Abstract
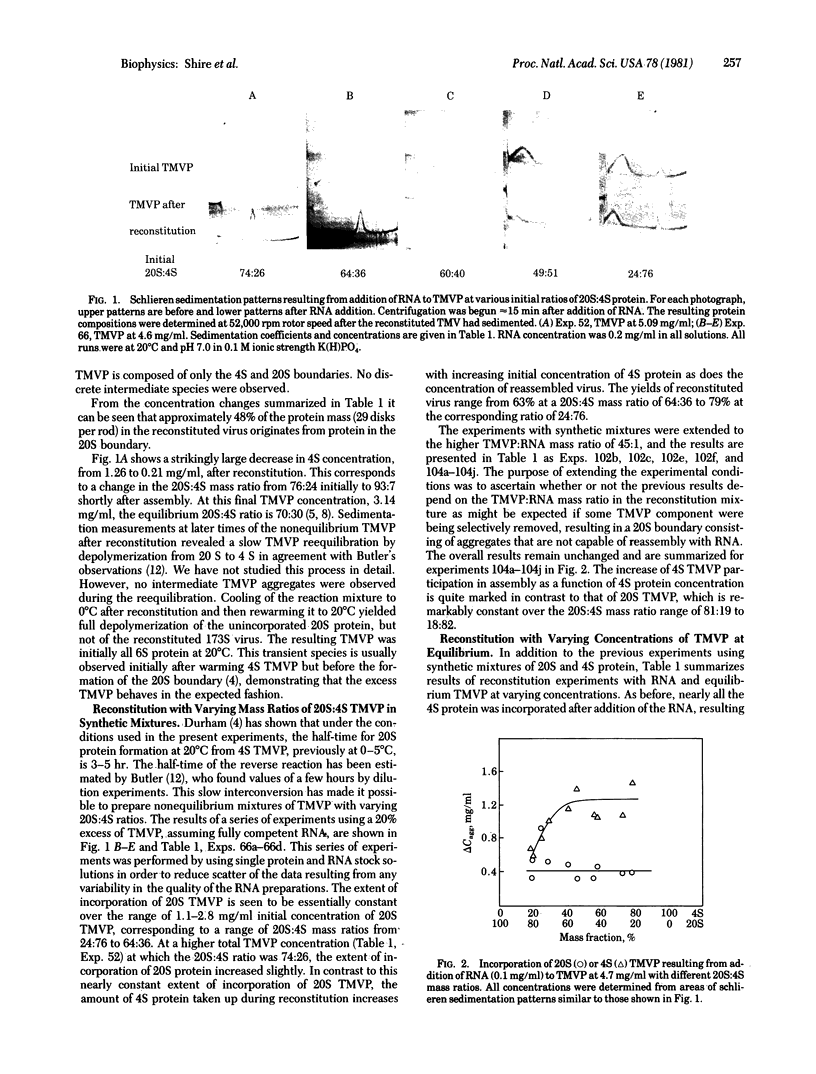
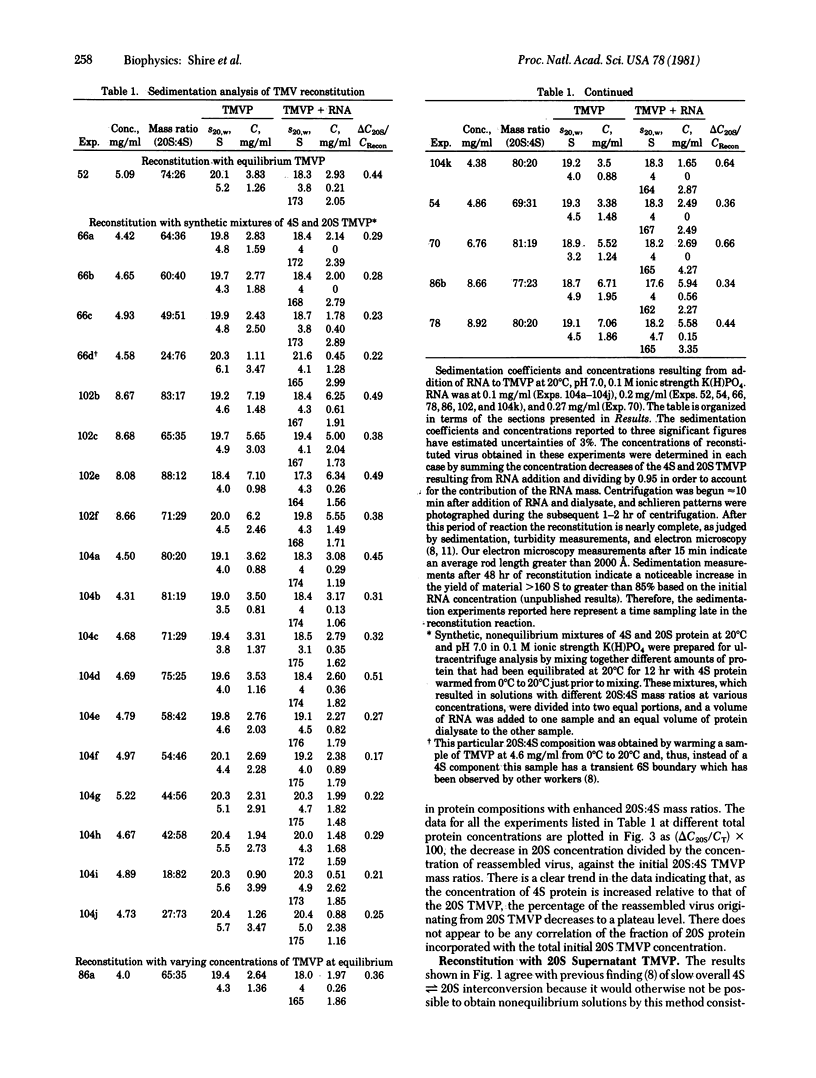
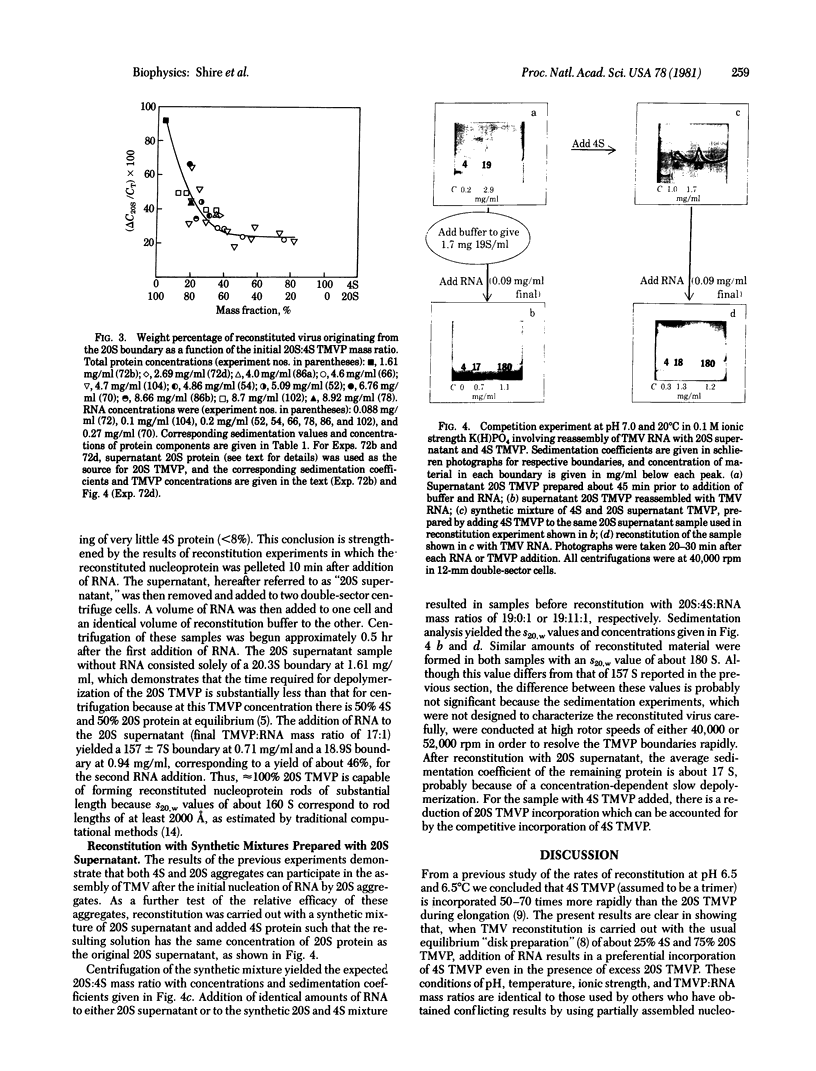
The mechanism of assembly of tobacco mosaic virus (TMV) has been investigated at pH 7.0 and 20°C by analytical ultracentrifugation. Under these conditions the overall rates of interconversion of 4S and 20S TMV coat protein are sufficiently slow to make possible measurements of the concentrations of remaining 4S and 20S TMV coat protein after addition of homologous RNA to solutions containing, initially, various mass ratios of 20S protein to 4S protein. It has been possible to measure, by schlieren boundary analysis, the relative rates of incorporation of 4S and 20S TMV protein into the growing nucleoprotein rod over the range of initial 20S:4S protein mass ratios from 93:7 to 18:82. The results show that the amount of incorporation of 20S TMV protein depends on the initial 20S:4S mass ratio between ≈100% and 60% 20S protein but that reconstitution can proceed with ≈100% 20S TMV protein to form full virus-size rods. However, when the initial protein solutions have less than 60% 20S protein, ≈80% of the reconstituted nucleoprotein is preferentially formed from 4S coat protein. The remaining ≈20% appears to require preformed 20S coat protein. These results suggest that a larger region of RNA than previously estimated is involved in the rate-limiting nucleation step in assembly and may explain previously conflicting results concerning the elongation phase of assembly when starting with partially assembled rods.
Keywords: protein-RNA interactions, initiation and elongation of reconstitution, metastable protein aggregates, analytical ultracentrifugation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler P. J. Assembly of tobacco mosaic virus. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):151–163. doi: 10.1098/rstb.1976.0106. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Lomonossoff G. P. RNA-protein interactions in the assembly of tobacco mosaic virus. Biophys J. 1980 Oct;32(1):295–312. doi: 10.1016/S0006-3495(80)84958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L. ASSEMBLY AND STABILITY OF THE TOBACCO MOSAIC VIRUS PARTICLE. Adv Protein Chem. 1963;18:37–121. doi: 10.1016/s0065-3233(08)60268-5. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Ohno T., Okada Y., Otsuki Y., Takebe I. Kinetics of biphasic reconstitution of tobacco mosaic virus in vitro. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1727–1730. doi: 10.1073/pnas.75.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonathan P., Butler G., Durham A. C. Tobacco mosaic virus protein aggregation and the virus assembly. Adv Protein Chem. 1977;31:187–251. doi: 10.1016/s0065-3233(08)60219-3. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Nicolaieff A., Richards K. E. Inside-out model for self-assembly of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):149–153. doi: 10.1073/pnas.74.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J. Location and encapsidation of the coat protein cistron of tobacco mosaic virus. A bidirectional elongation of the nucleoprotein rod. Eur J Biochem. 1979 Jan 2;93(1):157–164. doi: 10.1111/j.1432-1033.1979.tb12806.x. [DOI] [PubMed] [Google Scholar]
- Schuster T. M., Scheele R. B., Adams M. L., Shire S. J., Steckert J. J., Potschka M. Studies on the mechanism of assembly of tobacco mosaic virus. Biophys J. 1980 Oct;32(1):313–329. doi: 10.1016/S0006-3495(80)84959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire S. J., Steckert J. J., Adams M. L., Schuster T. M. Kinetics and mechanism of tobacco mosaic virus assembly: direct measurement of relative rates of incorporation of 4S and 20S protein. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2745–2749. doi: 10.1073/pnas.76.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire S. J., Steckert J. J., Schuster T. M. Mechanism of self-assembly of tobacco mosaic virus protein. II. Characterization of the metastable polymerization nucleus and the initial stages of helix formation. J Mol Biol. 1979 Feb 5;127(4):487–506. doi: 10.1016/0022-2836(79)90233-x. [DOI] [PubMed] [Google Scholar]
- Stubbs G., Warren S., Holmes K. Structure of RNA and RNA binding site in tobacco mosaic virus from 4-A map calculated from X-ray fibre diagrams. Nature. 1977 May 19;267(5608):216–221. doi: 10.1038/267216a0. [DOI] [PubMed] [Google Scholar]