptors. HEK293 cells were transfected with either the chimeric α7-nAChR/5HT3 receptor (A) or wild-type 5HT3 receptor (B) constructs and were examined 2 days later with whole-cell patch–clamp recording to compare responses elicited by ACh or 5-HT in the absence (Left) and presence (Right) of 100 nM Aβ1–42 for cells voltage-clamped at −60 mV. The black bar of the construct represents the α7 domain; the gray bar represents 5HT3 wild-type domain; M indicates transmembrane domain. The peptide produced significant (and equivalent) inhibition of chimeric responses elicited by either 0.3 or 1 mM ACh but not wild-type responses elicited by 1 mM 5HT (C). The peak amplitude of the response in Aβ1–42 was normalized to the initial response from the same cell in each case (n = 10 for each; P < 0.001).
The inhibition of the chimeric receptor appeared noncompetitive, as seen for hippocampal α7-nAChRs. Thus, Aβ1–42 (100 nM) was equally effective at inhibiting responses elicited by 0.3 and 1 mM ACh (Fig. 4 A and C), and 0.3 mM ACh was nonsaturating, i.e., it elicited a smaller response (1.3 ± 0.2 nA; n = 10) than did 1 mM ACh. Blockade of the chimeric receptor did not depend on receptor activation: maximal block was obtained with the first 0.4- to 1-s trial, as in the case of hippocampal α7-nAChR response, and further trials did not increase the blockade, despite continued application of Aβ1–42. No inhibition was seen for the wild-type 5HT3 receptor heterologously expressed in transfected cells (Fig. 4 B and C). Mean peak amplitudes in this case were 3.7 ± 0.9 and 3.7 ± 0.9 nA (n = 9) before and after Aβ1–42 application, respectively. The results strongly suggest that the blockade by Aβ1–42 is mediated by an interaction of the peptide with the extracellular N-terminal domain of the α7-nAChR. The results do not exclude the possibility that the interaction is indirect, i.e., that it is mediated by an interposed component, but the component would have to interact with the N-terminal extracellular domain of α7-nAChRs and be present not only on hippocampal and ciliary ganglion neurons, but also on HEK293 cells.
β-Amyloid Effects on Presynaptic Modulation of Transmitter Release.
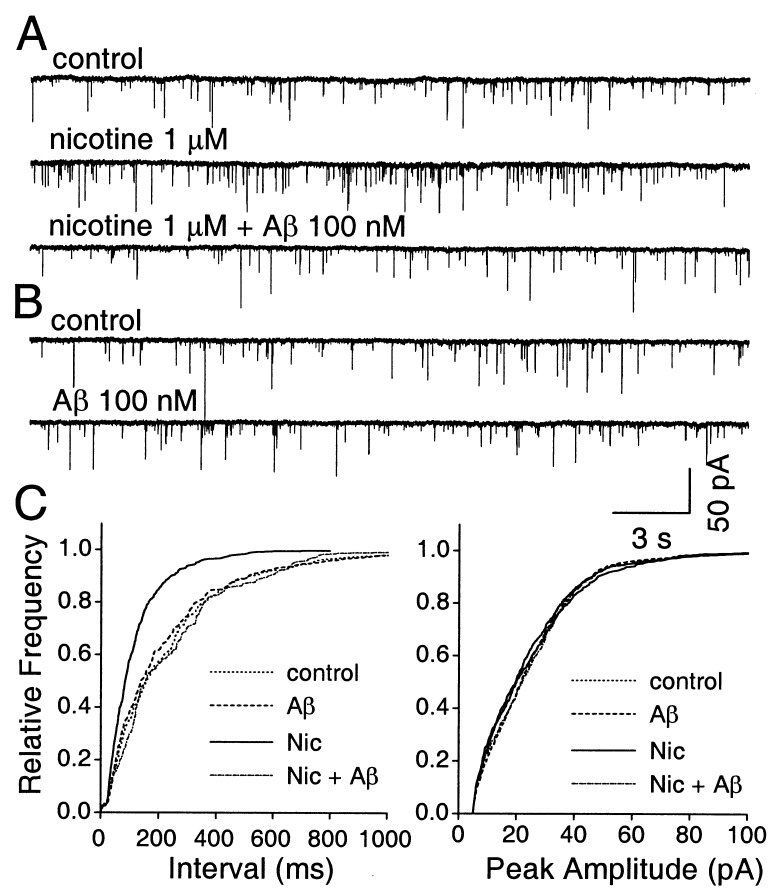
In the hippocampus, α7-nAChRs can act presynaptically to augment the release of excitatory neurotransmitters, suggesting a role for the receptors in synaptic modulation and information processing. Thus, nicotine increases the frequency but not the amplitude of spontaneously occurring mEPSCs in cultured hippocampal neurons, and the increase is blocked by αBgt (16). We tested the effects of Aβ1–42 on presynaptic hippocampal α7-nAChRs by determining whether the peptide prevented the nicotine-induced increase in mEPSC frequency. In a third of the neurons tested, bath application of 1 μM nicotine elicited a significant increase in the frequency of spontaneous mEPSCs (188 ± 23% of control values; 5/16 neurons; P < 0.01). This observation was made in the presence of 0.5 μM tetrodotoxin to block action potentials and 2 μM bicucculine to block GABAA receptors (Fig. 5A). The mEPSCs were blocked by 10 μM 6-cyano-7-nitroquinoxaline-2, 3-dione, as expected for glutamate responses (not shown). In all cases, the nicotine-induced increase in mEPSC frequency was blocked by 100 nM Aβ1–42 (103 ± 11% of control values in the absence of nicotine; n = 5), and the peptide had no effect on the basal rate of mEPSCs (96 ± 7% of controls; n = 5; Fig. 5B). Cumulative distribution plots showed the selective effect of Aβ1–42 on the nicotine-induced increase in mEPSC frequency, with no effect on either the basal rate or the amplitude of spontaneous mEPSCs, which is consistent with the peptide acting on presynaptic α7-nAChRs (Fig. 5C).
Figure 5.
Aβ1–42 blockade of nicotine-induced increases in spontaneous mEPSC frequency in hippocampal neurons. (A) Whole-cell perforated-patch–clamp recording from a neuron showing that the rate of spontaneous mEPSCs (Top) is increased by application of 1 μM nicotine (Middle) as shown previously (16) and that the nicotine-induced increase can be blocked by 100 nM Aβ1–42 (Aβ; Bottom). Tetrodotoxin and bicucculine were present to block action potentials and GABAA receptors, respectively. (B) Same neuron as in A, showing that Aβ1–42 does not depress the basal rate of spontaneous mEPSCs. (C) Cumulative distribution plots showing that 100 nM Aβ1–42 prevents 1 μM nicotine (Nic) from increasing the frequency of spontaneous mEPSCs but that the peptide has no effect on the basal rate (Left); in contrast, neither nicotine nor Aβ1–42 has any effect on the amplitude distribution of the spontaneous mEPSCs (Right).
Discussion
We have shown that β-amyloid peptides can block the function of α7-nAChRs specifically, reversibly, and with high affinity. The blockade is noncompetitive and is exerted through the N-terminal extracellular portion of the receptor. It is voltage-independent and does not appear to result from Aβ1–42 acting as an open channel blocker, because receptor activation is not required for the inhibition. The fact that α7-nAChRs on cell types as diverse as rat hippocampal neurons and chick ciliary ganglion neurons can be blocked by Aβ1–42 suggests that the property may be a common feature of such receptors. Moreover, the blockade is likely to have pleiotropic effects, because it applies both to somato-dendritic α7-nAChRs thought to mediate synaptic currents (22–25, 27) and to presynaptic α7-nAChRs thought to modulate transmitter release (16, 26). Given the widespread distribution of β-amyloid peptides in Alzheimer's disease (2, 3) and given the proposed roles for α7-nAChRs in learning and memory (31–33), the receptors may represent a significant molecular target of the disease in producing a cognitive deficit.
All of the α7-nAChR populations tested here were functionally blocked by nanomolar concentrations of Aβ1–42, but in no case was the blockade of the whole-cell response greater than 80%. Conceivably native α7-nAChRs are heterogeneous, with some being blocked and others being resistant, but this explanation is difficult to sustain for the partial block of chimeric α7/5HT3 receptors. Most likely, all rapidly desensitizing α7-nAChRs are incompletely blocked by the peptide. None of the receptor populations tested included the few cases of slowly desensitizing α7-nAChRs found on some cell types (52, 53). A recent study of nicotinic responses in hippocampal slices showed that Aβ1–42 can inhibit both α7-nAChR and non-α7-nAChR single-channel events; the extent of blockade predicted for the α7-nAChR portion of the whole-cell current (54) was less than that seen here. A more significant difference is the partial blockade of non-α7-nAChRs reported (54); no blockade of non-α7-nAChRs was seen here. Part of the explanation may be the 10- to 20-fold higher Aβ1–42 concentrations used in the previous study, which may affect non-α7-nAChRs. The other possibility is cell type: pyramidal neurons are the most likely target in hippocampal cell culture, whereas interneurons were being selected in the hippocampal slice work (54); different non-α7-nAChR subtypes may be expressed by the two populations.
Reports that Aβ1–42 competes with αBgt for binding to α7-nAChRs (35, 36) originally motivated the present studies, but no significant competition between Aβ1–42 and αBgt was seen here with intact neurons under conditions where Aβ1–42 was able to block α7-nAChR function. Clearly the competition reported earlier, which was biphasic and displayed components with both picomolar and nanomolar affinities, is different from the Aβ1–42/α7-nAChR interactions seen here. Possibly the competition previously reported depended critically on the aggregation state of the Aβ1–42 (55). No effort was made in the present studies to promote aggregation of the peptide, but we have no information about the physical state of the active species. An alternative explanation for the disparity is that the properties of Aβ1–42 binding to receptors in membrane fragments as used previously (35, 36) may differ from that of receptors on intact neurons.
What is the biomedical relevance of α7-nAChR blockade by β-amyloid peptides? β-Amyloid peptides have been advanced as key determinants of Alzheimer's disease (2, 3, 37). Aβ1–42 levels in cerebrospinal fluid from Alzheimer's patients are low (≤0.2 nM; ref. 56). Concentrations of β-amyloid peptides in brain tissue as a whole from Alzheimer's patients are in vast excess (2–20 μM) over those required for maximal α7-nAChR blockade (57), but such peptides are concentrated in neurofibrillary tangles or plaques, and their exchange with interstitial fluid and proximity to receptors are difficult to estimate. Mice genetically engineered to express β-amyloid peptides show little relationship between plaque load and behavioral deficits characteristic of the human disease (58). Such mice can, however, display synaptic toxicity that correlates with the Aβ1–42 level in the 10–100 nM range (59, 60), concentrations that are effective at blocking α7-nAChRs in the present experiments. In fact, the significance of the α7-nAChR blockade by β-amyloid peptides lies not so much in the possibility that the blockade produces the disease but rather that the blockade contributes importantly to the long-term behavioral consequences of the disease. Thus, the blockade can be expected to exacerbate cholinergic deficits associated with Alzheimer's disease and would put at additional risk the many cellular events the receptors influence.
Most, if not all, approved drug treatments at present for Alzheimer's disease involve compounds designed to augment cholinergic signaling. These include cholinesterase inhibitors to prolong the life of endogenous ACh and receptor agonists to stimulate cholinergic transmission (1). Compounds that enhance ACh levels or activate multiple cholinergic receptor subtypes, however, are broad spectrum and cause serious side effects. Designing compounds that distinguish individual receptor subtypes is a highly desirable therapeutic strategy for redressing some of the degenerative effects associated with Alzheimer's disease. To the extent that α7-nAChRs represent an early molecular casualty of the disease, they should be considered a high-priority target for drug design.
Acknowledgments
We thank Dr. William Green (University of Chicago) for generously providing the α7-nAChR-5HT3 receptor construct, Dr. David Julius (University of California, San Francisco) for the wild-type 5HT3 receptor construct, and Lynn Ogden for expert technical assistance with the binding studies. Grant support was provided by the National Institutes of Health (NS12601 and 35469) and the Tobacco-Related Disease Research Program (9RT-0221). Q.-s.L. and H.K. are American Heart Association Postdoctoral Fellows.
Abbreviations
- Aβ1–42
42-aa β-amyloid peptide
- α7-nAChRs
nicotinic acetylcholine receptors containing the α7 gene product
- αBgt
α-bungarotoxin
- ACh
acetylcholine
- GABA
γ-aminobutyric acid
- mEPSCs
miniature excitatory postsynaptic currents
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Francis P T, Palmer A M, Snape M, Wilcock G K. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe D J. Nature (London) 1999;399,(Suppl.):A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 3.Yankner B A. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 4.Whitehouse P J, Price D L, Struble R G, Clark A W, Coyle J T, DeLong M R. Science. 1982;215:1237–1239. [Google Scholar]
- 5.Wilcock G K, Esiri M M, Bowen D M, Smith C C. J Neurol Sci. 1982;57:407–417. doi: 10.1016/0022-510x(82)90045-4. [DOI] [PubMed] [Google Scholar]
- 6.Sims N R, Bowen D M, Allen S J, Smith C C, Neary D, Thomas D J, Davison A N. J Neurochem. 1983;40:503–509. doi: 10.1111/j.1471-4159.1983.tb11311.x. [DOI] [PubMed] [Google Scholar]
- 7.Hellstrom-Lindahl E, Mousavi M, Zhang X, Ravid R, Nordberg A. Mol Brain Res. 1999;66:94–103. doi: 10.1016/s0169-328x(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 8.Rinne J O, Myllykyla T, Lonnberg P, Marjamaki P. Brain Res. 1999;547:167–170. doi: 10.1016/0006-8993(91)90588-m. [DOI] [PubMed] [Google Scholar]
- 9.Burghaus L, Schutz U, Krempel U, de Vos R A I, Jansen Steur E N H, Wevers A, Lindstrom J, Schroder H. Mol Brain Res. 2000;76:385–388. doi: 10.1016/s0169-328x(00)00031-0. [DOI] [PubMed] [Google Scholar]
- 10.Court J A, Piggott M A, Lloyd S, Cookson N, Ballard C G, McKeith I G, Perry R H, Perry E K. Neuroscience. 2000;98:79–87. doi: 10.1016/s0306-4522(00)00071-3. [DOI] [PubMed] [Google Scholar]
- 11.Sargent P B. Annu Rev Neurosci. 1993;16:403–433. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 12.McGehee D S, Role L W. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand D, Galzi J L, Devillers-Thiery A, Bertrand S, Changeux J P. Proc Natl Acad Sci USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seguela P, Wadiche J, Dineley-Miller K, Dani J A, Patrick J W. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGehee D, Heath M, Gelber S, Role L W. Science. 1995;269:1692–1697. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 16.Gray R, Rajan A S, Radcliffe K A, Yakehiro M, Dani J A. Nature (London) 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 17.Vijayaraghavan S, Huang B, Blumenthal E M, Berg D K. J Neurosci. 1995;15:3679–3687. doi: 10.1523/JNEUROSCI.15-05-03679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugh P C, Berg D K. J Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu W-M, Liou H-C, Chen Y-H. J Neurosci. 1998;18:9954–9961. doi: 10.1523/JNEUROSCI.18-23-09954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger F, Gage F H, Vijayaraghavan S. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messi M L, Renganathan M, Grigorenko E, Delbono E. FEBS Lett. 1997;411:32–38. doi: 10.1016/s0014-5793(97)00600-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z-w, Coggan J S, Berg D K. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]
- 23.Ullian E M, McIntosh J M, Sargent P B. J Neurosci. 1997;17:7210–7219. doi: 10.1523/JNEUROSCI.17-19-07210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang K, Berg D K. J Neurosci. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frazier C J, Buhler A V, Weiner J L, Dunwiddie T V. J Neurosci. 1998;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkondon M, Pereira E F R, Eisenberg H M, Albuquerque E X. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hefft S, Hulo S, Bertrand D, Muller D. J Physiol (London) 1999;510:709–716. doi: 10.1111/j.1469-7793.1999.769ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 29.Mansvelder H D, McGehee D S. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 30.Paylor R, Nguyen M, Crawley J N, Patrick J, Beaudet A, Orr-Urtreger A. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer E M, Tay E T, Papke R L, Meyers C, Huang G-l, de Fiebre C M. Brain Res. 1997;768:49–56. doi: 10.1016/s0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- 32.Felix R, Levin E D. Neuroscience. 1997;81:1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- 33.Levin E D, Bettegowda C, Blosser J, Gordon J. Behav Pharmacol. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Wevers A, Monteggia L, Nowacki S, Bloch W, Schutz Y, Lindstrom J, Pereira E F R, Eisenberg H, Giacobini E, de Vos R A I, et al. Eur J Neurosci. 1999;11:2552–2565. doi: 10.1046/j.1460-9568.1999.00676.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang H-Y, Lee D H S, D'Andrea M R, Peterson P A, Shank R P, Reitz A B. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 36.Wang H-Y, Lee D H S, Davis C B, Shank R P. J Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 37.Auld D S, Kar S, Quirion R. Trends Neurosci. 1998;21:43–49. doi: 10.1016/s0166-2236(97)01144-2. [DOI] [PubMed] [Google Scholar]
- 38.Kar S, Issa A M, Seto D, Auld D S, Collier B, Quirion R. J Neurochem. 1998;70:2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- 39.Huettner J E, Baughman R W. J Neurosci. 1986;6:3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q-s, Berg D K. J Neurosci. 1999;19:10280–10288. doi: 10.1523/JNEUROSCI.19-23-10280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoop R D, Yamada N, Berg D K. J Neurosci. 2000;20:4021–4029. doi: 10.1523/JNEUROSCI.20-11-04021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisele J-L, Bertrand S, Galzi J-L, Devillers-Thiery A, Changeux J-P, Bertrand D. Nature (London) 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- 43.Maricq A V, Peterson A S, Brake A J, Myers R M, Julius D. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 44.Kassner P D, Berg D K. J Neurobiol. 1997;33:968–982. doi: 10.1002/(sici)1097-4695(199712)33:7<968::aid-neu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Rae J, Cooper K, Gates P, Watsky M. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 46.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Zw, Vijayaraghavan S, Berg D K. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 48.Vincent P, Marty A. Neuron. 1993;11:885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]
- 49.Van der Kloot W. Prog Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- 50.Alkondon M, Albuquerque E X. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- 51.Rangwala F, Drisdel R C, Rakhilin S, Ko E, Atluri P, Harkins A B, Fox A P, Salman S B, Green WN. J Neurosci. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuevas J, Berg D K. J Neurosci. 1998;18:10335–10344. doi: 10.1523/JNEUROSCI.18-24-10335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu C R, Role L W. J Physiol (London) 1998;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettit D L, Shao Z, Yakel J L. J Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. , 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorenzo A, Yankner B A. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jensen M, Schroder J, Blomberg M, Engvall B, Pantel J, Ida N, Basun H, Wahlund L W, Werle E, Jauss M, et al. Ann Neurol. 1999;45:504–511. doi: 10.1002/1531-8249(199904)45:4<504::aid-ana12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 57.Gravina S A, Ho L, Eckman C B, Long K E, Otvos Jr L, Younkin L H, Suzuki N, Younkin S G. J Biol Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- 58.Holcomb L A, Gordon M N, Jantzen P, Hsiao K, Duff K, Morgan D. Behav Genet. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- 59.Hsia A Y, Masliah E, McConlogue L, Yu G-Q, Tatsuno G, Hu K, Kholodenko D, Malenka R C, Nicoll R A, Mucke L. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein E M, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]