Abstract
Careful examination of liver, kidney or heart transplants in human recipients has revealed small numbers of host bone marrow derived stem cells in the graft. If the limited recipient repopulation of a donor graft that is currently observed could be facilitated, it is possible that conversion to a predominantly host phenotype would permit long term graft function without immunosuppression. We proposed to “engineer” repopulation after transplant in a strain combination (DA to Lewis GFP+) which rejects liver grafts strongly, a model which more closely resembles the situation in humans. Treatment on days 0,1,2,3 and 7 after transplantation with low-dose (0.1mg/kg) tacrolimus (T) designed to blunt rejection combined with plerixafor (P) to mobilize host stem cells resulted in greater than 180 day graft survival with extensive albeit spotty conversion of a small (50%) DA graft to the recipient Lewis GFP+ genotype. Subsequent skin grafting revealed donor specific graft prolongation. T plus P treatment resulted in higher levels of Lin-Thy1+CD34+CD133+ stem cells and Foxp3+ regulatory T cells in the blood and liver at day 7. Thus, pharmacological mobilization of host stem cells sustains liver allografts by two mechanisms: repopulation of injured donor cells, and regulation of the immune response.
Keywords: CXCR4, Plerixafor, CD133, Regulatory T cells, Tacrolimus
INTRODUCTION
Despite careful examination of human liver (1–7), kidney (8, 9) and heart (10, 11) transplants very few host cells are present in the grafts. Therefore, we were surprised when we found that Lewis rat livers slowly converted to the host DA genotype after transplantation (12). This was associated with lifelong acceptance despite MHC differences, an early rejection crisis, and the complete absence of immunosuppression. Host bone marrow derived c-Kit+ CXCR4+ CD34+ stem cells were observed in the transplanted liver and host cells contained albumen. The process of host stem cell recruitment was retarded by cyclosporine, and was not present in syngeneic transplants. To reconcile the rat and human findings (2–7), we designed the current studies hoping to “engineer“ repopulation after transplantation in a strain combination (DA to Lewis GFP+) which rejects liver grafts strongly, a model which more closely resembles the situation in humans.
We hypothesized that transplantation of a small liver and treatment with brief low-dose tacrolimus and plerixafor to mobilize host stem cells would result in repopulation and long term acceptance without further drug treatment. We now report that long term liver graft acceptance can be fashioned in an aggressive rejection model of liver transplantation by promoting regeneration responses, permitting low grade rejection, and stimulating stem cell mobilization.
MATERIALS AND METHODS
Rat Strains and Care
Lewis (LEW, RT11) and dark agouti (DA, RT1A) rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and used at 8–12 weeks of age. The green fluorescent protein (GFP) transgenic Lewis rat strain was obtained from the National Institutes of Health (NIH)-funded Rat Resource and Research Center (RRRC), University of Missouri, Columbia, MO. Animals were maintained in the specific pathogen-free facility of the Johns Hopkins Medical Institutions. Animals were cared for according to NIH guidelines and under a protocol approved by the Johns Hopkins University Animal Care Committee. In some transplanted rats, plerixafor (1mg/kg) or tacrolimus (0.1mg/kg) in various combinations were injected subcutaneously at postoperative days 0, 1, 2, 3 and 7. Animals received 0.1mg/kg subcutaneous injection with tacrolimus levels range of 4 to 7ng/ml.
Liver Transplantation
Reduced sized (50%) orthotopic liver transplants from DA to Lewis rats were performed under isoflurane (Abbott Laboratories, North Chicago, IL) inhalation anesthesia according to methods described previously (12).
Skin Grafts
DA or BN rats were used as skin donors and liver transplanted Lewis rats were used as recipients. A graft bed (2.5×3 cm) was prepared on the back of a recipient rat. A 2.5×3cm2 full thickness graft was prepared from the trunk of a donor and attached to the back of the recipient with interrupted sutures of 5-0 silk. The graft was covered with a protective tape, and the first inspection was done 2 days after skin grafting and daily thereafter. Rejection was defined as the day when the skin graft acquired a red-brown color and hard consistency.
Preparation of non-parenchymal cell suspensions from rat liver with Collagenase D treatment
The liver was excised and cut gently into small pieces with a scalpel blade. The pieces were put in a beaker containing 5 mL of HEPES buffer (10 mM HEPES-NaOH pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2). The collagenase D solution was added to make the final Collagenase D concentration 2 mg/mL. The sample of rat liver was incubated for 45 minutes at 37 degrees under slow continuous rotation. Dissociated tissue was removed and the remnants were pressed through a 100 μm mesh placed over a 50 mL tube. The cells were suspended in 30–40 mL of Hank's balanced salt solution (HBSS) and centrifuged at 400×g for 6 minutes. The supernatant was then aspirated completely. The cell pellet was re-suspended in 5 mL HBSS and pressed through a 70 μm mesh placed over a 50 mL tube. The same procedure was repeated 3–4 times with the remaining liver fragments and the cells were released and washed twice after re-suspension in 4 degree HBSS. After repeated washes in HBSS the final pellet was re-suspended in FBS containing 10% dimethyl sulfoxide (DMSO) and was then frozen and kept in −80 degree until analysis.
Preparation of Cell Suspensions from Blood and Spleen
Mononuclear cells were isolated from peripheral blood and spleen by Ficoll-Hypaque (1.077 g/liter) density gradient centrifugation. Isolated cells were frozen in FBS containing 10%DMSO and kept in −80 degree until analysis.
Flow Cytometry
Single-cell suspensions (1 × 106) of blood, spleen and liver were analyzed for expression of lineage negative Thy-1+, CD34+ and CD133+ stem cell markers, as well as expression of CD4+CD25+Foxp3+ and CD8+Foxp3+ regulatory T cells. All antibodies used were from commercial sources: CD133 (abcam), CD34 (R&D System), Thy-1 (PharMingen), CD3 (FITC), CD4 (PE), CD25 (APC, Biolegend), FoxP3 (PerCP, eBioscience), CD8 (Santa Cruz), Cy3 (Rabbit), FITC (goat), anti-rat CD3, biotin anti-rat CD11b/c (eBioscience). Nonspecific antibody binding was blocked with donkey, mouse, and rat serum (Sigma) for 30 minutes. Cells were incubated with antibodies for 1 hour at 4°C, and the positive cells were counted by flow cytometry (fluorescence activated cell sorting [FACS]) using CELLQuest software (Becton-Dickinson).
Immunohistochemistry
Five μm serially cut, frozen sections were fixed with acetone at (−20°C) for 10 minutes and dried for 1 hour at room temperature. The streptavidin-biotin-peroxidase method with the DAKO Kit (Carpinteria, CA) was used to detect CD133 and c-Met antigens. After inactivation of endogenous peroxidase and blocking of nonspecific antibody binding, the specimens were treated with biotinylated antibodies specific for CD133 (1:100, ab19898, abcam) or c-Met (1:100, ab61024, abcom) at 4°C overnight. Subsequently, sections were incubated with streptavidin-biotin-peroxidase complex reagent for 30 minutes at room temperature. Diaminobenzidine tetrahydrochloride was used as the chromogen, and hematoxylin was used for counterstaining.
Immunofluorescence Staining
Frozen sections (5-μm) were fixed with acetone (−20°C) for 10 minutes and dried for 1 hour at room temperature. A Tris-based buffer containing 0.5% casein and 5% normal rat and rabbit serum was used for blocking nonspecific background and for dilution of antibodies. Sections were incubated for 1.5 hours at room temperature with a mixture of a mouse monoclonal antibody specific for Foxp3 (1:100; BioLegend), goat polyclonal antibody specific for CD3 (1:100, Santa Cruz) and rabbit polyclonal antibody specific for c-Met (1:100; Abcam) followed by treatment with Cy3 donkey anti-mouse IgG (1:100; Jackson ImmunoResearch Inc.), Cy3 donkey anti-goat IgG (1:100, Jackson ImmunoResearch Inc.) or Cy3 donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch Inc.) for 1 hour at room temperature. Cell nuclei were stained blue with DAPI. Tissue sections were analyzed by confocal fluorescence microscopy.
Semi-quantitative reverse transcription (RT)-PCR analysis
Liver specimens were kept frozen at −80°C until homogenized for RNA extraction using the TRIzol Reagent (Invitrogen, Carlsbad, CA). First-strand cDNA synthesis was then performed on 5μg of total RNA using the Superscript First-Strand Synthesis system for RT-PCR (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Polymerase chain reactions (PCR) contained 1 μL of deoxynucleoside triphosphate mix (10 mM each dNTP), 1 μl of 10 μM each primer (Table 1), 0.4 μL (5 IU/μL) of Platinum Taq polymerase (Invitrogen, Carlsbad, CA), 1.5 μl of 50 mM MgCl2 and 2 μL total DNA as template in a 50 μL reaction solution. Thermal cycling was started with one cycle at 94°C for 4 minutes. This was followed by 30 cycles at 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 30 seconds, and 72°C for final extension for 3 minutes. PCR products were electrophoresed on 1.5% agarose gels and visualized with ethidium bromide staining. (See primer sets for amplification in Supplemental Materials Table S1).
Western blot analysis
Tissues were homogenized in lysis buffer (30 mmol/L Tris, pH 7.5, 150 mmol/L sodium chloride, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate, 1% Nonidet P-40, 10% glycerol) at 4°C, vortexed and centrifuged at 16,000 rpm at 4°C for 10 minutes. The supernatants were mixed in SDS sample loading buffer (NuPAGE, Invitrogen), 70°C for 10 minutes, and then subjected to SDS-PAGE. After electrophoresis, proteins were transferred onto PVDF membranes and blotted against primary antibody (SDF-1, 1:1000, Santa Cruz) for 1 hour at room temperature. Membranes were washed with TPBS (0.05% [vol/vol] Tween 20 in phosphate-buffered saline [pH 7.4]) and incubated with a 1:4000 dilution of horseradish peroxidase-conjugated secondary antibody for 45 minutes. Protein bands were visualized by an enhanced chemiluminescence reaction (ECL-plus, Bio-Rad).
In Situ Imaging of GFP Expression in Livers
Liver grafts were flushed with cold saline (4°C, 10 mL) and fixed with 4% paraformaldehyde via portal vein perfusion. The fluorescence of GFP in liver grafts was measured by the Xenogen IVIS Imaging system and Living Image software (Xenogen Biosciences).
Statistics
Continuous variables were presented as the mean ± SD. Dichotomous variables were presented as both number and percentage values. Data of flow cytometry were analyzed using the Student's t test (two-tailed), with dichotomous variables analyzed by the Fisher's exact test (two-tailed). All analyses were performed using SPSS® (SPSS; Chicago, IL). P < 0.05 was considered significant.
RESULTS
Brief low-dose tacrolimus and plerixafor treatment results in long term liver transplant survival in an acute rejection rat model
We performed liver transplants from dark agouti (DA) rat donors into green fluorescence protein (GFP) transgenic Lewis rat hosts. We employed three strategies: transplantation of reduced sized (50%) liver grafts to increase the regenerative stimuli, provision of low-dose tacrolimus (0.1mg/kg) to lessen the severity of acute rejection and thirdly, concomitant pharmacologic mobilization of recipient stem cells. Transplanted rats were divided into four groups: 1) a control group treated with saline, 2) animals treatment with low dose tacrolimus (0.1mg/kg) alone, 3) animals treated with stem cell mobilizer (plerixafor, 1mg/kg) alone, 4) animals treated with the combination of low dose tacrolimus and plerixafor (T and P). Plerixafor, tacrolimus or both were injected subcutaneously immediately after reperfusion and on days 1, 2, 3, and 7 following liver transplantation.
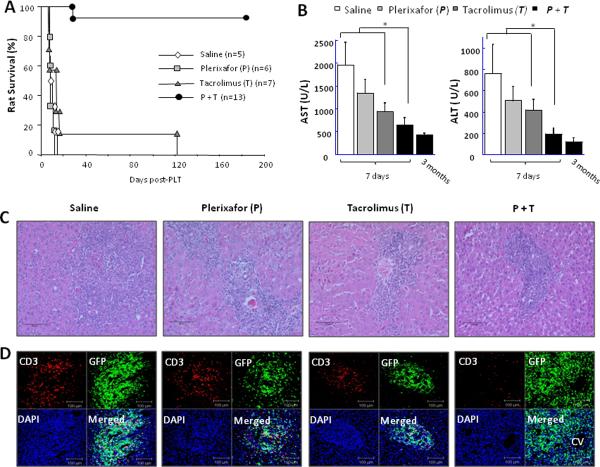
Combined tacrolimus and plerixafor treatment (group 4) resulted in long term graft function in 12 of 13 rats while all the rest underwent rejection within twelve days after transplantation, with the exception of one rat which survived 120 days on only low dose tacrolimus (Fig. 1A). Liver enzyme elevations were partially mitigated in dual treated rats at 7 days after transplantation (Fig. 1B) as was portal inflammation. H&E staining of saline treated hosts of liver allografts (group 1) showed heavy mononuclear cell infiltration in expanded portal areas with disruption of liver architecture (Fig. 1C). This pattern of cellular infiltration was similar in groups 2 and 3, but the number of infiltrating cells was reduced. In contrast, there were far fewer cells in the portal areas of animals in group 4. Anti-CD3 staining revealed far fewer cells in group 4 than found in the control groups 1, 2 and 3. Further, in group 4 confocal microscopy showed that most of the GFP fluorescent cells were CD3 negative (Fig. 1D).
Figure 1. Plerixafor treatment with brief low-dose tacrolimus results in long term liver transplant survival.
Transplanted rats were divided into four groups including a control group treated with saline, treatment with low dose tacrolimus (0.1mg/kg/dose) alone, plerixafor (1mg/kg/dose) monotherapy, or a combination of low dose tacrolimus and plerixafor. (A) Animal survival. (B) Blood levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Data represent mean ± SE of n=3 or 4 animals per group. *P<0.05. (C) H&E staining of liver allografts at 7 days after transplantation. Images were photographed with a 40× objective. (D) Double fluorescence staining for CD3 (red) and host-derived GFP-positive cells (green) in the parenchyma of liver allografts at 7 days after transplantation. Cell nuclei were stained blue with DAPI. Representative photographs of n = 3 or 4 individual samples per group.
Host repopulation of liver allograft in the animals displaying long term acceptance
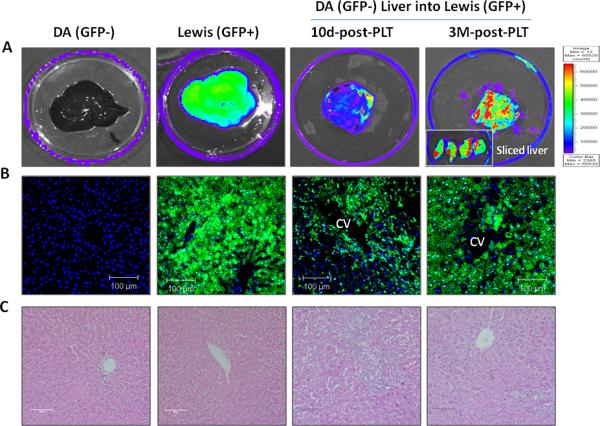
To determine the extent of liver allograft replacement by host-derived cells, GFP whole-organ fluorescence was measured using the Xenogen system. Donor DA livers showed no GFP expression, as expected. However, the transplanted donor GFP-negative liver graft showed moderate green fluorescence at 10 days after transplantation in group 4 which became extensive three months after transplantation (Fig. 2A). However, there were still patches of GFP negative areas (Fig. 2B). Traditional H&E staining of liver allografts at this time point (Fig. 2C) showed bile duct regeneration and many small size hepatocytes in central vein areas indicating that repopulation and/or remodeling was ongoing. Liver chemistries also indicated that the liver was still undergoing cellular turnover as the liver enzymes, although less elevated, remained abnormal at 3 months after transplantation (Fig. 1B).
Figure 2. Host repopulation of liver allograft in the animals displaying long term acceptance.
(A) The Xenogen imaging system was used to study DA livers into GFP Lewis hosts with dual drug treatment after partial (50%) liver transplantation. The non-transgenic donor DA liver has no GFP expression. However, the transplanted donor liver graft shows GFP positive at 10 days and a high degree of fluorescence at three months after transplant into a GFP+ Lewis recipient treated with plerixafor and tacrolimus. (B) Liver tissue sections analyzed by fluorescent microscopy. GFP positive cells were present in central vein areas on day 10 after transplantation. Most of the hepatocytes and non-parenchyma cells are GFP positive indicating virtually complete host repopulation_at 3 months after transplantation. Representative photographs of n = 3 individual transplant samples. Images were photographed with a 40× objective. (C) H&E staining showed bile duct regeneration (10 days) and small size hepatocytes in the central vein areas (3 months) suggesting that repopulation and/or remodeling is ongoing. Representative photographs of n = 3 individual transplant samples. Images were photographed with a 20× objective.
Prolongation of donor skin graft survival in transplanted rats treated with dual drug therapy
To test if the combination of plerixafor and low-dose Tacrolimus treatment (group 4) induced donor specific immune suppression/tolerance, donor strain and third party skin allografts were performed at one month, which was three weeks after cessation of drug therapy. In these animals, donor strain skin allografts survived for more than one month after which there was slow progressive loss of skin. Third party grafts were rejected aggressively with normal kinetics (7 days) (Supplemental Supporting Figure S1) indicating that combination treatment induced self perpetuating antigen specific immunosuppression but not immune tolerance.
Tacrolimus reacted synergistically with plerixafor in the recruitment of lineage negative (Lin-) Thy-1+, CD34+, CD133+ stem cells
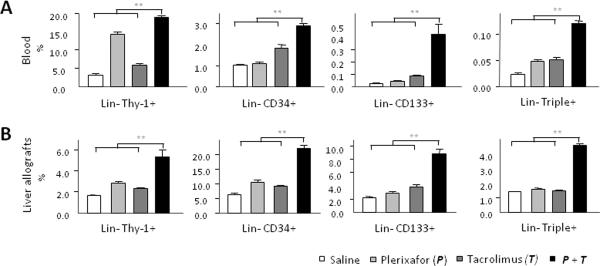
We performed flow cytometry to determine the stem cell constituents mobilized by treatment with plerixafor and tacrolimus. The percentage of lineage negative (Lin-) Thy-1+, CD34+, CD133+ cells as well as Lin-Triple positive (Thy-1+CD34+CD133+) cells were significantly greater in both peripheral blood (Fig. 3A) and cell suspensions made from the liver allografts (Fig. 3B) in the animals receiving dual drug treatment at 7 days after transplantation
Figure 3. Quantitative analysis of stem cells in peripheral blood and allografts at 7 days after liver transplantation.
Quantitative analysis of Lineage negative (Lin-) Thy-1+, CD34+ and CD133+ cells in blood and liver allografts by flow cytometry at 7 days after transplantation. The percentage of Lin-Thy-1+, CD34+, CD133+ cells as well as Lin-Triple positive (Thy-1+CD34+CD133+) cells was significantly greater in both peripheral blood (A) and cell suspensions made from the liver allografts (B) in the animals receiving dual drug treatment. Quantitative data are represented as group means (bars) (n=3). **P<0.01.
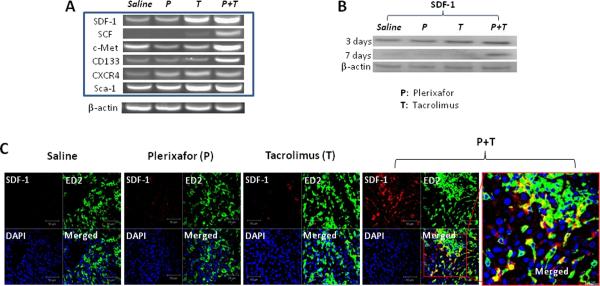
Semi-quantitative PCR analysis of the liver transplant showed that mRNA expression of the attractor molecules stromal cell-derived factor (SDF)-1 and stem cell factor (SCF), and important cellular markers, c-Met and CD133 were all significantly increased in the group 4 rats compared to groups 1, 2, 3 at day 7 after transplantation (Fig. 4A).
Figure 4. Increased SDF-1 expressions in liver allografts recovered from rats treated with dual drug therapy.
(A) Semi-quantitative RT-PCR analysis of liver allografts at 7 days after transplantation. The mRNA expression of the attractor molecules SDF-1 and SCF, and important cellular markers, c-Met and CD133 were all significantly increased in the animals receiving dual drug treatment._Representative graphs of three individual samples. (B) Liver lysates were western blotted for SDF-1 on day 3 or day 7 after transplantation, and β-actin used as internal control. SDF-1 protein concentrations in liver homogenates were also significantly higher in animals in the dual treatment group compared to other groups, especially at 7 days after transplantation. Representative graphs of three individual samples. (C) Double fluorescence staining for SDF-1 and ED2 (a marker for Kupffer cells) at 7 days after transplantation. Sections stained with both anti-SDF-1 and anti-ED2 antibodies show some ED2 positive cells (green) stained for SDF-1 (red) in rats treated with dual drug therapy. Representative photographs of n = 3 individual transplant samples per group. Images were photographed with a 40× objective.
SDF-1 protein concentrations in liver homogenates (Fig. 4B) were also significantly higher in animals in the dual treatment group compared to other groups, especially at 7 days after transplantation. Immunofluorescence staining demonstrated that SDF-1 co-stained with some of the ED2+ cells indicating that these Kupffer cells were producing SDF-1(Fig. 4C).
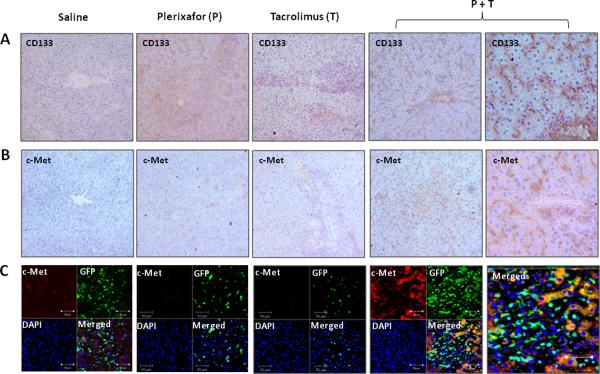
Immunohistochemistry also showed that the number of CD133+ was increased by dual treatment as were c-Met+ cells (Fig. 5A and B). Immunofluorescence staining demonstrated that the c-Met+ cells were host derived as shown in figure 5C.
Figure 5. Recruitment of host bone marrow stem cells to rat liver transplants following treatment with dual drug therapy.
Immunohistochemistry staining for CD133 (A) and c-Met (B) in tissue sections from liver allografts at 7 days after transplantation. CD133 or c-Met positive cells are brown. Representative photographs of n = 3 individual transplant samples per group. Images were photographed with a 20× or 40× (right panels) objective. (C) Double fluorescence staining for c-Met and GFP at 7 days after transplantation. Sections stained with both anti-c-Met and anti-GFP antibodies show all c-Met positive cells (red) stained for recipient GFP (green) in rats treated with dual drug therapy. Representative photographs of n = 3 individual transplant samples per group. Images were photographed with a 40× objective.
Increased Foxp3+ regulatory T cells in transplanted rats treated with dual drug therapy
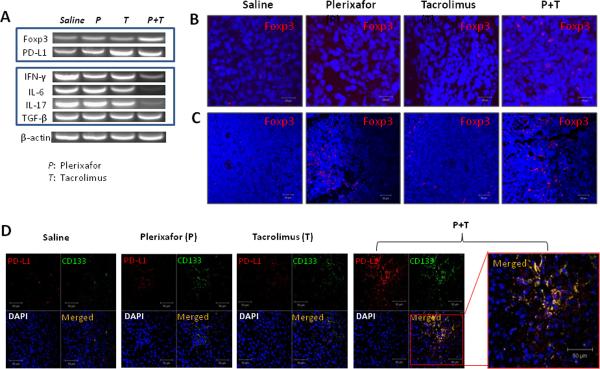
To learn whether the prolongation of liver allograft and donor skin graft survival observed in dual drug treated rats was associated with development of regulatory T cells (Treg), we performed semi-quantitative RT-PCR analysis of the liver transplant. The mRNA expression of programmed cell death ligand-1 (PD-L1) and Treg marker Foxp3 was significantly increased in the group 4 compared to all other groups at day 7 after transplantation. Interestingly, IFN-γ (a cytokine of Th1), IL-17 (a cytokine of Th17) and IL-6 (a promoter of Th17 development) were all significantly decreased in the dual treatment group, although TGF-β remained unchanged (Fig. 6A). The involvement of Foxp3 in suppressing donor specific immunity was confirmed by immunofluorescent staining. The number of Foxp3 positive cells was significantly higher in tissue sections from liver allografts (Fig. 6B) and spleens (Fig. 6C) in group 4 seven days after transplantation. Interestingly, the number of Foxp3 positive cells was also increased in tissue sections from spleens in the plerixafor treatment group (group 2) seven days after transplantation.
Figure 6. Increased Foxp3 and PD-L1 expressions in transplanted rats treated with dual drug therapy.
(A) Semi-quantitative PCR analysis of liver allografts at 7 days after transplantation. Representative graphs of three individual samples. The mRNA expression of programmed cell death ligand-1 (PD-L1) and Treg marker Foxp3 was significantly increased in the animals receiving dual drug treatment. Interestingly, IFN-γ, IL-17 and IL-6 were all significantly decreased in the dual treatment group, although TGF-β remained unchanged. (B) Detection of Foxp3+ cells in liver allografts and spleens by immunofluorescence staining. Cell nuclei were stained blue with DAPI. The number of Foxp3 positive cells (red) was significantly higher in tissue sections from liver allografts and spleens in the dual treatment group at 7 days after transplantation. (C) Double fluorescence staining for PD-L1 and CD133 at 7 days after transplantation. Sections stained with both anti-PD-L1 and CD133 antibodies show all PD-L1 positive cells (red) stained for CD133 (green) in rats treated with dual drug therapy. Representative photographs of n=3 individual transplant samples per group. Images were photographed with a 40× objective.
The elevation in Foxp3 cells was supported by finding increased numbers of PD-L1 cells which co-stained for CD133. Programmed cell death ligand-1 (PD-L1) has a pivotal role in regulating induced Treg (iTreg) cell development and in sustaining iTreg cell function (13). This pathway is reported to be involved in MSC-mediated immunomodulation and requires cell-to-cell contact (14). The number of PD-L1 positive cells was significantly higher in tissue sections from liver allografts in the dual treatment group at 7 days after transplantation. Interestingly, these PD-L1 positive cells co-stained with CD133 (Fig. 6D).
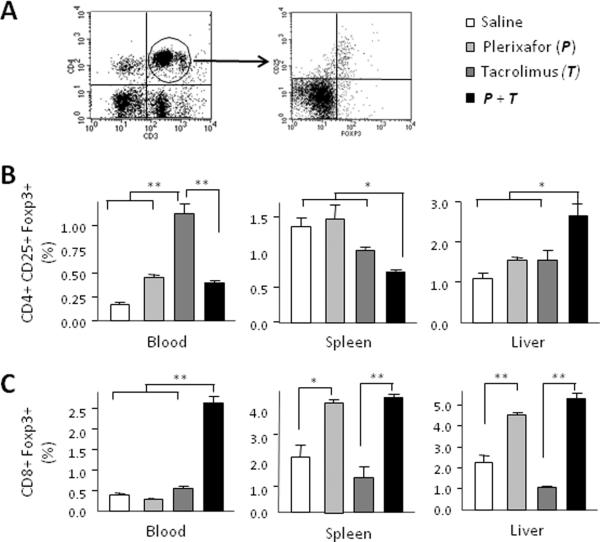
Foxp3+ cells isolated from the blood, spleen and liver grafts were further characterized by flow cytometry and there were complex compartmental relationships. Dual treatment resulted in greater numbers of CD4+CD25+Foxp3+ regulatory T cells in the liver than single drug or no treatment at 7 days after transplantation (Fig. 7B). It is interesting that low dose tacrolimus treatment alone increased levels of CD4+CD25+Foxp3+ regulatory T cells in peripheral blood. Also of interest, plerixafor treatment alone significantly increased the percentage of CD8+Foxp3+ T cells in spleens and liver allografts at 7 days. However the highest concentration of CD8+Foxp3+ T cells in peripheral blood, spleen, and in liver allografts was found in animals receiving dual treatment (Fig. 7C). The increase of CD8+Foxp3+ T cells, but not CD4+CD25+Foxp3+ regulatory T cells in the spleen in animals destined for survival suggests that this change in the Treg phenotype may be an important mechanism suppressing the effecter limb of donor specific immunity.
Figure 7. Increased Foxp3+ regulatory T cells in transplanted rats treated with dual drug therapy.
Further characterization of Foxp3+ cells was performed by flow cytometry of cells isolated from blood, spleen, and liver graft seven days after transplantation. (A) Gated CD3+ and CD4+ or CD8+ double positive cells were further analyzed for CD25 and Foxp3 expression. (B) CD4+CD25+Foxp3+ regulatory T cells. Dual treatment resulted in greater numbers of CD4+CD25+Foxp3+ regulatory T cells in the liver than single drug or no treatment. Interestingly, low dose tacrolimus treatment alone increased levels of CD4+CD25+Foxp3+ regulatory T cells in peripheral blood. (C) CD8+Foxp3+ regulatory T cells. Plerixafor treatment alone significantly increased the percentage of CD8+Foxp3+ T cells in spleens and liver allografts at 7 days. However the highest concentration of CD8+Foxp3+ T cells in peripheral blood, spleen, and in liver allografts was found in animals receiving dual treatment. Quantitative data are represented as group means (bars) (n=3). *P < 0.05 and **P < 0.01.
The failure of G-CFS and transferred CD-133 cells to salvage liver transplants
Substituting G-CSF (Neupogen, 300μg/kg) for plerixafor resulted in acute rejection (Supplemental Supporting Figure S2). In normal rats G-CSF mobilized stem cells lacked the CD133 phenotype (Supplemental Supporting Figure S3). However infusions of syngeneic CD133 cells (106) on days 0,1,2,3 and 7 along with low dose tacrolimus produced a moderate but unsustained effect in 2 of 4 rats.
DISCUSSION
Conventional immunosuppression (IS) is designed to keep the recipient in a “state of ignorance” of the graft. This approach is responsible for the success seen in clinical transplantation but has significant limitations. When IS coverage is inadequate, rejection episodes occur which are treated with increased levels of IS which leads to an increased risk of developing certain cancers and opportunistic infection. Lifelong treatment is needed to avoid chronic rejection. A second paradigm of graft-recipient interaction results when the recipient takes on some donor properties and becomes chimeric (15, 16). This scenario may be unintentional, i.e. when passenger leukocytes are observed to migrate from the donor graft to recipient, or may be facilitated therapeutically by donor hematopoietic stem cell (HSC) transfer in order to generate a state of donor specific unresponsiveness or “transplant tolerance” (17–20). A third possible donor-recipient interaction is suggested by our results here and occurs when a recipient repopulates a transplanted donor graft, the conceptual reverse of mixed chimerism. This phenomenon was first reported in an aortic allograft model over forty years ago (21, 22) and has been observed to a limited extent in liver (1–7), kidney (8, 9) and heart (10, 11) transplants. In the case of the liver we propose that current IS interferes with the repopulation process as it obliterates the rejection response which both limits the immunological injury and allows donor derived resident cells to repair such injury obviating the need for major host stem cell repair.
Compared to other organ allografts, liver allografts have an immunological advantage, and several mechanisms have been proposed including antigen overload, and overall plasticity of the liver tissues permitting host repopulation of portions of the graft setting up chimeric conditions The possibility that the whole liver is able to regenerate from extra-hepatic sources was raised in Lewis to DA rat strain transplants (12) which are accepted without immunosuppression. This finding was associated with recruitment and differentiation of host progenitor cells which predominated at three months when a reduced-size (50%) liver was transplanted (12). To extend this finding closer to clinical relevance we performed transplantation in a rat strain combination (DA to Lewis GFP+) which rejects liver grafts strongly. We employed three strategies to promote long term graft survival: 1. transplantation of half sized livers to stimulate regeneration; 2. provision of low dose tacrolimus during the first week to prevent outright acute rejection; and 3. provision of agents which free bone marrow stem cells. The results enable us to report that plerixafor mobilized the appropriate bone marrow derived stem cells; that the addition of low dose tacrolimus prevented outright rejection; and that the combination provided for just the first week after transplantation resulted in long term liver transplant survival without further drug treatment.
Plerixafor (Mozobil) acts as an antagonist of the alpha chemokine receptor CXCR4. This receptor and one of its ligands, SDF-1, are one mechanism involved in attraction of hematopoietic stem cells. Usually, SDF-1 draws stem cells into the bone marrow where they are quiescent. Plerixafor disrupts this relationship, and it has been used clinically to drive HSCs out of the bone marrow into the peripheral blood of humans where they can be harvested and preserved until the completion of ablative chemotherapy. We found that plerixafor had equivalent mobilization effects in rats. Plerixafor treatment alone did not prolong animal survival after liver transplantation presumably because mobilization and differentiation could not occur fast enough to sustain liver viability in the face of severe immunological attack. However, tacrolimus immunosuppression at one tenth the clinical dosage proved effective when combined with plerixafor as twelve out of thirteen recipient animals survived for 6 or more months.
The question invariably arises about which type of bone marrow cell differentiates into liver tissue. To this end we substituted G-CSF for plerixafor. While the number of CD34+ cells was equivalent to those released by plerixafor, the number of Lin- CD34+CD133+Thy-1+ cells was significantly lower. Since cells with these phenotypes, particularly CD133, were increased in the blood and liver at 7 days in rats treated with plerixafor and tacrolimus and destined to survive, we propose that they are the stem cells which are crucial for differentiation into liver tissues. However, when we sought confirmation by passive transfer of these cells, we found weak effects in two and no effect in two of four rats studied. This outcome is best explained by the bone marrow competing with the liver for the transferred cells. By contrast, plerixafor “pushes” the cells from the bone marrow into the blood, eliminating competition and increasing the quantity of stem cells for tissue repairs. Additionally, the attractor molecule SDF-1 was significantly increased in the liver allografts from surviving animals. The SDF-1 signal was localized to Kuppfer cells. Thus dual treatment resulted in “pushing” CXCR-4 cells from the bone marrow and “pulling” them into the liver. Within this group of CXCR-4 cells are CD34, CD133, and Thy-l positive cells which become the key responders to the injured (rejecting) liver.
The extent of liver allograft repopulation by host-derived cells was dramatized by measuring GFP whole-organ fluorescence and fluorescent microscopy. There were significant numbers of host GFP+ cells at 10 days while at three months GFP+ cells clearly predominated. Immunofluorescent studies demonstrated that the majority of the GFP positive cells lacked the CD3 “inflammatory” phenotype. Sustaining the SDF-1 signal is likely to be important because the liver had persistent patches of donor cells at three months, and small host cells continued to be abundant in the central vein areas. SDF-1 signaling was supported acutely by tacrolimus perhaps by limiting outright rejection. Once in the liver the host stem cells must differentiate and the mechanisms, while very important, are not addressed and remain speculative. Clues exist from in vitro studies which have shown that bone marrow stem cells can be converted by a series of cultures in the presence of growth factors including HGF to cells containing albumen (23–25).
In the complex interaction of the early immune response, stem cell attraction and differentiation in the host rats became less aggressive to donor tissues. Donor strain skin grafts survived for greater than 30 days while third party grafts were rejected at 7 days. Therefore dual treatment with tacrolimus-plerixafor did more than allow host replacement of donor cells, as it created down regulation of the specific immune response as well. In this group of rats we found increased numbers of Foxp3+ cells in the liver and spleen at seven days. Kared et al have demonstrated that murine HSCs stimulate peripheral Foxp3+ Treg expansion through both cell-cell contact activation of Notch signaling and through soluble factors, such as GM-CSF, which is produced at high levels by hematopoietic progenitors (26). The CD8+ Foxp3+ phenotype was increased in the liver and spleen while the CD4+Foxp3+ phenotype was increased only in the liver. The programmed cell death ligand PD-L1 was also elevated in the liver and spleen while the aggressive cytokines were diminished. Thus this combination therapy resulted in several immunosuppressive pathways.
We intentionally sought a dose of tacrolimus (0.1mg/kg) which allowed rejection putting donor regenerating mechanisms at severe disadvantage and creating an advantage for host stem cells. We found that at 0.1 mg/kg tacrolimus alone permitted increased levels of CD4+CD25+Foxp3+ T cells (Figure 7B) in the blood but this effect was insufficient to sustain the graft. After being activated, conventional human CD4 (+) T cells transiently up-regulate Foxp3 without acquiring a regulatory function (27). We speculate that stem cells mobilized in greater numbers by pleraxifor promote the development and distribution of regulatory T cells which are responsible for suppression of the allograft response. However, the regulatory phenotype of the abundant Foxp3+ T cells produced by combined treatment needs further investigation before we assign them the role of mediating the donor specific immune-modulation we observed.
The immunological status of the host subjected to the liver transplant and combined treatment is intriguing. True liver tolerance may exist, and as the finding of donor strain skin being slowly rejected may be the result of recognition and response to skin specific antigens not present in the liver (28). Alternatively, the progressive loss of donor liver tissue argues that low grade rejection is present and is reasonable for the phenomenon of host repopulation present in the liver. We suspect that the application of this treatment strategy to other organ transplants will be successful or not depending on the immunological status produced and less on host replacement
Implementation of these findings poses difficulties. First, the repopulation observed may be a phenomenon limited to rodents. Second, two findings appear to be counter-intuitive: small livers work better than whole ones; and low dose short term immunosuppression rather than high dose long term immunosuppression is essential. Indeed the findings we report are provocative as this is the first report documenting the success of an entirely new concept in managing liver transplantation. The approach must be tested in large animals, and the long term studies will be expensive, but it is worthwhile, as dual therapy has the great advantage of short term exposure to one immunosuppressive drug in low doses, and durable graft function. It is also possible that greater use will be made of fatty and reduced sized livers from deceased donors.
Supplementary Material
Acknowledgement
This work was supported by US National Institutes of Health (R21AI065488 and UO1 AA018113 to Z.S.) and departmental start-up fund (Z.S.) and a grant from Genzyme Inc. (Z.S.).
Footnotes
Contributions: Z.S. and G.M.W. designed all experiments; T.O. carried out all experiments except liver transplantation (Z.S.) and immunohistochemistry (M.H.); T.O. and Z.S. conducted statistical analysis of the data; Z.S., G.M.W., A.M.C. and R.A.M. supervised the project; Z.S. wrote the manuscript and Z.S., G.M.W and A.M.C. edited the manuscript. Z.S. and G.M.W. have filed patent related to this work (PCT/US10/59877).
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Gao Z, McAlister VC, Williams GM. Repopulation of liver endothelium by bone-marrow-derived cells. Lancet. 2001;357:932–933. doi: 10.1016/s0140-6736(00)04217-3. [DOI] [PubMed] [Google Scholar]
- 2.Kleeberger W, Rothämel T, Glöckner S, Flemming P, Lehmann U, Kreipe H. High frequency of epithelial chimerism in liver transplants demonstrated by microdissection and STR-analysis. Hepatology. 2002;35:110–116. doi: 10.1053/jhep.2002.30275. [DOI] [PubMed] [Google Scholar]
- 3.Fogt F, Beyser KH, Poremba C, Zimmerman RL, Khettry U, Ruschoff J. Recipient-derived hepatocytes in liver transplants: a rare event in sex-mismatched transplants. Hepatology. 2002;36:173–176. doi: 10.1053/jhep.2002.33994. [DOI] [PubMed] [Google Scholar]
- 4.Hove WR, van Hoek B, Bajema IM, Ringers J, van Krieken JH, Lagaaij EL. Extensive chimerism in liver transplants: vascular endothelium, bile duct epithelium, and hepatocytes. Liver Transpl. 2003;9:552–556. doi: 10.1053/jlts.2003.50116. [DOI] [PubMed] [Google Scholar]
- 5.Ng IO, Chan KL, Shek WH, Lee JM, Fong DY, Lo CM, Fan ST. High frequency of chimerism in transplanted livers. Hepatology. 2003;38:989–998. doi: 10.1053/jhep.2003.50395. [DOI] [PubMed] [Google Scholar]
- 6.Idilman R, Erden E, Kuzu I, Ersoz S, Karasu Z, Karayalcin K, et al. Recipient-derived hepatocytes in sex-mismatched liver allografts after Liver transplantation: early versus late transplant biopsies. Transplantation. 2004;78:1647–1652. doi: 10.1097/01.tp.0000144055.78462.4f. [DOI] [PubMed] [Google Scholar]
- 7.Idilman R, Erden E, Kuzu I, Ersoz S, Karayalcin S. The fate of recipient-derived hepatocytes in sex-mismatched liver allograft following liver transplantation. Clin Transplant. 2007;21:202–206. doi: 10.1111/j.1399-0012.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 8.Lagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JH. Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet. 2001;357:33–37. doi: 10.1016/S0140-6736(00)03569-8. [DOI] [PubMed] [Google Scholar]
- 9.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 10.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 11.Bayes-Genis A, Salido M, Solé Ristol F, Puig M, Brossa V, Campreciós M, et al. Host cell-derived cardiomyocytes in sex-mismatch cardiac allografts. Cardiovasc Res. 2002;56:404–410. doi: 10.1016/s0008-6363(02)00597-7. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Zhang X, Locke JE, Zheng Q, Tachibana S, Diehl AM, Williams GM. Recruitment of host progenitor cells in rat liver transplants. Hepatology. 2009;49:587–597. doi: 10.1002/hep.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Demetris AJ, Trucco M, Ricordi C, Ildstad S, Terasaki PI, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type 1 gaucher's disease. N Engl J Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosimi AB, Sachs DH. Mixed chimerism and transplantation tolerance. Transplantation. 2004;77:943–946. doi: 10.1097/01.tp.0000117779.23431.3f. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-Mismatched Renal Transplantation without Maintenance Immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA. Tolerance and Chimerism after Renal and Hematopoietic-Cell Transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 20.Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, et al. Chimerism and Tolerance in a Recipient of a Deceased-Donor Liver Transplant. N Engl J Med. 2008;358:369–74. doi: 10.1056/NEJMoa0707255. [DOI] [PubMed] [Google Scholar]
- 21.Williams GM, Alvarez CA. Host repopulation of the endothelium in allografts of kidneys and aorta. Surg Forum. 1969;20:293–294. [PubMed] [Google Scholar]
- 22.Williams GM, Krajewski CA, Dagher FJ, ter Haar AM, Roth JA, Santos GW. Host repopulation of the endothelium. Transplant Proc. 1971;3:869–872. [PubMed] [Google Scholar]
- 23.Nishino T, Hisha H, Nishino N, Adachi M, Ikehara S. Hepatocyte growth factor as a hematopoietic regulator. Blood. 1995;85:3093–100. [PubMed] [Google Scholar]
- 24.Miyazaki M, Masaka T, Akiyama I, Nakashima E, Sakaguchi M, Huh NH. Propagation of adult rat bone marrow-derived hepatocyte-like cells by serial passages in vitro. Cell Transplant. 2004;13:385–91. doi: 10.3727/000000004783983800. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. Transdifferentiation of mouse BM cells into hepatocyte-like cells. Cytotherapy. 2006;8:381–9. doi: 10.1080/14653240600735800. [DOI] [PubMed] [Google Scholar]
- 26.Kared H, Adle-Biassette H, Foïs E, Masson A, Bach JF, Chatenoud L, et al. Jagged2-expressing hematopoietic progenitors promote regulatory T cell expansion in the periphery through notch signaling. Immunity. 2006;25:823–34. doi: 10.1016/j.immuni.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 28.Steinmuller D, Lofgreen JS. Differential survival of skin and heart allografts in radiation chimaeras provides further evidence for Sk histocompatibility antigen. Nature. 1974;248:796–7. doi: 10.1038/248796a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.