Abstract
During peripheral nerve development, each segment of a myelinated axon is matched with a single Schwann cell. Tight regulation of Schwann cell movement, proliferation and differentiation is essential to ensure that these glial cells properly associate with axons. ErbB receptors are required for Schwann cell migration, but the operative ligand and its mechanism of action have remained unknown. We demonstrate that zebrafish Neuregulin 1 (Nrg1) type III, which signals through ErbB receptors, controls Schwann cell migration in addition to its previously known roles in proliferation and myelination. Chimera analyses indicate that ErbB receptors are required in all migrating Schwann cells, and that Nrg1 type III is required in neurons for migration. Surprisingly, expression of the ligand in a few axons is sufficient to induce migration along a chimeric nerve constituted largely of nrg1 type III mutant axons. These studies also reveal a mechanism that allows Schwann cells to fasciculate axons regardless of nrg1 type III expression. Time-lapse imaging of transgenic embryos demonstrated that misexpression of human NRG1 type III results in ectopic Schwann cell migration, allowing them to aberrantly enter the central nervous system. These results demonstrate that Nrg1 type III is an essential signal that controls Schwann cell migration to ensure that these glia are present in the correct numbers and positions in developing nerves.
Keywords: Myelin, Neuregulin, Schwann cell, Zebrafish
INTRODUCTION
In the vertebrate nervous system, rapid communication occurs among diverse cell types separated by large distances. Many studies have illuminated the mechanisms that direct the outgrowth of axons to distant targets (Raper and Mason, 2010). Much less is known about the cues that guide migrating Schwann cells, the glial cells that associate with axons in peripheral nerves (Nave and Salzer, 2006). Schwann cells derive from multipotent neural crest cells (Jessen and Mirsky, 2005). In zebrafish, some neural crest cells coalesce at the ganglion of the posterior lateral line nerve (PLLn) and become Schwann cell precursors that migrate with growing axons (Gilmour et al., 2002; Grant et al., 2005). In the PLLn, migrating Schwann cells each associate with many axons and bundle them into one fascicle (Gilmour et al., 2002; Raphael et al., 2010). After axonal and Schwann cell migration is completed, Schwann cells proliferate to generate the large number of cells required to myelinate the many axonal segments in a nerve. As they mature, myelinating Schwann cells insert processes into the bundle, pluck out one axon to ensheathe, and then wrap their membrane around the axon to form myelin (Jessen and Mirsky, 2005). The balance among survival, proliferation and migration of Schwann cells is tightly regulated by neurons to achieve the 1:1 relationship between myelinating Schwann cells and their associated axonal segments (Sherman and Brophy, 2005). Although recent studies have made progress in defining the pathways that initiate Schwann cell differentiation, the molecular nature of the axonal signals that coordinate Schwann cell migration in vivo has not been defined (Britsch et al., 2001; Dong et al., 1995; Garratt et al., 2000).
Recent studies in zebrafish have demonstrated that signaling through ErbB receptor tyrosine kinases is required continuously for directed migration of Schwann cells along the PLLn. When ErbB signaling was inhibited during migration, some Schwann cells retained their motility but deviated from their original path (Lyons et al., 2005). This suggests that ErbB signaling has a specific role in directed migration in addition to other known roles in Schwann cell development, including proliferation and myelination (Garratt et al., 2000). Proliferation is not required for migration, and thus it seems likely that these two processes, which must be tightly coupled in nerve development, are regulated distinctly (Lyons et al., 2005).
Neuregulin 1 (Nrg1) signals through ErbB receptors and functions throughout Schwann cell development, influencing cell fate specification, motility, proliferation, survival and myelination (Garratt et al., 2000; Mahanthappa et al., 1996; Meyer et al., 1997; Michailov et al., 2004; Taveggia et al., 2005). In mammals, there are over 15 isoforms of Nrg1 (Falls, 2003). In the peripheral nervous system (PNS), an important isoform is Nrg1 type III, which signals through the ErbB2-ErbB3 heterodimer (Ho et al., 1995; Meyer et al., 1997; Nave and Salzer, 2006). Its unique cysteine-rich domain (CRD) functions as a transmembrane domain, allowing the type III isoform to act primarily as a juxtacrine signal (Falls, 2003; Wang et al., 2001). Mouse mutants revealed that Nrg1 type III is required for Schwann cells to fully populate spinal nerves (Meyer et al., 1997; Wolpowitz et al., 2000), although it is not known whether this is due to aberrant Schwann cell migration or diminished proliferation (Jessen and Mirsky, 2005). Nrg1 type III regulates whether an axon is ensheathed by a non-myelinating or myelinating Schwann cell, and also controls myelin sheath thickness (Michailov et al., 2004; Nave and Salzer, 2006; Taveggia et al., 2005). Additionally, in vitro experiments implicate an Nrg1 ligand in Schwann cell migration, although the specific isoform and mechanism are unknown (Cornejo et al., 2010; Eckert et al., 2009; Grossmann et al., 2009; Hayworth et al., 2006; Meintanis et al., 2001).
In this study, we report that nrg1 type III is expressed in the zebrafish posterior lateral line ganglion (PLLg) and is required for Schwann cell proliferation and migration. Analysis of genetic chimeras shows that Nrg1 type III is required in neurons, and that ErbB receptors are required in all Schwann cells for migration. Schwann cells can, however, migrate along and fasciculate nrg1 type III mutant axons if wild-type neurons are also present in a chimeric nerve. Furthermore, ectopic pan-neuronal expression of human NRG1 type III can draw Schwann cells into the spinal cord, indicating that this signal can direct migration and also instruct Schwann cells to overcome boundaries that normally exclude them from the central nervous system (CNS) (Coulpier et al., 2010; Fraher, 1992; Kucenas et al., 2009; Vermeren et al., 2003). These results define a key role for Nrg1 type III in controlling migration, show that this signal is sufficient to allow Schwann cells to trespass within the CNS, and provide novel insights into the control of axonal fasciculation.
MATERIALS AND METHODS
Fish strains
S1101:Gal4, Foxd3:GFP and Olig2:dsRed transgenic fish and erbb2st50 mutants were described previously (Gilmour et al., 2002; Kucenas et al., 2008; Lyons et al., 2005; Scott et al., 2007).
Genotyping
A derived cleaved amplified polymorphic sequence (dCAPS) assay using the restriction enzyme Hpy81 was used to score the erbb2st50 mutation (the wild-type fragment is shorter than the mutant). The nrg1z26 mutation eliminates an HpyCH4V restriction enzyme recognition site, and was scored with primers flanking the lesion (see Table S1 in the supplementary material).
In situ hybridization and immunohistochemistry
In situ hybridization and immunohistochemistry were performed using standard procedures. sox10 (Dutton et al., 2001), foxd3 (Odenthal and Nüsslein-Volhard, 1998), krox20 (Oxtoby and Jowett, 1993), olig2 (Park et al., 2002), mbp (Lyons et al., 2005) and nrg1 type III (Monk et al., 2009) probes were previously described. After in situ hybridization, embryos were equilibrated in 30% sucrose and frozen in O.C.T. embedding medium (Tissue-Tek). Transverse sections (10 μm) were prepared on a cryostat microtome and imaged on a Zeiss Axioplan 2 fluorescence microscope.
An antisense riboprobe recognizing all zebrafish nrg1 isoforms (Honjo et al., 2008) was PCR amplified (909 bp) from 48 hpf zebrafish cDNA, cloned into pBluescript II KS+ (Stratagene), sequenced, linearized with EcoRV and transcribed with T7 RNA polymerase.
The human NRG1 type III (Open Biosystems clone ID #3161700, accession number BC007675) open reading frame was cloned into pBluescript KS II+; an antisense probe was made by cutting with SalI and transcribing with T7 RNA polymerase.
For primers and probes described above, see Table S1 in the supplementary material.
The following antibodies were used: anti-acetylated tubulin at 1:1000 (Sigma), anti-Mbp at 1:50 (Lyons et al., 2005), anti-phospho-Histone H3 (pH3) (Ser10) at 1:500 (Millipore) and anti-Sox10 at 1:100 (Anaspec). Fluorescent images were collected on a Zeiss Pascal LSM5 or Leica DM 6000B confocal microscope.
Morpholino injections and small-molecule inhibitor
An antisense morpholino oligonucleotide that knocks down all isoforms of Nrg1 [NCBI ref seq NM_001044911.1 (Honjo et al., 2008)] targets the exon-intron boundary of the first exon of the common EGF domain (Gene Tools) (Milan et al., 2006). Nrg1 type III [GenBank ID FJ593489.1 (Honjo et al., 2008)] was knocked down by a morpholino (Gene Tools) that targets the boundary of the type III isoform-specific exon and following intron. Embryos were injected with Phenol Red and Danieau’s buffer or 6.4 ng of morpholino. For morpholino sequences, see Table S1 in the supplementary material.
ErbB signaling was inhibited by treating fish with AG1478 (EMD Chemicals; 2 μM in 1% DMSO) from 10-26 hpf (Lyons et al., 2005).
Molecular analysis of nrg1z26
Standard methods (Talbot and Schier, 1999) established linkage between the nrg1z26 mutation and the nrg1 gene on linkage group 10. Sequence analysis of wild-type and mutant genomic DNA revealed the lesion.
Transmission electron microscopy
Electron microscopy was performed as previously described (Lyons et al., 2008). At least three nerves from three distinct embryos were observed in each condition. Images were obtained using a JEOL TEM1230 microscope.
Transplantation
Genetic chimeras were generated by labeling donor embryos with 1% Texas Red-Dextran (Molecular Probes) and transplanting labeled cells into erbb2st50 or nrg1z26 mutant hosts. Embryos were analyzed for the presence of Foxd3:GFP+ Schwann cells along the PLLn, imaged at 57 hpf and genotyped. A subset of nrg1z26 chimeras was imaged live at 76 hpf and then processed for electron microscopy.
Generation of hNRG1type III:UAS transgenic fish
Human NRG1 type III was cloned into pBH mcs (Williams et al., 2010) that was modified to include 14 UAS recognition sites and an E1b basal promoter (Matthew G. Voas and W.S.T., unpublished). Supercoiled plasmid and transposase RNA (25 pg each) were injected into embryos at the one-cell stage (Kawakami, 2004). All experiments were performed with the same transgenic line, which contains more than one insertion of the transgene.
Time-lapse imaging
Embryos were anesthetized in 0.016% (w/v) Tricaine, mounted in 1.5% low-melting-point agarose and imaged on a Zeiss LSM 700 confocal microscope. Time-lapse imaging took place at room temperature with scans obtained every 5 minutes for at least 6 hours. The yz plane was visualized using the Ortho-Select function of Zen 2009 software (Zeiss).
RESULTS
Nrg1 is required for Schwann cell migration
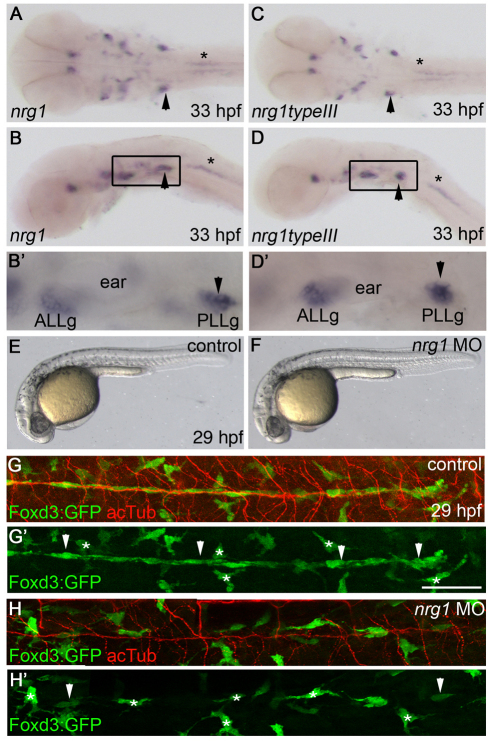
To determine whether Neuregulin 1 (Nrg1) ligands are expressed during Schwann cell migration, we performed in situ hybridization using a probe that recognizes all known zebrafish nrg1 isoforms. During migration (33 hpf), the probe strongly labeled the PLLg, which contains the cell bodies of the neurons that form the PLLn (Fig. 1A,B,B′, arrowhead). A probe specific for the type III isoform revealed an almost identical staining pattern (Fig. 1C,D,D′). Common region probes and type III-specific probes also detected similar expression patterns at 24 hpf, 48 hpf, 72 hpf and 4 dpf (data not shown). These results indicate that nrg1 type III is expressed at the right time and place to control Schwann cell migration and myelination in zebrafish.
Fig. 1.
Neuregulin 1 is required for Schwann cell migration. (A-D′) nrg1 (A,B,B′) and nrg1 type III (C,D,D′) mRNA expression at 33 hpf, shown in dorsal (A,C) and lateral (B,D) views. (B′,D′) Higher magnification of the boxed regions in B,D. Arrowheads mark the PLLg and asterisks mark motoneurons. (E,F) Lateral views of control-injected (E) and nrg1 morpholino-injected (F) zebrafish embryos at 29 hpf. (G-H′) Lateral views of control-injected (G,G′) and morphant (H,H′) embryos carrying the Foxd3:GFP transgene (green) and stained with anti-acetylated tubulin (acTub, red) at 29 hpf. Schwann cells are marked by arrowheads and stellate-shaped pigment cells by asterisks. In lateral views, anterior is to the left and dorsal is up. In dorsal views, anterior is to the left. ALLg, anterior lateral line ganglion; PLLg, posterior lateral line ganglion. Scale bar: 50 μm.
To determine whether Nrg1 is required for Schwann cell migration, all nrg1 isoforms were knocked down by an antisense morpholino oligonucleotide that targets the common EGF domain (Holmes et al., 1992; Honjo et al., 2008). Embryos injected with the nrg1 morpholino had comparable morphology to control-injected embryos, but RT-PCR analysis demonstrated that the nrg1 transcripts were improperly spliced (Fig. 1E,F; see Fig. S1A in the supplementary material). The Foxd3:GFP transgene, which marks a subset of neural crest derivatives, was used to visualize Schwann cells (Gilmour et al., 2002). Schwann cells are detected as elongated, Foxd3:GFP+ cells (Fig. 1, white arrowheads) that are associated with PLLn axons; nearby pigment cells also express GFP but are not associated with the nerve and have a stellate morphology (Fig. 1, white asterisks). There is a dramatic reduction of Schwann cells along the PLLn of morphants (Fig. 1H,H′; n=68/68) as compared with control-injected embryos (Fig. 1G,G′; n=48/48). Neural crest cells, which are the precursors of Schwann cells, were able to migrate to the ganglion, as has been observed in erbb mutants (Lyons et al., 2005), but most did not migrate from the ganglion. This indicates that Nrg1 signaling is required for Schwann cells to occupy the PLLn, supporting the possibility that Nrg1 signals direct Schwann cell migration via ErbB receptors.
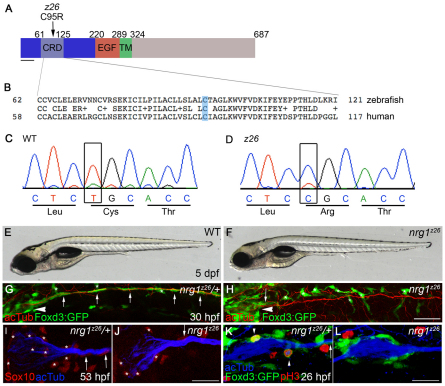
nrg1z26 specifically disrupts the Nrg1 type III isoform
In the absence of Schwann cells, supernumerary neuromasts form in the posterior lateral line (Grant et al., 2005). A screen for mutants with supernumerary neuromasts uncovered a mutation, nrg1z26, that mapped to the nrg1 locus. Sequence analysis showed that nrg1z26 mutants harbor a T-to-C transition that converts a cysteine in the CRD to an arginine (Fig. 2A-D). This cysteine, which is conserved from fish to human, is within the N-terminal transmembrane segment required for membrane localization of Nrg1 type III (Cabedo et al., 2002; Ho et al., 1995; Wang et al., 2001). The presence of the bulky charged residue in the transmembrane segment is likely to disrupt folding or localization of the mutant Nrg1 type III protein. In genotyping studies, all nrg1z26 mutants were homozygous for the T-to-C mutation (n=134 mutants), confirming that this lesion is tightly linked to the nrg1z26 mutation. Gross morphology of mutants is normal at 5 dpf (Fig. 2E,F) and a few homozygous mutant adult fish are viable, although small. Schwann cells are absent from the PLLn in nrg1z26 mutants at all stages examined from 26 hpf to 5 dpf (n=156/156). During initial axon outgrowth, Schwann cells are present at the PLLg in nrg1z26 mutants, but fail to migrate along growing axons (Fig. 2H; n=45/45). A morpholino targeted specifically to the type III isoform knocked down the transcript and similarly reduced Schwann cell migration in otherwise morphologically normal animals (see Fig. S1B,D,F in the supplementary material; n=51/51). Taken together, these data indicate that the z26 mutation specifically disrupts the nrg1 type III isoform, and that Nrg1 type III is required for Schwann cell migration.
Fig. 2.
nrg1z26 specifically disrupts the Nrg1 type III isoform. (A) Protein structure of zebrafish Nrg1 type III. The z26 lesion in the cysteine-rich domain (CRD) of the type III-specific domain (dark blue) changes a conserved cysteine (C) at position 95 to an arginine (R). TM, transmembrane domain. Scale bar: 40 amino acids. (B) Comparison of zebrafish and human Nrg1 type III CRD sequences. The conserved cysteine mutated by the z26 lesion is shaded in blue. (C,D) Sequence traces from wild-type and z26 mutant zebrafish larvae illustrating the T-to-C lesion. (E,F) Lateral views of 5 dpf wild-type and nrg1z26 mutant sibling embryos. (G,H) Lateral views of 30 hpf nrg1z26/+ siblings (G) and nrg1z26 mutants (H) stained with anti-acetylated tubulin (acTub, red). Arrowheads mark the PLLg, asterisks mark pigment cells. Embryos were genotyped after imaging. (I,J) PLLg of a 53 hpf nrg1z26/+ sibling (I) and nrg1z26 mutant (J) stained with anti-Sox10 (red) and anti-acTub (blue). Asterisks mark Schwann cells associated with the ganglion. (K,L) PLLg of 26 hpf nrg1z26/+ sibling (K) and nrg1z26 mutant (L) stained with anti-phospho-Histone H3 (pH3) (red) and anti-acTub (blue). Arrowhead marks Foxd3:GFP+ cells that are co-labeled with pH3. (G-L) Schwann cells are marked by Foxd3:GFP (green, arrows). Anterior is to left, dorsal is up. (I-L) Embryos were genotyped before imaging and images are a projection of either three (I,J) or four (K,L) 3 μm sections taken at the middle of each PLLg. Scale bars: 50 μm in G,H; 25 μm in I-L.
To further compare the phenotypes of erbb and nrg1z26 mutants, we examined marker expression and proliferation in Schwann cells. As in erbb mutants (Pogoda et al., 2006), early (sox10, see Fig. S2C,D in the supplementary material) and late (mbp mRNA, see Fig. S2E,F in the supplementary material; Mbp protein, see Fig. S2G,H in the supplementary material) markers of Schwann cells are absent along the PLLn of nrg1z26 mutants. In the PLLg at 53 hpf, Sox10-expressing cells are present at reduced numbers in nrg1z26 mutants (Fig. 2I,J; see Fig. S3A in the supplementary material; an average of 16.2 cells in controls versus 8.3 cells in mutants; P<0.0001). To determine whether diminished Schwann cell proliferation causes this reduction in Schwann cell number, we examined the proliferation marker phospho-Histone H3. At 26 hpf, there were fewer proliferating Schwann cells in the ganglia of nrg1z26 mutants (Fig. 2L; see Fig. S3B in the supplementary material) as compared with control siblings (Fig. 2K; see Fig. S3B in the supplementary material; an average of 1.3 cells in controls versus 0.9 cells in mutants; P<0.05). This reduction in proliferating Schwann cells is similar to that observed in embryos that lack all ErbB signaling or all Nrg1 function (see Fig. S3B in the supplementary material). These data show that Nrg1 type III is required for Schwann cell proliferation and further indicate that Nrg1 type III is the major Schwann cell mitogen acting through ErbB receptors at this stage.
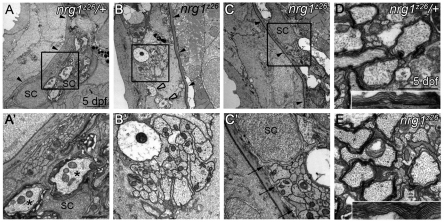
Ultrastructural analysis of nrg1 type III mutants
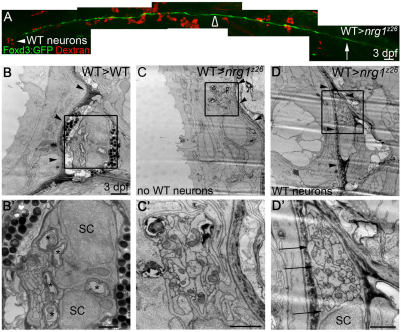
The PLLn initially grows out within the epidermis above the basement membrane, and Schwann cells are required for the nerve to transition to its mature location beneath this basement membrane (Raphael et al., 2010). At 5 dpf the nerve is normally located beneath the basement membrane and Schwann cells have begun to myelinate axons (Fig. 3A,A′; n=10). In the absence of Schwann cells, erbb mutant nerves remain in the epidermis and become defasciculated (Raphael et al., 2010). To determine whether nrg1z26 mutant nerves resemble erbb mutant nerves at the ultrastructural level, we examined transverse sections of the PLLn by transmission electron microscopy (TEM). As in other mutants lacking Schwann cells (Raphael et al., 2010; Voas et al., 2009), nrg1z26 nerves fail to transition across the basement membrane and are defasciculated (Fig. 3B,B′; n=4).
Fig. 3.
Nrg1 type III is required for myelin formation in the peripheral but not central nervous system. (A-C′) Transmission electron microscopy (TEM) images of transverse sections through the posterior lateral line nerve (PLLn) of zebrafish larvae of the indicated genotypes. (A,A′) At 5 dpf, nrg1z26/+ nerves are beneath the epidermal basement membrane (black arrowheads) and Schwann cells (SC) have begun to myelinate axons (asterisks). (B,B′) nrg1z26 mutant nerves that lack Schwann cells fail to transition across the basement membrane (black arrowheads) and remain in the epidermis where they are unmyelinated and defasciculated (open arrowheads). (C,C′) nrg1z26 mutant nerves with ‘escaper’ Schwann cells are correctly positioned beneath the epidermal basement membrane (black arrowheads), but Schwann cell processes (arrows) fail to myelinate axons within the bundle. (A′,B′,C′) Higher magnification of the boxed regions in A,B,C. (D,E) TEM of transverse sections through the ventral spinal cord of nrg1z26/+ (D) and nrg1z26 mutant (E) siblings. Insets show myelin around the Mauthner axon. All images were taken at the level of the fifth somite. Scale bars: 2 μm in A,B,C; 1 μm in A′,B′; 0.5 μm in C′; 1 μm in D,E; 0.5 μm in D,E insets.
By Foxd3:GFP transgene analysis we observed a small number of ‘escaper’ Schwann cells in the nrg1z26 mutants at 2 dpf. In wild-type larvae, more than 75 Foxd3:GFP+ Schwann cells stretch from the ganglion to the end of the PLLn, whereas in nrg1z26 mutants an average of 2.3 Schwann cells reach, on average, somite 3. The greatest number of Schwann cells observed in a mutant nerve was seven, and the farthest somite reached was somite 7 (data not shown; n=25 nrg1z26 mutants). Similarly, by TEM we occasionally observed these escaper Schwann cells along nrg1z26 mutant nerves at the level of somite 5. These Schwann cells were sufficient to transition the nerve across the basement membrane, but did not myelinate the axons within their bundle (Fig. 3C,C′; n=2). These results indicate that mutants for erbb receptors and nrg1 type III have similar ultrastructural phenotypes.
In contrast to Schwann cells in the PNS, oligodendrocytes did form myelin in the CNS of nrg1z26 mutants. We did not observe a difference in myelination between the spinal cords of nrg1z26 mutants and control siblings (Fig. 3D,E; n=3 for both). We cannot, however, exclude a role for Nrg1 type III in CNS myelination in other locations or at different stages.
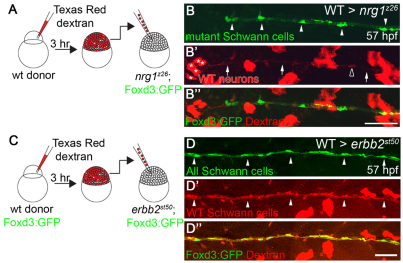
Nrg1 type III is required in neurons and ErbB2 receptors are required in all Schwann cells for Schwann cell migration
Expression analyses alone do not distinguish whether Nrg1 type III is required in neurons or in glia. Although we (Fig. 1C,D) and others (Ho et al., 1995; Meyer et al., 1997) primarily observed nrg1 type III expression in neurons, nrg1 type III expression has been detected in rat Schwann cells (Rosenbaum et al., 1997) and in adult zebrafish nerve, in which most mRNAs derive from Schwann cells (data not shown). To determine whether Nrg1 type III is required in neurons or Schwann cells, we made genetic chimeras. Wild-type cells labeled with a lineage tracer were transplanted into nrg1z26 mutant hosts carrying the Foxd3:GFP transgene to mark mutant Schwann cells (Fig. 4A). As a few Schwann cells are seen in nerves of nrg1z26 mutants (never more than seven Schwann cells, an average of 2.3), we only counted a chimera as having rescued Schwann cell migration if at least ten Schwann cells were present in the nerve. All chimeras with at least ten nrg1z26 mutant Schwann cells in the nerve had wild-type neurons in the PLLg (Fig. 4B-B′; n=8 nerves from eight different chimeras). The rescued chimeras had, on average, 3.8 wild-type neurons (range 1-6) and 15.4 Schwann cells (range 11-29), which extended on average to somite 15.9 (range 11-27). The number of rescued Schwann cells did not directly correlate with the number of wild-type neurons (e.g. six neurons rescued 11 or 29 Schwann cells in different chimeras). In addition, six of the eight chimeras with rescued mutant Schwann cells had no wild-type Schwann cells, indicating that the Nrg1 type III signals that direct migration come from neurons, not Schwann cells.
Fig. 4.
Nrg1 type III is required in neurons and ErbB receptors are required in all Schwann cells. (A) Wild-type cells marked with a Texas Red-Dextran lineage tracer were transplanted into nrg1z26 mutant hosts carrying the Foxd3:GFP (green) transgene at the blastula stage. (B-B′) Lateral view of genetic chimera generated by transplanting wild-type cells (red) into the nrg1z26 mutant. Arrowheads point to nrg1z26 mutant Schwann cells, which have migrated along axons (arrows) of wild-type neurons (asterisks). Open arrowhead marks a wild-type Schwann cell. (C) Wild-type cells carrying the Foxd3:GFP transgene (green) labeled with a Texas Red-Dextran lineage tracer were transplanted into erbbst50 mutant hosts also carrying the Foxd3:GFP transgene at the blastula stage. (D-D′) Lateral view of genetic chimera generated by transplanting wild-type cells (red) into the erbbst50 mutant. All Schwann cells (arrowheads) are labeled by the wild-type lineage tracer. Anterior is to the left and dorsal is up in all images; embryos were genotyped after imaging. Scale bars: 50 μm.
In complementary experiments, we also analyzed chimeras to determine the site of action of ErbB receptors during Schwann cell migration. Schwann cells that are directly migrating with axonal growth cones exhibit more protrusions, which is characteristic of cells that are actively migrating, whereas those that are migrating behind them show no protrusions (Gilmour et al., 2002; Gompel et al., 2001). This has led to the suggestion that the dynamic Schwann cells at the leading edge are directing the following Schwann cells (Gilmour et al., 2002), but it has been unclear whether these different Schwann cell populations have distinct genetic requirements during migration. To determine if ErbB receptors are required in all Schwann cells or only in a subset, we analyzed genetic chimeras. Dye-labeled cells from Foxd3:GFP+ embryos were transplanted into erbb2st50 mutant hosts, which also carried the Foxd3:GFP transgene (Fig. 4C). All Schwann cells that migrated in mutant hosts were wild-type (i.e. labeled with the Dextran lineage tracer; Fig. 4D-D′; n=8 nerves from six different chimeras). This analysis indicates that all Schwann cells autonomously require ErbB2 receptors to migrate, and that wild-type Schwann cells are not able to rescue the migration of their erbb2st50 mutant neighbors. Collectively, the chimera analyses indicate that all Schwann cells autonomously require ErbB2 receptors, which respond to neuronal Nrg1 type III ligands.
Overexpressing human NRG1 type III leads to ectopic expression of Schwann cell markers
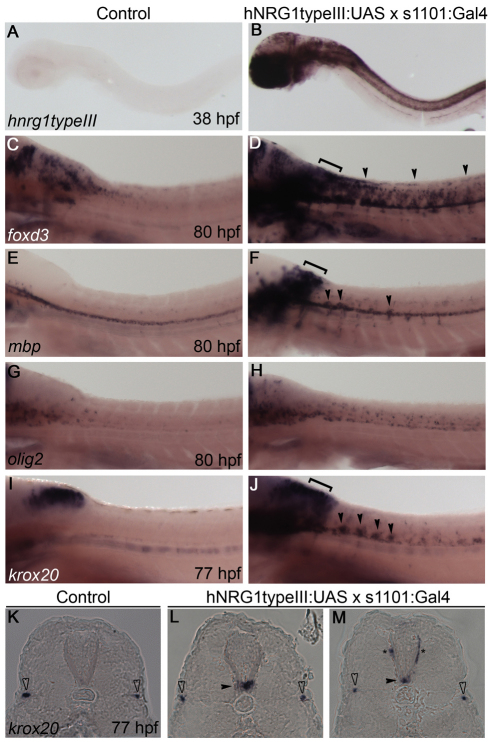
Although previous studies (Lyons et al., 2005) supported a role for ErbB receptors in directing Schwann cell migration, it has not been determined whether Nrg1 ligands act to promote Schwann cell motility, direct their migration, or both. To investigate if Nrg1 type III is an instructive signal for migration, we tested whether ectopic expression could misdirect Schwann cell migration. We expressed human NRG1 (hNRG1) type III in all neurons under the control of a pan-neuronal GAL4 driver (Scott et al., 2007). Zebrafish embryos with both the hNRG1typeIII:UAS and the s1101:GAL4 pan-neuronal driver transgenes displayed robust expression of hNRG1 type III in the brain and spinal cord, although there was variability in expression between embryos and within individuals (Fig. 5B; n=18/18). Control embryos that lacked either transgene showed no hNRG1 type III expression (Fig. 5A; n=125/125).
Fig. 5.
Overexpression of human NRG1 type III in all neurons leads to expression of Schwann cell markers in the spinal cord. (A-J) Lateral views of human NRG1 (hNRG1) type III-overexpressing transgenic zebrafish embryos (B,D,F,H,J) and control siblings (A,C,E,G,I) stained by in situ hybridization for the indicated genes. Anterior is to the left and dorsal is up. Embryos were genotyped before imaging. Transgenic embryos have expanded foxd3 (C,D), mbp (E,F) and krox20 (I,J) expression in dorsal hindbrain (brackets) and spinal cord (arrowheads). olig2 is only slightly expanded in hNRG1 type III-overexpressing embryos (G,H). (K-M) Cryosections of embryos showing krox20 expression. krox20-expressing cells are present within the neural tube (black arrowheads) and along nearby nerves (asterisks) of hNRG1 type III-overexpressing embryos (L,M), but not in control embryos (K). The PLLn is marked by open arrowheads.
Double-transgenic embryos also had expanded expression of the neural crest marker foxd3 around the hindbrain and spinal cord as compared with control embryos (Fig. 5C,D; n=7/7 controls, n=6/6 double transgenics). mbp expression was similarly expanded in double-transgenic embryos (Fig. 5E,F; n=7/7 controls, n=7/7 double transgenics). Expression of the oligodendrocyte marker olig2 was somewhat increased in the spinal cord, but this expansion was not sufficient in extent or location to account for the expanded mbp expression (Fig. 5G,H; n=8/8 controls, n=4/4 double transgenics).
These expression patterns raised the possibility that Schwann cells were present in the spinal cord of embryos overexpressing Nrg1 type III. Accordingly, expression of krox20 (egr2 – Zebrafish Information Network), a marker of Schwann cells initiating myelination, was strongly expanded in the spinal cord, correlating with the upregulation of mbp (Fig. 5I,J; n=18/18 controls, n=19/20 double transgenics). Sectioned double-transgenic embryos had krox20-expressing cells located within the ventral neural tube (Fig. 5L,M, black arrowheads), as well as more dorsally located krox20-expressing cells just outside the neural tube (Fig. 5L,M, asterisks). Together, these data indicate that expressing hNRG1 type III in all neurons leads to the ectopic presence of Schwann cells in the CNS.
Overexpressing human NRG1 type III leads to ectopic Schwann cell migration
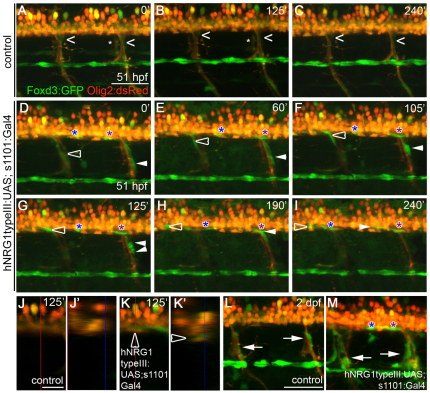
The above analysis suggests that ectopic Neuregulin expression can either direct Schwann cells to migrate into the CNS or direct CNS-resident cells to differentiate as myelinating Schwann cells (Zawadzka et al., 2010). To determine whether Schwann cells enter the spinal cord via ectopic migration upon neuronal overexpression of hNRG1 type III, we performed time-lapse imaging. Schwann cell migration along the lateral line is complete by ∼40 hpf. In control embryos, we observed little Schwann cell movement along the lateral line or motor nerves from 51-55.5 hpf (Fig. 6A-C; see Movie 1 in the supplementary material; n=4 time-lapse movies). By contrast, Schwann cells in embryos overexpressing hNRG1 type III exhibited unusual migratory behaviors during the same time (Fig. 6D-I; n=9 time-lapse movies). Strikingly, Schwann cells moved up motor nerves and into the spinal cord, bypassing the normal barriers that exclude Schwann cells from the CNS (Coulpier et al., 2010; Fraher, 1992; Kucenas et al., 2009; Vermeren et al., 2003) (see Movie 2 and Fig. S4 in the supplementary material; n=16/39 motor nerves in eight time-lapse movies). For example, the Schwann cell marked by the open arrowhead in Fig. 6D-I can be seen within the Olig2:dsRed-expressing spinal cord in an orthogonal view of the yz plane of a single scan at 125 minutes (Fig. 6K,K′; another example is shown in Fig. S4B,B′ in the supplementary material). No Schwann cells are seen within the spinal cords of control embryos (Fig. 6J,J′; see Fig. S4A,A′ in the supplementary material).
Fig. 6.
Overexpression of hNRG1 type III in all neurons leads to the ectopic migration of Schwann cells into the spinal cord. (A-K′) Images from in vivo time-lapse movies. Schwann cells are marked by the Foxd3:GFP transgene (green) and oligodendrocytes and motoneurons are marked by Olig2:dsRed (red). Numbers at top right denote time (minutes) elapsed since the start of the movie. (A-C) Schwann cells (carets) along motor nerves undergo little movement in the control embryo; asterisks mark motor nerves on the contralateral side. (D-I) Embryos overexpressing hNRG1 type III in all neurons. The open arrowhead follows a Schwann cell that migrates up a motor nerve and into the spinal cord; the white arrowhead follows a Schwann cell that divides and produces one daughter that moves into the spinal cord; and blue and purple asterisks follow Schwann cells already present in the spinal cord as they migrate along spinal axons. (J,K) Lateral views of single scans from in vivo time-lapse movies of control (J) and transgenic (K) embryos at 125 minutes. (J′,K′) Orthogonal view of the yz plane indicated by the vertical red lines in J and K. The open arrowhead marks the Schwann cell that is tracked by the open arrowhead in D-I. (L,M) Excess Schwann cells (arrows) are present along motor nerves of embryos overexpressing hNRG1 type III (M) as compared with control embryo (L) at 2 dpf. Anterior is to the left, dorsal is up. Comparable regions over the yolk extension were imaged. Embryos were genotyped after imaging. Scale bars: 50 μm in A-I,L-M; 20 μm in J,K.
After entering the spinal cord, individual Schwann cells moved back and forth within it (n=9 time-lapse movies). In two time-lapse movies, Schwann cells moved from the lateral line nerve onto motor nerves and into the spinal cord (data not shown). None of these behaviors was seen in control movies. Thus, it seems that hNRG1 type III not only increases the motility of Schwann cells, but also directs their migration into the spinal cord. In addition, 15% of motor nerves in embryos overexpressing hNRG1 type III (88 among 607 motor nerves, n=23 embryos) had excess Schwann cells compared with controls (0 among 604 motor nerves, n=23 embryos), which is likely to be due to a combination of misdirected migration and excess proliferation (Fig. 6L,M). Motor nerves with excess Schwann cells in transgenic embryos were distributed at many points along the anteroposterior axis. These data indicate that Nrg1 type III can promote Schwann cell motility and proliferation, direct ectopic migration of Schwann cells, and allow Schwann cells to bypass boundaries that normally exclude them from the CNS.
Schwann cells can migrate along and fasciculate chimeric nerves that are mostly mutant for nrg1 type III
Using genetic chimeras, we found that Nrg1 type III is required in neurons to control the migration of Schwann cells. However, we did not know whether the Schwann cells selectively migrated along wild-type axons, or whether the presence of wild-type axons could rescue migration along neighboring nrg1z26 mutant axons. To address this question, we generated chimeras as described above, live-imaged Schwann cells in chimeric nerves via the Foxd3:GFP transgene (Fig. 7A), and analyzed the ultrastructure of the nerves in the same embryos by TEM. To avoid confounding effects from escaper Schwann cells in the mutants (which never migrated farther than somite 7), the TEM data were acquired at the level of somite 10 (Fig. 7A, open arrowhead).
Fig. 7.
Schwann cells can migrate along nrg1z26 mutant axons in the presence of wild-type neurons. (A) Lateral view of 3 dpf genetic chimera generated by transplanting Dextran-labeled wild-type cells (red) into an nrg1z26 mutant with Foxd3:GFP-labeled Schwann cells (green). White arrowhead marks wild-type neurons in the ganglion; arrow marks the most posterior Schwann cell; open arrowhead marks the region that was examined by TEM in C,D. Anterior to left, dorsal is up; embryo genotyped after imaging. Scale bar is 50 μm. (B-D′) TEM of transverse sections of the PLLn of larvae of the indicated genotypes at somite 10. (B,B′) The PLLn of the wild-type chimera is in its mature location beneath the basement membrane (arrowheads), and Schwann cells (SC) have begun to myelinate axons (asterisks). (C,C′) The contralateral nerve of the rescued nrg1z26 mutant chimera lacks wild-type neurons, has no visible Schwann cell nuclei or processes, and fails to properly transition across the basement membrane (arrowheads). (D,D′) The PLLn of nrg1z26 mutant chimera with wild-type neurons successfully transitioned across the epidermal basement membrane (arrowheads), and Schwann cell processes (arrows) envelop all axons in the bundle. (B′,C′,D′) Higher magnifications of boxed regions in B,C,D. Scale bars: 50 μm in A; 2 μm in B,C,D; 1 μm in B′,C′,D′.
At 3 dpf, nerves of control genetic chimeras (in which wild-type cells were transplanted into wild-type or heterozygous hosts) were properly localized with respect to the basement membrane and Schwann cells had already begun to myelinate axons within the nerve (Fig. 7B,B′; n=3). In nrg1z26 mutant chimeras with wild-type neurons, the nerves on the contralateral side, which lacked wild-type neurons, did not contain visible Schwann cells or Schwann cell processes and did not transition across the basement membrane (Fig. 7C,C′; n=3). By contrast, Schwann cell bodies or processes did associate with the nerves that contained wild-type neurons, as expected from the analysis of the transgenic reporter (Fig. 7A,D,D′; n=3). In nerves with visible Schwann cell bodies or processes, some or all of the axons were properly localized beneath the basement membrane. In the mutant genetic chimera shown in Fig. 7A, for example, there were six wild-type neurons transplanted into the otherwise mutant PLLg (arrowhead). Strikingly, Schwann cell processes in this chimera extended around many more than six axons (Fig. 7D′), indicating that Schwann cells can extend processes around nrg1z26 mutant axons in the presence of wild-type axons. These chimera analyses therefore indicate that the presence of Nrg1 type III-expressing axons within a bundle can allow Schwann cells to associate with and fasciculate nrg1z26 mutant axons.
DISCUSSION
Before Schwann cells can myelinate peripheral nerves, they must migrate along outgrowing axons and then proliferate to ensure that there is a Schwann cell to wrap each segment of every axon to be myelinated (Jessen and Mirsky, 2005). Previously, we determined that ErbB receptor tyrosine kinases are essential for the migration of Schwann cells in developing nerves (Lyons et al., 2005). Here, we show that ErbB receptors are required in all Schwann cells to respond to Nrg1 type III, which is required in neurons for Schwann cell migration. Nrg1 type III stimulates Schwann cell proliferation and motility and serves as an attractant that directs migration, in some cases instructing Schwann cells to overcome PNS-CNS boundaries. In embryos overexpressing Nrg1 type III in all neurons, some Schwann cells abandon peripheral nerves and preferentially migrate towards high levels of expression in the spinal cord. Further analysis of chimeric nerves reveals a mechanism that ensures that all axons are fasciculated, regardless of Nrg1 type III expression.
There are many distinct Nrg1 isoforms (Falls, 2003), and a priori one might postulate that different isoforms control different aspects of Schwann cell development. The type III isoform, however, has been implicated in the control of Schwann cell proliferation (Morrissey et al., 1995; Salzer et al., 1980), initiation of myelination and in the regulation of myelin thickness (Michailov et al., 2004; Taveggia et al., 2005). Our analysis in zebrafish defines Nrg1 type III as an endogenous guidance cue controlling Schwann cell migration. Thus, the collective evidence implicates Nrg1 type III in many aspects of Schwann cell development, including proliferation, differentiation (Birchmeier and Nave, 2008) and, now, migration.
It remains unclear how one signal can control so many distinct processes in Schwann cell development. Nonetheless, the level of Nrg1 type III expressed on an axon is likely to be a critical determinant of different outcomes. Previous reports show that an axon must have a certain level of Nrg1 type III expression to be myelinated by a Schwann cell, and that Nrg1 type III levels beyond this threshold determine the thickness of its myelin sheath (Michailov et al., 2004; Taveggia et al., 2005). Recent studies have determined that even higher levels of Nrg1 can inhibit myelin formation (Syed et al., 2010). We propose that low levels of Nrg1 type III, beneath the threshold that allows myelination, control Schwann cell migration along an axon, defining a new threshold level of Nrg1 type III signaling. Our genetic chimera data demonstrate that a few wild-type neurons are sufficient to rescue migration, but not myelination, around a bundle of mostly nrg1z26 mutant axons. It therefore seems that these chimeric nerves have enough Nrg1 type III to support migration, but not enough to promote myelination. Together, the present analysis and previous studies suggest that low levels of axonal Nrg1 type III are required for Schwann cell migration, intermediate levels are required for Schwann cells to select single axons, and higher levels are required for a Schwann cell to initiate myelination (Michailov et al., 2004; Raphael et al., 2011; Syed et al., 2010; Taveggia et al., 2005). In this sense, different threshold levels of Nrg1 type III over time might govern the developmental progression of a Schwann cell in a manner somewhat analogous to increasing concentrations of a morphogen determining different cell fates (Dale and Jones, 1999). Whereas a classical morphogen acts at a distance to differentially specify cell fates by varying concentration across a field, the combined data suggest that Nrg1 type III is a juxtacrine signal that varies in concentration on the surface of an axon over time to coordinate the developmental progression of an axon and its closely associated Schwann cell. Although there is good evidence that different levels of Nrg1 type III are important for different aspects of Schwann cell development, it is also likely that other stage-specific factors, such as cAMP signaling, influence the response of Schwann cells to Nrg1 type III (Arthur-Farraj et al., 2011; Monje et al., 2008; Monk et al., 2009; Newbern and Birchmeier, 2010).
Chimeric nerve analysis not only revealed that lower levels of Nrg1 type III within a nerve are sufficient to support migration, but also demonstrated that expression in a subset of neurons could affect the organization of an entire nerve. Our experiments uncover a mechanism that allows axons with different levels of Nrg1 type III to remain fasciculated. We were surprised to observe that Schwann cells do not preferentially migrate along the subset of wild-type axons in genetic chimeras (Fig. 7). Rather, Schwann cell processes extended around the entire bundle of wild-type and mutant axons. Intriguingly, all Schwann cells require ErbB receptors to migrate, but Nrg1 type III is only required in a subset of axons to support the migration and fasciculation of all axons. It therefore appears that there is a mechanism that allows Schwann cells to establish and maintain the integrity of the axon bundle that does not require all axons to express nrg1 type III. In a developing wild-type nerve, this mechanism might ensure that all axons, regardless of Nrg1 type III levels, associate with Schwann cells.
Our analysis provides some insight into the mechanisms that control Schwann cell migration. In principle, Nrg1 type III signaling could act in several ways to allow Schwann cells to move into the developing nerve. Possible modes of action, which are not necessarily mutually exclusive, include: promoting Schwann cell proliferation, which could populate nerves by directed division; stimulating Schwann cell motility; and serving as a directional cue. Nrg1 type III is required for proliferation, but our previous work has shown that proliferation itself is not required for migration (Lyons et al., 2005). These data support the possibility that Nrg1 type III has parallel functions in proliferation and migration, such that Nrg1 type III might control the balance between these two processes during peripheral nerve development. Examination of Schwann cell migration in embryos overexpressing Nrg1 type III indicates that excess ligand does stimulate increased motility of Schwann cells along nerves, but increased motility alone is not sufficient to fully explain the migratory behavior of these cells. Misexpression of Nrg1 type III causes Schwann cells to move from one axon to another, towards high levels of ectopic signal in the CNS. During their ectopic migration, Schwann cells always remained associated with axons, consistent with previous evidence that Nrg1 type III functions as a juxtacrine signal (Nave and Salzer, 2006). Our analysis therefore indicates that Nrg1 type III acts as a juxtacrine signal that promotes Schwann cell motility and also attracts Schwann cells to ectopic locations.
Although it is clear that Nrg1 type III acts in neurons to control Schwann cell migration, it is also possible that Schwann cell-to-Schwann cell communication reinforces this signal and propagates the directional information along the chain. Communication between Schwann cells might be part of the mechanism that directs Schwann cells towards the end of the PLLn, even if they are located far from the neuronal cell bodies or axonal growth cones. Furthermore, there are two morphologically distinct populations of Schwann cells, termed leaders and followers, and it has been proposed that the actively migrating leaders are the pathfinders for the followers (Gilmour et al., 2002). Our analysis of chimeric nerves, however, demonstrates that all migrating Schwann cells autonomously require ErbB2, indicating that all Schwann cells directly respond to ErbB ligands. This result does not exclude a role for Schwann cell-to-Schwann cell communication, but does highlight the essential role of ErbB ligands originating from the axon.
Myelinating Schwann cells have been reported in the spinal cord in some cases of CNS remyelination (Kaneko et al., 2006), and recent work has shown that oligodendrocyte progenitors can differentiate as myelinating Schwann cells in this context (Zawadzka et al., 2010). Our analysis demonstrates that Schwann cells are also capable of moving from the periphery into the CNS under the influence of misexpressed guidance cues, bypassing boundaries that normally delineate the border of the PNS and CNS. These results highlight the possibility that restricted expression of Nrg1 type III could be part of the mechanism that distinguishes PNS and CNS domains, and demonstrate that ectopic expression of this signal can induce Schwann cells to migrate into normally nonpermissive territory. Together, these results demonstrate a role for neuronal Nrg1 type III in directing Schwann cell migration within the PNS, as well as in directing ectopic migration, even allowing peripheral glia to trespass within the CNS.
Supplementary Material
Acknowledgments
We thank Dave Lyons, Alex Schier and W.S.T. laboratory members for helpful discussions and comments on the manuscript; Tuky Reyes for excellent fish care; John Perrino for expert TEM technical advice; Felicity Jones for statistical help; Herwig Baier, Darren Gilmour, Bruce Appel, Michael L. Nonet and Matthew G. Voas for kindly providing fish strains and plasmid vectors; and Joanna Wysocka, James Chen, Roel Nusse and Mark Krasnow for sharing microscopes.
Footnotes
Funding
J.R.P. is supported by the Stanford Genome Training Program and a Stanford Graduate Fellowship. This work was supported by the National Institutes of Health [grant NS050223 to W.S.T.]; a National Science Foundation Grant [No.0519462 to T.P.]; and a National Institutes of Health Multidisciplinary Cancer Training Grant [T32CA093247 to M.E.L.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.068072/-/DC1
References
- Arthur-Farraj P., Wanek K., Hantke J., Davis C. M., Jayakar A., Parkinson D. B., Mirsky R., Jessen K. R. (2011). Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia 59, 720-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C., Nave K.-A. (2008). Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491-1497 [DOI] [PubMed] [Google Scholar]
- Britsch S., Goerich D. E., Riethmacher D., Peirano R. I., Rossner M., Nave K. A., Birchmeier C., Wegner M. (2001). The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabedo H., Luna C., Fernandez A. M., Gallar J., Ferrer-Montiel A. (2002). Molecular determinants of the sensory and motor neuron-derived factor insertion into plasma membrane. J. Biol. Chem. 277, 19905-19912 [DOI] [PubMed] [Google Scholar]
- Cornejo M., Nambi D., Walheim C., Somerville M., Walker J., Kim L., Ollison L., Diamante G., Vyawahare S., de Bellard M. E. (2010). Effect of NRG1, GDNF, EGF and NGF in the migration of a Schwann cell precursor line. Neurochem. Res. 35, 1643-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulpier F., Decker L., Funalot B., Vallat J.-M., Garcia-Bragado F., Charnay P., Topilko P. (2010). CNS/PNS boundary transgression by central glia in the absence of Schwann cells on Krox20/Egr2 function. J. Neurosci. 30, 5958-5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L., Jones C. M. (1999). BMP signalling in early Xenopus development. BioEssays 21, 751-760 [DOI] [PubMed] [Google Scholar]
- Dong Z., Brennan A., Liu N., Yarden Y., Lefkowitz G., Mirsky R., Jessen K. R. (1995). Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron 15, 585-596 [DOI] [PubMed] [Google Scholar]
- Dutton K. A., Pauliny A., Lopes S. S., Elworthy S., Carney T. J., Rauch J., Geisler R., Haffter P., Kelsh R. N. (2001). Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128, 4113-4125 [DOI] [PubMed] [Google Scholar]
- Eckert J. M., Byer S. J., Clodfelder-Miller B. J., Carroll S. L. (2009). Neuregulin-1 beta and neuregulin-1 alpha differentially affect the migration and invasion of malignant peripheral nerve sheath tumor cells. Glia 57, 1501-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls D. L. (2003). Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 284, 14-30 [DOI] [PubMed] [Google Scholar]
- Fraher J. P. (1992). The CNS-PNS transitional zone of the rat. Morphometric studies at cranial and spinal levels. Prog. Neurobiol. 38, 261-316 [DOI] [PubMed] [Google Scholar]
- Garratt A. N., Britsch S., Birchmeier C. (2000). Neuregulin, a factor with many functions in the life of a schwann cell. BioEssays 22, 987-996 [DOI] [PubMed] [Google Scholar]
- Gilmour D. T., Maischein H.-M., Nüsslein-Volhard C. (2002). Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron 34, 577-588 [DOI] [PubMed] [Google Scholar]
- Gompel N., Dambly-Chaudière C., Ghysen A. (2001). Neuronal differences prefigure somatotopy in the zebrafish lateral line. Development 128, 387-393 [DOI] [PubMed] [Google Scholar]
- Grant K. A., Raible D. W., Piotrowski T. (2005). Regulation of latent sensory hair cell precursors by glia in the zebrafish lateral line. Neuron 45, 69-80 [DOI] [PubMed] [Google Scholar]
- Grossmann K. S., Wende H., Paul F. E., Cheret C., Garratt A. N., Zurborg S., Feinberg K., Besser D., Schulz H., Peles E., et al. (2009). The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc. Natl. Acad. Sci. USA 106, 16704-16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayworth C. R., Moody S. E., Chodosh L. A., Krieg P., Rimer M., Thompson W. J. (2006). Induction of neuregulin signaling in mouse schwann cells in vivo mimics responses to denervation. J. Neurosci. 26, 6873-6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W. H., Armanini M. P., Nuijens A., Phillips H. S., Osheroff P. L. (1995). Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J. Biol. Chem. 270, 14523-14532 [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Sliwkowski M. X., Akita R. W., Henzel W. J., Lee J., Park J. W., Yansura D., Abadi N., Raab H., Lewis G. D. (1992). Identification of heregulin, a specific activator of p185erbB2. Science 256, 1205-1210 [DOI] [PubMed] [Google Scholar]
- Honjo Y., Kniss J., Eisen J. S. (2008). Neuregulin-mediated ErbB3 signaling is required for formation of zebrafish dorsal root ganglion neurons. Development 135, 2615-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. (2005). The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6, 671-682 [DOI] [PubMed] [Google Scholar]
- Kaneko S., Iwanami A., Nakamura M., Kishino A., Kikuchi K., Shibata S., Okano H. J., Ikegami T., Moriya A., Konishi O., et al. (2006). A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 12, 1380-1389 [DOI] [PubMed] [Google Scholar]
- Kawakami K. (2004). Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 77, 201-222 [DOI] [PubMed] [Google Scholar]
- Kucenas S., Takada N., Park H.-C., Woodruff E., Broadie K., Appel B. (2008). CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 11, 143-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucenas S., Wang W.-D., Knapik E. W., Appel B. (2009). A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. J. Neurosci. 29, 15187-15194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D. A., Pogoda H.-M., Voas M. G., Woods I. G., Diamond B., Nix R., Arana N., Jacobs J., Talbot W. S. (2005). erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr. Biol. 15, 513-524 [DOI] [PubMed] [Google Scholar]
- Lyons D. A., Naylor S. G., Mercurio S., Dominguez C., Talbot W. S. (2008). KBP is essential for axonal structure, outgrowth and maintenance in zebrafish, providing insight into the cellular basis of Goldberg-Shprintzen syndrome. Development 135, 599-608 [DOI] [PubMed] [Google Scholar]
- Mahanthappa N. K., Anton E. S., Matthew W. D. (1996). Glial growth factor 2, a soluble neuregulin, directly increases Schwann cell motility and indirectly promotes neurite outgrowth. J. Neurosci. 16, 4673-4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintanis S., Thomaidou D., Jessen K. R., Mirsky R., Matsas R. (2001). The neuron-glia signal beta-neuregulin promotes Schwann cell motility via the MAPK pathway. Glia 34, 39-51 [PubMed] [Google Scholar]
- Meyer D., Yamaai T., Garratt A., Riethmacher-Sonnenberg E., Kane D., Theill L. E., Birchmeier C. (1997). Isoform-specific expression and function of neuregulin. Development 124, 3575-3586 [DOI] [PubMed] [Google Scholar]
- Michailov G. V., Sereda M. W., Brinkmann B. G., Fischer T. M., Haug B., Birchmeier C., Role L., Lai C., Schwab M. H., Nave K.-A. (2004). Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700-703 [DOI] [PubMed] [Google Scholar]
- Milan D. J., Giokas A. C., Serluca F. C., Peterson R. T., MacRae C. A. (2006). Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development 133, 1125-1132 [DOI] [PubMed] [Google Scholar]
- Monje P. V., Soto J., Bacallao K., Wood P. M. (2008). Protein kinaseA-mediated gating of neuregulin-dependent ErbB2-ErbB3 activation underlies the synergystic action of cAMP on Schwann cell proliferation. J. Biol. Chem. 283, 34087-34100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk K. R., Naylor S. G., Glenn T. D., Mercurio S., Perlin J. R., Dominguez C., Moens C. B., Talbot W. S. (2009). A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325, 1402-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey T. K., Levi A. D., Nuijens A., Sliwkowski M. X., Bunge R. P. (1995). Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl. Acad. Sci. USA 92, 1431-1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K.-A., Salzer J. L. (2006). Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 16, 492-500 [DOI] [PubMed] [Google Scholar]
- Newbern J., Birchmeier C. (2010). Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin. Cell Dev. Biol. 21, 922-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenthal J., Nüsslein-Volhard C. (1998). fork head domain genes in zebrafish. Dev. Genes Evol. 208, 245-258 [DOI] [PubMed] [Google Scholar]
- Oxtoby E., Jowett T. (1993). Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 21, 1087-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-C., Mehta A., Richardson J. S., Appel B. (2002). olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev. Biol. 248, 356-368 [DOI] [PubMed] [Google Scholar]
- Pogoda H. M., Sternheim N., Lyons D. A., Diamond B., Hawkins T. A., Woods I. G., Bhatt D. H., Franzini-Armstrong C., Dominguez C., Arana N., et al. (2006). A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev. Biol. 298, 118-131 [DOI] [PubMed] [Google Scholar]
- Raper J., Mason C. (2010). Cellular strategies of axonal pathfinding. Cold Spring Harb. Perspect. Biol. 2, 1-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael A. R., Perlin J. R., Talbot W. S. (2010). Schwann cells reposition a peripheral nerve to isolate it from postembryonic remodeling of its targets. Development 137, 3643-3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael A. R., Lyons D. A., Talbot W. S. (2011). ErbB signaling has a role in radial sorting independent of Schwann cell number. Glia 59, 1047-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum C., Karyala S., Marchionni M. A., Kim H. A., Krasnoselsky A. L., Happel B., Isaacs I., Brackenbury R., Ratner N. (1997). Schwann cells express NDF and SMDF/n-ARIA mRNAs, secrete neuregulin, and show constitutive activation of erbB3 receptors: evidence for a neuregulin autocrine loop. Exp. Neurol. 148, 604-615 [DOI] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P., Glaser L. (1980). Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J. Cell Biol. 84, 767-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E. K., Mason L., Arrenberg A. B., Ziv L., Gosse N. J., Xiao T., Chi N. C., Asakawa K., Kawakami K., Baier H. (2007). Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4, 323-326 [DOI] [PubMed] [Google Scholar]
- Sherman D. L., Brophy P. J. (2005). Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6, 683-690 [DOI] [PubMed] [Google Scholar]
- Syed N., Reddy K., Yang D. P., Taveggia C., Salzer J. L., Maurel P., Kim H. A. (2010). Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 30, 6122-6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot W. S., Schier A. F. (1999). Positional cloning of mutated zebrafish genes. Methods Cell Biol. 60, 259-286 [DOI] [PubMed] [Google Scholar]
- Taveggia C., Zanazzi G., Petrylak A., Yano H., Rosenbluth J., Einheber S., Xu X., Esper R. M., Loeb J. A., Shrager P., et al. (2005). Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeren M., Maro G. S., Bron R., McGonnell I. M., Charnay P., Topilko P., Cohen J. (2003). Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron 37, 403-415 [DOI] [PubMed] [Google Scholar]
- Voas M. G., Glenn T. D., Raphael A. R., Talbot W. S. (2009). Schwann cells inhibit ectopic clustering of axonal sodium channels. J. Neurosci. 29, 14408-14414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Miller S. J., Falls D. L. (2001). The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J. Biol. Chem. 276, 2841-2851 [DOI] [PubMed] [Google Scholar]
- Williams P. R., Suzuki S. C., Yoshimatsu T., Lawrence O. T., Waldron S. J., Parsons M. J., Nonet M. L., Wong R. O. L. (2010). In vivo development of outer retinal synapses in the absence of glial contact. J. Neurosci. 30, 11951-11961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpowitz D., Mason T. B., Dietrich P., Mendelsohn M., Talmage D. A., Role L. W. (2000). Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron 25, 79-91 [DOI] [PubMed] [Google Scholar]
- Zawadzka M., Rivers L. E., Fancy S. P. J., Zhao C., Tripathi R., Jamen F., Young K., Goncharevich A., Pohl H., Rizzi M., et al. (2010). CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6, 578-590 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.